ABSTRACT
Objectives
Our objective is to retrospectively analyze the response to low dose of homoharringtonine (HHT) and cytarabine-based priming induction regimens in patients above 70 years with de novo acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS).
Patients and methods
We retrospectively analyzed these very elderly newly diagnosed patients with AML and high-risk MDS, who received low dose of HHT and cytarabine-based priming induction regimens between March 2006 and September 2019.
Results
Of the 24 patients, 11 patients (47.8%) achieved complete remission (CR) and 3 (13%) partial remission, and the overall response rate was 60.9%. The estimated median overall survival (OS) time was 12 months and the 1-year OS rate was 47.8%. Patients without CR and Charlson’s Comorbidity Index > 2 may be the two independent prognostic factors. The median OS was significantly higher for patients with CR after induction chemotherapy than those without CR (22.93 vs. 8.5 months, p < .01).
Conclusion
Our study provides a hint of the efficacy of low dose of HHT and cytarabine-based priming induction regimens for patients aged over 70 years with de novo AML and high-risk MDS should be further studied.
Introduction
The incidence of acute myeloid leukemia (AML) increases with age, with a median age of ≥65 years at the time of diagnosis [Citation1,Citation2]. Although much progress has been made in the younger patients over the past decades, the survival of elderly AML patients remains poor [Citation3–5]. Elderly AML is different from young AML, including more frequently unfavorable cytogenetics, biologically somatic mutations, chemotherapy resistance and/or relatively poor tolerance of chemotherapy owing to comorbidity [Citation5,Citation6]. Therefore, elderly AML is difficult to cure and often has a poor outcome. AML in the elderly population (aged ≥ 70 years) has a high incidence [Citation7]. There is no standard treatment regimen for these elderly AML patients, and they are often recommended to enroll the clinical trials. However, in reality, patients aged over 70 years are usually excluded in the randomized controlled trials. There is no large-scale randomized control of intensive chemotherapy and hypomethylating agent for these patients. Optimal front-line therapies for this subset of patients remain controversial. Several retrospective studies suggested intensive chemotherapy did not benefit these elderly patients’ survival [Citation8–11]. Decitabine was effective in the treatment of newly diagnosed AML aged over 65 years [Citation12]. Talati et al. [Citation13] retrospectively analyzed the clinical survival data in 980 elderly (≥70 years) patients with AML, and found significant longer median overall survival (OS) with hypomethylating agent (14.4 months) compared to high-intensity therapy (10.8 months). Homoharringtonine (HHT) is a natural cephalotaxine alkaloid derived from a Chinese Yew species called Cephalotaxus herringtonia. Several studies confirmed HHT-based intensive induction regimens had promising results in young patients and elderly patients with AML [Citation14–16]. A low dose of HHT and cytarabine combined with a granulocyte colony-stimulating factor (CHG regimen) were often used to treat elderly patients with AML [Citation17–19]. However, there were a few patients aged over 70 years and often had a poor median overall survival. In this study, we retrospectively analyzed survival outcomes of HHT and cytarabine-based priming induction regimens for de novo AML and high-risk myelodysplastic syndrome (MDS) patients aged over 70 years in our center.
Patients and methods
Subjects
A retrospective chart review was performed to evaluate newly diagnosed patients with AML and MDS aged over 70 years who presented to First Hospital of Tsinghua University between March 2006 and September 2019. The diagnosis criteria of AML and MDS were according to the World Health Organization (WHO) classification systems [Citation20]. Patients with acute promyelocytic leukemia were excluded from this study. At the time of diagnosis, functional status was assessed using the Barthel activity of daily living (ADL) scale [Citation21]. Comorbidity was evaluated using Charlson’s Comorbidity Index (CCI) (leukemia, MDS, and HIV excluded) [Citation22]. The Eastern Cooperative Oncology Group (ECOG) performance status (PS) information and body mass index (BMI) [Citation23] was obtained at the diagnosis.
Treatment
The patients received the following HHT and cytarabine-based priming induction regimens determined by the physician’s experience: (1) priming regimen CHG (cytarabine 10 mg/m2/12 h, subcutaneously, for 10–14 days, HHT 1 mg/day, continuously infused, for 10–14 days, and G-CSF 300 μg/day, subcutaneously, from day 0 until white blood cell (WBC) count > 20 × 109/L); or (2) decitabine combination with an adjusted CHG (decitabine 7 mg/m2, continuously infused, for 6–9 days, cytarabine 10 mg/m2/12 h, subcutaneously, for 8–12 days, HHT 1 mg/day, continuously infused, for 8–12 days, and G-CSF 300 μg/day, subcutaneously, from day 0 until WBC count > 20 × 109/L). If the treatment response was achieved after 1 cycle, the previous regimen would be consolidated for one or two cycles. Otherwise, a different regimen would be used in the next cycle.
