ABSTRACT
Objective
This study was carried out to explore clinical treatment and prognosis of patients with AA with different economic status. Methods: We retrospectively analyzed the clinical outcome of 301 patients with AA in our center from April 2008 to November 2017.
Results
Treatments included anti-thymocyte globulin (ATG) or anti-lymphocyte globulin (ALG) combined with cyclosporineA (CsA) (9%), allogeneic hematopoietic stem cell transplantation (allo-HSCT) (7%), CsA combined with androgen or CsA alone (hereinafter referred to as CsA group) (77%), no specific therapy (7%). The 5-year overall survival (OS) was higher in patients with non-severe AA (94.6%) compared with those with severe AA (SAA) (66.6%, P <.001), very severe AA (VSAA) (41.3%, P <.001). The 5-year OS was 76.5% in patients with SAA/VSAA treated with ATG/ALG combined with CsA, 75% in allo-HSCT group(P =.936), 63.6% in CsA group (P =.557), which was significantly higher than no specific therapy group (21.8%, P =.002). For those who responded to CsA , the duration of CsA (median follow-up time: 27 months, 1–101 months) was positively correlated with progression-free survival (r=0.603, P <.001). Multivariate analysis revealed that 36–65 years of age, SAA/VSAA, and no specific therapy were independent risk factors for inferior survival.
Conclusion
The treatment of elderly patients with AA still faces challenges. CsA is benefit to the survival of SAA/VSAA patients. AA patients, who responded to initialy CsA treatment, may benefit from prolonged CsA treatment. In view of the side effects of CsA, the timing of withdrawal is worth further exploration.
Introduction
Aplastic anemia (AA) is a rare bone marrow failure syndrome characterized by pancytopenia and a markedly hypocellular marrow. The underlying pathophysiology is believed to be immune-mediated hematopoietic stem cell destruction [Citation1]. The application of allogeneic hematopoietic stem cell transplantation (allo-HSCT) and ATG/ALG had improved the prognosis of AA patients greatly with 5-year overall survival (OS) rates of up to approximately 70%−80% [Citation2–5]. Owing to differences of regional and race, the results from foreign studies may not truly reflect the treatment and prognosis of our local AA patients. Especially limited by economic conditions, some AA patients cannot accept allo-HSCT or ATG/ALG combined with cyclosporineA (CsA) recommended by the guidelines. Therefore, we sought to review our clinical experiences with treating AA patients about 10 years at Department of Hematology and Pediatrics, The Affiliated Hospital of ******. To ensure the integrity and accuracy of the data, the personal information of each patient, diagnosis of the disease, treatment received, changes of the condition during hospitalization, follow-up after discharge and other detailed data were uniformly registered, so as to conduct a detailed study on the treatment and prognosis of local AA patients. The purpose of this retrospective study was to assess the clinical treatment and prognosis of AA patients in our area and provided some supplements for the treatment of patient population in the development area.
Patients and methods
Diagnosis
This study recruited 301 patients with AA (both children and adults) newly diagnosed in the Affiliated Hospital of ****** from April 2008 to November 2017. Personal information of all AA patients, including their names, genders, ages, ethnicities, occupations, telephones, addresses, ID numbers, histories of exposure to hazardous chemicals and radioactive materials or no, and medical data are recorded in the case system of our institute in details.
A diagnosis of AA was made and severity graded as per currently accepted criteria [Citation6]; disease diagnosis: pancytopenia with a hypocellular bone marrow in the absence of an abnormal infiltrate or marrow fibrosis, plus at least two of the following haemoglobin concentration (Hb) <100 g/L, platelet count <50 × 109/L, neutrophil count <1.5 × 109/L. Disease severity was classified as follows: severe AA (SAA), marrow cellularity <25% (or 25–50% with <30% residual hematopoietic cells), plus at least 2 of: neutrophils <0.5 × 109/L, platelets <20 × 109/L, reticulocyte count <20 × 109/L; very severe AA (VSAA), as for SAA but neutrophils <0.2 × 109/L; non-severe AA (NSAA), AA not fulfilling the criteria for SAA or VSAA. Patients were excluded if (1) patients with congenital bone marrow failure, including Fanconi anemia (FA), dyskeratosis congenital, Shwachman-Diamond syndrome, Dimaond–Blackfan anemia, (2) the disease was not newly diagnosed, and (3) they received therapy previously.