Study assessments
The time to hematopoietic recovery was measured from the last day of chemotherapy to the day when the neutrophil count was more than 0.5 × 109/L, and the platelet count was more than 20 × 109/L in the patients who achieved complete remission (CR). Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0.
Efficacy
Bone marrow assessments were performed after each cycle recovery. The CR achieved by induction chemotherapy was assessed according to the AML response criteria [Citation24]. The morphologic CR was defined as normal hematopoiesis of bone marrow blasts (≤5% blasts), peripheral blood counts (absolute neutrophil count ≥ 1.0 × 109/L and platelet ≥ 100 × 109/L), and there were no blasts in peripheral blood and extramedullary disease. OS and disease-free survival (DFS) were estimated by the Kaplan–Meier method. OS was defined as the time from the start of treatment to the date of death from any cause or the termination of observation. DFS was evaluated from the date of CR to an event, including partial response (PR), relapse, or death. Early toxic death (ED) was defined as the 30 days mortality after an induction cycle.
Statistical analysis
OS was analyzed by the Kaplan–Meier method using SPSS software (19.0). Survival among different categories was compared by the log-rank test. Missing data were imputed as censored. In univariate analysis, the possible risk factors were expressed by hazard ratio (HR) with 95% and 90% confidence interval (CI) and p-value. Multivariate analyses of prognostic factors of survival were used by the Cox proportional hazard method. p < .05 was considered statistically significant. All graphs were made using Prism software (GraphPad, San Diego, CA, USA).
Results
Patient characteristics
Clinical characteristics of AML and MDS patients, such as age, sex, ECOG PS, CCI, ADL, BMI, and so on, are recorded in . Ten females and 14 males, with a median age of 75 years (range 70–84) were included. The mean WBC count was 5.54 × 109/L, ranging from 0.66 to 153.24 × 109/L. Of the 24 patients, 18 patients received a CHG induction regimen and 6 patients with decitabine combination with an adjusted CHG treatment. The study was approved by the ethical committee of First Hospital of Tsinghua University (No.2021-07), and informed consent was obtained from all patients.
Table 1. Baseline patient characteristics.
Response to treatment
Among 24 patients, one did not undergo the bone marrow examination to evaluate response after chemotherapy despite hematologic recovery. Of the 23 analyzable cases, 7 patients (30.4%) achieved CR after the first cycle of induction chemotherapy, 5 patients (21.7%) PR, overall response rate (ORR) was 52.2%. 2 PR patients achieved CR after two cycles, and 2 PR patients achieved CR after three cycles. Therefore, 11 patients (47.8%) achieved CR and 3 (13%) PR, ORR was 60.9%.
Overall survival
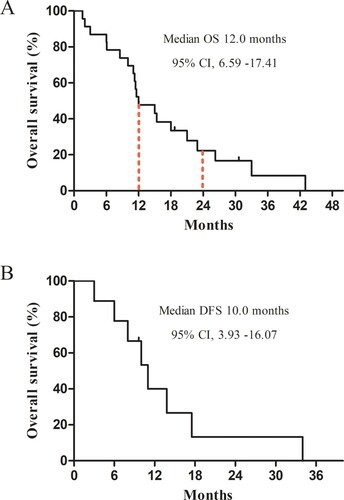
The terminal point of the follow-up was September 2020. The median follow-up time was 12.0 months (1.6–43.0 months). One of 24 patients (4%) was lost to follow-up. For all the patients, the estimated median OS was 12.0 months, 95% CI, 6.59–17.41, with an OS rate of 47.8% at 1 year and 22.3% at 2 years ( (A)). Also, in CR patients, the median duration of remission was 10.0 months, 95% CI, 3.93–16.07 ((B)). In CR patients, the estimated 1- and 2-year OS rate was 90% and 40%, respectively. However, the estimated 1- and 2-year OS rate of patients without the CR group was significantly lower, at 1-year OS rate of 17% and 2-year OS rate of 8%, respectively.
Figure 1. Kaplan–Meier curves of survival for AML and MDS patients aged over 70 years. (A) OS for all patients; (B) DFS for patients who achieved CR after treatment. OS, overall survival; DFS, disease-free survival; CI, confidence interval; CR, complete remission; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome.
Prognostic factors for overall survival
In univariate analysis, patients who did not achieve CR and CCI (>2) may be the two worse prognostic factors to estimate the poor OS. Also, WBC (> 5 × 109/L) tended to estimate the poor OS (). Furthermore, multivariate analysis showed patients who did not achieve CR and CCI (>2) might be the two independent prognostic factors for the poor survival (). The median OS of the CCI (>2) group (3.0 months, 95% CI 1.40–4.60) was significantly shorter than the CCI (≤2) group (12.0 months, 95% CI 4.17–19.83) (p < .05). Compared to patients who achieved CR, patients who did not achieve CR had a shorter median OS (8.50 months, 95% CI 1.88–15.12 vs. 22.93 months, 95% CI 13.34–32.52) (p < .01) ().
Figure 2. Kaplan–Meier curves of OS for AML and MDS patients aged over 70 years. (A) CCI subgroup in all patients; (B) WBC subgroup in all patients; (C) OS for patients who achieved CR and those none-CR. OS, overall survival; CCI, Charlson’s comorbidity index; WBC, white blood cell; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; CR, complete remission.
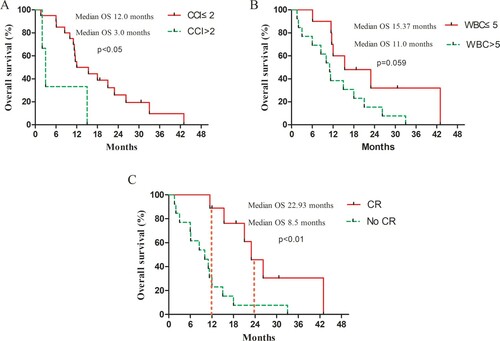
Table 2. Univariate analysis for overall survival.
Table 3. Multivariate analysis for overall survival.
Treatment-related toxicity
Bone marrow suppression was the most common AEs during induction therapy. Among patients who achieved CR, the median time was 8 days (ranging from 0 to 14 days) for granulocyte recovery and 7 days (range from 0 to 20 days) for platelet recovery. No ED occurred in this study. Non-hematological toxicities were mild. Twenty-one (87.5%) patients had grade 2–4 infectious toxicity (19% grade 3/4). Of these infectious patients, 18 patients had pulmonary infections, 3 had bacteremia, and 1 septic shock. Grade 1–2 increased alanine aminotransferase was observed in 3 patients. Cardiac events were observed in 2 patients (1 atrial fibrillation and 1 angina pectoris).
Discussion
The treatment of AML in elderly patients remains a big challenge. Previous most clinical trials had a few patients aged over 70 years. In the present study, we retrospectively analyzed these very elderly AML and MDS patients aged over 70 years in real world. Also, many patients had several chronic coexisting diseases and poor functional status PS at the time of diagnosis.
Up to now, there is no standard treatment regimen for these elderly AML patients aged over 70 years. Kantarjian et al. showed that intensive chemotherapy might not be beneficial to most AML patients older than 70 years. The median OS was only 4.6 months, and the 4-week mortality was 26% [Citation9]. A similar result was also observed in Ross’ study [Citation25]. However, Heiblig et al. suggested intensive chemotherapy was effective for these elderly patients with a median OS of 12.4 months. They also showed similar OS between intensive chemotherapy and lower intensity treatments [Citation26]. Budziszewska prospectively estimated outcomes in 509 elderly AML patients. They stratified patients into fit (ECOG 0–2 and CCI 0–2) or frail (ECOG > 2 and/or CCI > 2) groups. Their data showed a longer OS in fit patients received chemotherapy than those frail subjects with the best supportive therapy. However, the median OS in this study was unsatisfactory. Although many young patients aged < 70 years were included, the median OS in the fit and frail patients was only 30.3 and 13.8 weeks, respectively [Citation27]. Recently, Talati et al. retrospectively evaluated survival outcomes in 980 elderly (≥70 years) AML patients. Pairwise comparisons result indicated a significant median OS benefit with hypomethylating agents (14.4 months) compared to high-intensity (10.8 months) or supportive care (2.1 months). The estimated survival probability at 1 year with HMA treatment was significantly greater than high-intensity therapy (55.4% versus 42.7%), and the supportive care group was still 14.2% [Citation13]. They also showed that any treatment was superior to no treatment in these patients aged over 70 years. Although a high OS rate was achieved in this retrospective study, there was a high selective bias in this study. Most of patients (79.3%) had a good ECOG PS (0–1), and only 13.4% of patients had a high CCI of ≥3. In a leukemia registry, Juliusson demonstrated even older patients with poor ECOG PS seemed to benefit from chemotherapy compared with patients with a supportive treatment [Citation28]. Consistent with Talati and Juliusson’s study, our data showed a low dose of HHT and cytarabine-based chemotherapy might be effective in these AML and MDS patients aged over 70 years.