Statistics
All analyses and graphs were obtained using the statistical software SPSS Statistics 19.0(SPSS Inc, Chicago, IL). Non-normally distributed data were expressed as median. Rates and proportions were compared using Pearson’s χ2 test. The Kaplan–Meier method was used to evaluate OS, and comparisons were based on the Log-rank test. Spearman analysis was used to evaluate correlation. Cox proportional hazards regression was used to carry out multivariate survival analyses, including age, type of treatment and disease severity. All statistical tests were two-sided, with P values ≤.05 indicating statistical significance.
Results
Basic data and treatments
The clinical features of 301 AA patients are summarized in , including 178 males (59%) and 123 females (41%). The median age at diagnosis was 27 years (range: 2−93). One fifty seven patients had NSAA, 78 patients had SAA, and 66 patients had VSAA. Younger patients accounted for a higher proportion of the total follow-up cohort. Among the 301 patients, 233 (77%) AA patients were initially treated with CsA combined with androgen or CsA alone(CsA group). Of those AA patients who did not respond to CsA or no specific therapy, two were treated with allo-HSCT and seven were treated with ATG/ALG combined with CsA. A small number of AA patients chose allo-HSCT (20 cases, 7%) or ATG/ALG combined with CsA (26 cases, 9%), and most of them were SAA/VSAA patients less than 35-years-old. All AA patients aged older than 65 years of age chose CsA-based treatment or no specific therapy.
Table 1 . Clinical characteristics.
Survival
The median time of follow-up was 38 months (range: 1–144 months). Fifty (19%) died during follow-up, 23 died within 3 months after diagnosis and the survival time of 40 were less than 12 months. As the main causes of death, 40 (69%) AA patients died from infection. Throughout the follow-up period, the paroxysmal nocturnal hemoglobinuria clone were detected in 24 patients, 3 case patients eventually transformed into myelodysplastic syndrome. Multivariate Cox-regression analysis showed that 36–65 years of age, SAA/VSAA, and no specific therapy were independent risk factors for inferior survival ().
Table 2. Uni- and multivariable Cox-regression.
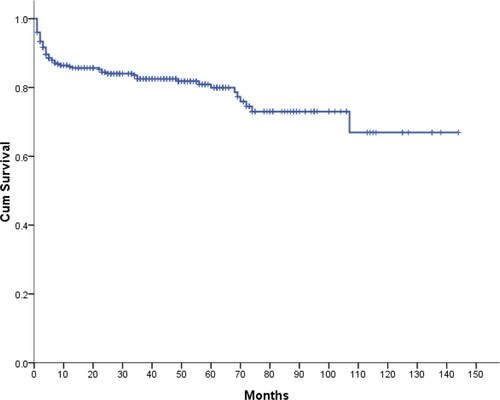
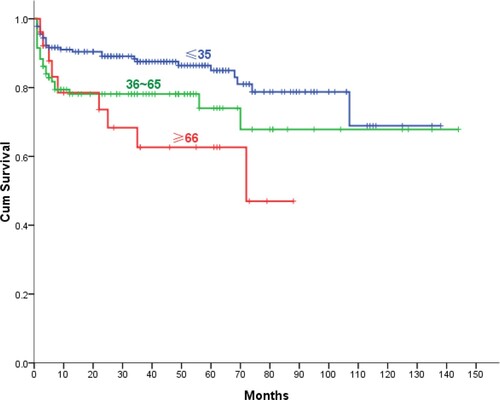
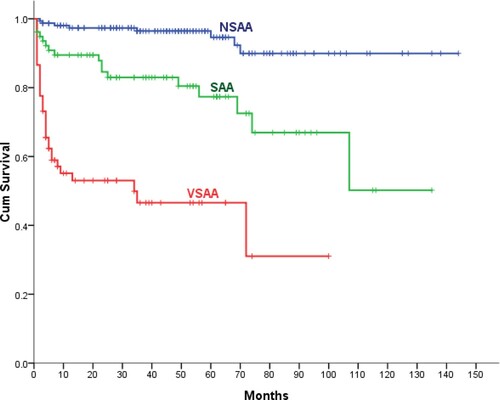
A 5-year OS of all patients were 79.9% after diagnosis (), with no gender differences (P = .64). The 5-year OS of AA patients in the 1–18-year-old group and the 19–35-year-old group were 82.2% and 87.1%, respectively, with no significant difference (P = .481). When grouped by age, patients with aged ≤35 years had 85% 5-year OS, and the survival rate dropped to 74% (P = .027) in 36–65 years, and 62.7%(P < .001) in ≥66 years age groups. (). There was no significant difference of 5-year OS in the last two age groups (P = .35). A 5-year OS was higher in patients with NSAA (94.6%) compared with those with SAA (66.6%, P < .001), VSAA (41.3%, P < .001) (). The early mortality rate (at 3 months) of NSAA (1.3%) was significantly lower than that of SAA (6.4%, P < .001) and VSAA (27.2%, P < .001), respectively.
Figure 3. Overall survival according to disease severity at diagnosis (very severe, severe, and non-severe AA).
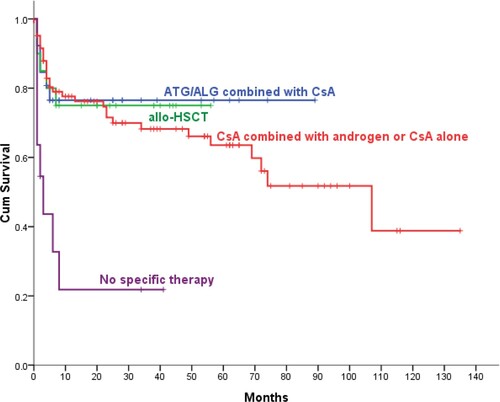
The 5-year OS was 76.5% in patients with SAA/VSAA treated with ATG/ALG combined with CsA, 85.4% in the allo-HSCT group (P = .797), 63.6% in the CsA group (P = .557), which was significantly higher than no specific therapy group (21.8%, P = .002) (). In our study, the 5-year OS and 10-year OS of 144 patients with SAA/VSAA were 63% and 39.4%, respectively. Only 46 cases (32%) of them accepted ATG/ALG combined with CsA or allo-HSCT as first-line treatment. Thirty-one cases (75.6%) of 41 SAA/VSAA patients aged ≤18 years old were initially treated with CsA or no specific therapy, 11 (35.5%) died within 12 months after diagnosis. Objective response rate (ORR), including complete remission (CR) rate and partial remission rate, of SAA/VSAA patients who were initially treated with ATG/ALG combined with CsA was 57.7%, lower than the allo-HSCT group(80%), but the difference was not statistically significant (P = .2). There was no significant difference in ORR between AA patients initially receiving ATG/ALG combined with CsA or allo-HSCT compared and those receiving ATG/ALG combined with CsA or allo-HSCT as the second-line treatment regimen (67.4%:44.4%, P = 35.2%).