Li et al. [Citation29] designed a D-CAG induction treatment regimen for elderly patients with newly diagnosed AML and showed a promising clinical efficacy in elderly patients with AML. The OS for patients aged ≥ 70 years and 60–69 years was 10 and 12 months, respectively. However, myelosuppression of adverse effects was severe. The median time of granulocyte recovery (> 0.5 × 109/L) was 23 days (range, 16–46 days) and 16 days (range, 5–50 days) for platelet recovery (> 20 × 109/L) in patients with CR. Chen showed CHG regimen was effective in 18 patients aged 70–80 years, with the median OS of 6.0 (95% CI 4.8–7.2) months [Citation18]. In our study, a low dose of HHT and cytarabine-based priming induction regimens may be effective for AML and high-risk MDS patients aged over 70 years. Compared with previous studies, the estimated median OS in the present data was significantly higher (12.0 months, 95% CI 6.59–17.41), and the estimated 1-year OS rate was 47.8%. Recently, B-cell lymphoma 2 inhibitor venetoclax combined with hypomethylating agents showed a good response for elderly AML patients. The CR + CRi rate for the venetoclax 400 mg + HMA cohort was 73%, the median OS for all patients was 17.5 months. One-year OS rates for all patients were 59%. However, the median time to the first response was longer (1.2 months, range 0.8–13.5 months) [Citation30]. Also, the drug is costly and could not be applied as a common regimen in China.
Emerging evidence suggested that chronologic age was not a fundamental factor in determining the available treatment options [Citation31]. However, in the real world, age remains an important factor while taking the treatment decision. The best supportive care might be the only reasonable option for patients age >80 years [Citation31]. In the present study, the univariate analysis suggested age (> 80 years) might not be the worse prognostic factor to estimate the poor OS. However, given only 1 patient > 80 years in this study, it may be an incidental finding. Further study is needed to collect cases to explore the prognostic factor of age. Comorbidity scoring was important to predict prognosis and useful for decision-making for elderly AML [Citation28]. Francis et al. found haematopoietic cell transplantation comorbidity index score was a predictive factor of early death and survival in elderly AML patients receiving induction therapy [Citation32]. In Budziszewska’s study, elderly AML patients with CCI > 2 were assigned to the frail group to receive a supportive treatment with a median OS of 13.8 weeks [Citation27]. Our data showed CCI (>2) might be an independent prognostic factor for the survival of elderly patients aged over 70 years. Although these patients with CCI (>2) were treated with the HHT-based chemotherapy, they had a lower median OS (3.0 months) compared with CCI (≤2)(12.0 months). Of note, the general health condition of the elderly patients (aged over 70 years) in China seems to be worse than that in American and European countries, which shows good tolerability to induction chemotherapy. Because of limited cases in our study, although statistically significant CCI was shown in multivariable analysis,the 95% CI was significantly wide, which might be attributed to a random error. In the future, we need to collect cases to confirm the poor prognostic factor of CCI (>2).
In the real world, our results suggest a low dose of HHT and cytarabine-based priming induction regimens may be effective and safe for the elderly AML and high-risk MDS patients aged over 70 years. Decision-making in these patients might be based on biological features and comorbidity rather than only on age. Given the limitations of a few cases and single institution cohort in this study, further study is needed to enlarge cases and perform a multicenter, prospective cohort study to investigate the low dose of HHT and cytarabine-based priming induction regimens for elderly AML patients aged over 70 years.
Acknowledgments
The authors would like to thank Prof. Wenjing Gao (School of Public Health, Peking University, China) and Guifen Liu (School of Public Health, Shanxi Medical University, China) for the help in the statistical analysis of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- United Nations. Department of economic and social affairs. Population DivisionWorld population prospects. The 2010 Revision. New York (NY): United Nations; 2011. (Report no. ST/ESA/SER.A/313)
- Colovic M, Colovic N, Radojkovic M, et al. Induction chemotherapy versus palliative treatment for acute myeloid leukemia in a consecutive cohort of elderly patients. Ann Hematol. 2012;91(9):1363–1370.