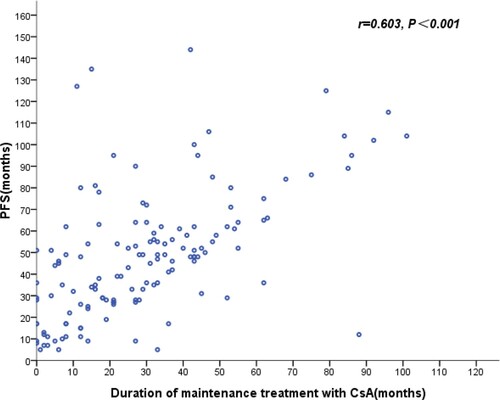
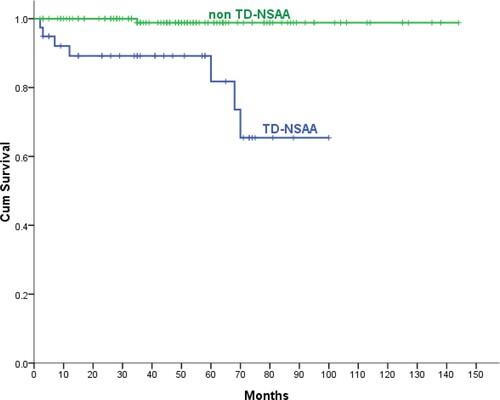
The 5-year OS of NSAA patients treated with CsA combined with androgen or CsA alone was significantly higher than the no specific therapy group (97.8%:75%, P = .013), and there was the higher ORR in the CsA group than the no specific therapy group (69.7%:20%, P = .004). For those AA patients who responded to initialy CsA treatment, the duration of maintenance treatment with CsA (median follow-up time: 27 months, 1–101 months) was positively correlated with progression-free survival (r = 0.603, P < .001) (). In the CsA group, the 10-year OS of the patients aged ≤50 years was significantly higher than the patients aged ≥51 years(73.7%:62.9%, P = .025). During the follow-up, 15 NSAA patients progressed to SAA, and 5 of them died within the next 6 months. Among the aforementioned 15 patients, 11 cases were transfusion dependent (TD) (defined as ≥2 transfusion events within an 8-week period) [Citation7] NSAA (TD-NSAA), including red cell transfusion dependence and/or platelet transfusion dependence, accounting for 28.2% of the total TD-NSAA (39 cases). Further studies showed that TD-NSAA patients had lower 5-year OS than non–TD-NSAA patients (81.8%:98.8%, P < .001) ().
Discussion
The results from our study showed that 5-year OS of all patients were 79.9%, which appears to be superior in comparison with data from the previous population-based AA study [Citation8], on Swedish patients diagnosed from 2000 to 2011, where the reported 5-year survival was about 60.7%. Compared with our follow-up results, the proportion of SAA/VSAA patients was higher in the study from Sweden. Age may have an important impact on the survival of patients with AA. The 5-year OS of patients with AA aged ≤35 years old was 85%, which is similar to results from clinical study about allo-HSCT or ATG [Citation9,Citation10]. Compared with those aged ≤35 years old, the 5-year OS was lower in patients aged ≥36 years old patients (85%:71.8%, P = .006). Further analysis showed that there was no significant difference in 5-year OS between patients aged ≤18 years and patients aged 19–35 years, which was consistent with the data from the clinical study in Swedish region [Citation7].
The application of allo-HSCT and ATG had improved the prognosis of AA patients greatly. In accordance with prior studies [Citation11–13], there was no significant difference in 5-year OS between SAA/VSAA patients treated with ATG/ALG combined with CsA and SAA/VSAA patients treated with allo-HSCT(76.5%:75%, P = 93.6%). Limited by economic situation, low percentage of SAA/VSAA patients was treated with first-line regimen. The study results from Japan showed that 10-year OS of younger (0−16 years) SAA patients, receiving allo-HSCT or ATG as first-line treatment, was more than 80% [Citation14]. Compared with Japan study results, the prognosis of younger SAA/VSAA patients in our area was unsatisfactory.
Thirty-two (96%) SAA/VSAA patients aged ≥ 51 years old were treated with cyclosporine-based regimens or no specific therapy. Their 5-year OS was only 49.1% and 10 (30.3%) patients among them died within 12 months after diagnosis. A study about AA from European Bone Marrow Transplantation (EBMT) showed that the 5-year survival rate of the elderly SAA patients, treated with ALG, cyclosporine, or both, was about 50%–57%, which was lower than 72% of SAA patients aged ≤50 years old [Citation15]. With the improvement of medical conditions, although the upper age limit for allo-HSCT is increasing [Citation6,Citation16–18], the survival outcomes of elderly SAA patients undergoing an allo-HSCT is poor and mortality risks increased with age [Citation19–22]. Some clinical data had indicated that eltrombopag had achieved good results in the treatment of SAA [Citation23–25]. In view of the poor prognosis, the elderly SAA patients, who are not eligible for allo-HSCT or ATG/ALG combined with CsA, can attempt to be treated with eltrombopag if they can afford.