- Hiddemann W, Kern W, Schoch C, et al. Management of acute myeloid leukemia in elderly patients. J Clin Oncol. 1999;17:3569–3576.
- Lowenberg B, Zittoun R, Kerkhofs H, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 65 > years with acute myeloid leukemia: a randomized phase III of the European Organization for Research and Treatment of Cancer Leukemia Group. J Clin Oncol. 1989;7:1268–1274.
- Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485.
- Kantarjian H, O'brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106(5):1090–1098.
- Estey EH. Older adults: should the paradigm shift from standard therapy? Best Pract Res Clin Haematol. 2008;21(1):61–66.
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Kantarjian H, Ravandi F, O'Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116(22):4422–4429.
- Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361(13):1235–1248.
- Gardin C, Turlure P, Fagot T, et al. Postremission treatment of elderly patients with acute myeloid leukemia in first complete remission after intensive induction chemotherapy: results of the multicenter randomized Acute Leukemia French Association (ALFA) 9803 trial. Blood. 2007;109(12):5129–5135.
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–2677.
- Talati C, Dhulipala VC, Extermann MT, et al. Comparisons of commonly used front-line regimens on survival outcomes in patients aged 70 years and older with acute myeloid leukemia. Haematologica. 2020;105(2):398–406.
- Wang J, Lü S, Yang J, et al. A homoharringtonine-based induction regimen for the treatment of elderly patients with acute myeloid leukemia: a single center experience from China. J Hematol Oncol. 2009;2(32) doi:https://doi.org/10.1186/1756-8722-2-32.
- Jin J, Jiang DZ, Mai WY, et al. Homoharringtonine in combination with cytarabine and aclarubicin resulted in high complete remission rate after the first induction therapy in patients with de novo acute myeloid leukemia. Leukemia. 2006;20(8):1361–1367.
- Jin J, Wang JX, Chen FF, et al. Homoharringtonine-based induction regimens for patients with de-novo acute myeloid leukaemia: a multicentre, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2013;14(7):599–608.
- Wu L, Li X, Su J, et al. Efficacy and safety of CHG regimen (low-dose cytarabine, homoharringtonine with G-CSF priming) as induction chemotherapy for elderly patients with high-risk MDS or AML transformed from MDS. J Cancer Res Clin Oncol. 2011;137(10):1563–1569.
- Chen C, Xu W, Yang J. Low-dose homoharringtonine and cytarabine in combination with granulocyte colony-stimulating factor for elderly patients with de novo acute myeloid leukemia. Leuk Lymphoma. 2015;56(1):141–146.
- Liu H, Zhang J, Ren S, et al. Using low-dose homoharringtonine and cytarabine in combination with granulocyte colony-stimulating factor in a priming induction therapy for acute myeloid leukemia: a retrospective study of 29 cases in China. Leuk Lymphoma. 2017;58(11):2758–2761.
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302.
- Stewart AL, Ware JE Jr. Measuring functioning and well-being: the medical outcomes study approach. Durham (NC): Duke University Press; 1992.
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251.
- Landi F, Onder G, Gambassi G, et al. Body mass index and mortality among hospitalized patients. Arch Intern Med. 2000;160:2641–2644.
- Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21(24):4642–4649.
- Ross K, Gillespie-Twardy AL, Agha M, et al. Intensive chemotherapy in patients aged 70 years or older newly diagnosed with acute myeloid leukemia. Oncol Res. 2015;22(2):85–92.
- Heiblig M, Le Jeune C, Elhamri M, et al. Treatment patterns and comparative effectiveness in elderly acute myeloid leukemia patients (age 70 years or older): the Lyon-university hospital experience. Leuk Lymphoma. 2017;58(1):110–117.
- Budziszewska BK, Pluta A, Sulek K, et al. Treatment of elderly patients with acute myeloid leukemia adjusted for performance status and presence of comorbidities: a Polish adult Leukemia Group study. Leuk Lymphoma. 2015;56(8):2331–2338.
- Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish acute leukemia registry. Blood. 2009;113(18):4179–4187.
- Li J, Chen Y, Zhu Y, et al. Efficacy and safety of decitabine in combination with G-CSF, low-dose cytarabine and aclarubicin in newly diagnosed elderly patients with acute myeloid leukemia. Oncotarget. 2015;6(8):6448–6458.
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17.
- Estey EH. Treatment of acute myeloid leukemia. Haematologica. 2009;94:10–16.
- Giles FJ, Borthakur G, Ravandi F, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136:624–627.