Compared with allo-HSCT group or ATG/ALG combined with CsA group, the 5-year OS of SAA/VSAA patients in CsA group was slightly lower, which was significantly higher than the no specific therapy group (63.6%:21.8%, P < .001). SAA/VSAA patients in developing areas, who cannot afford allo-HSCT or ATG/ALG combined with CsA, can benefit from CsA. Both 5-year OS and ORR of NSAA patients in CsA group were superior to the no specific therapy group. For those AA patients who responded to initialy CsA treatment, the duration of maintenance treatment with CsA was positively correlated with progression-free survival. These results showed that this patient population may benefit from long-term treatment with CsA. In view of the side effects of CsA, the timing of withdrawal is worth further exploration. Clinical studies showed that red cell TD has an negative effect on OS of patients with some hematologic malignances [Citation26,Citation27]. Our study results revealed that TD were independent risk factors for inferior survival. Compared with non TD-NSAA group, the 5-year OS was lower and the risk of progressing to SAA was higher in TD-NSAA group. A report from EBMT showed that the ATG + CsA had a 74% ORR for NSAA [Citation28]. which was significantly higher than the ORR in NSAA patients treated with CsA. Compared with CSA alone, the combination of ATG and CSA resulted in a significantly higher median hemoglobin level and platelet count at 6 months. TD-NSAA patients should be treated with more aggressive treatment regimen to be freed from transfusion dependence as soon as possible.
In summary, the treatment of elderly patients with AA still faces challenges and can attempt to be treated with new treatment regimens to improve prognosis. CsA is benefit to the survival of SAA/VSAA patients who cannot afford to receive allo-HSCT or ATG/ALG combined with CsA. AA patients, who responded to initialy CsA treatment, may benefit from prolonged CsA treatment. In view of the side effects of CsA, the timing of withdrawal is worth further exploration.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108(8):2509–2519.
- Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. The German aplastic anemia study group. N Engl J Med. 1991;324(19):1297–1304.
- Speck B, Gluckman E, Haak HL, et al. Treatment of aplastic anaemia by antilymphocyte globulin with and without allogeneic bone-marrow infusions. Lancet. 1977;2(8049):1145–1148.
- Bacigalupo A, Bruno B, Saracco P, et al. Antilymphocyte globulin, cyclosporine, prednisolone, and granulocyte colony-stimulating factor for severe aplastic anemia: an update of the GITMO/EBMT study on 100 patients. European Group for Blood and Marrow Transplantation (EBMT) working party on severe aplastic anemia and the Gruppo Italiano Trapianti di Midolio Osseo (GITMO). Blood. 2000;95(6):1931–1934.
- Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2007;92(1):11–18.
- Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172(2):187–207.
- Frytak JR, Henk HJ, De Castro CM, et al. Estimation of economic costs associated with transfusion dependence in adults with MDS. Curr Med Res Opin. 2009;25(8):1941–1951.
- Vaht K, Goransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000−2011. Haematologica. 2017;102(10):1683–1690.
- Bacigalupo A, Socie G, Lanino E, et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA working party. Haematologica. 2010;95(6):976–982.
- Marsh JC, Pearce RM, Koh MB, et al. Retrospective study of alemtuzumab vs ATG-based conditioning without irradiation for unrelated and matched sibling donor transplants in acquired severe aplastic anemia: a study from the British society for blood and marrow transplantation. Bone Marrow Transplant. 2014;49(1):42–48.
- Bacigalupo A, Giammarco S, Sica S. Bone marrow transplantation versus immunosuppressive therapy in patients with acquired severe aplastic anemia. Int J Hematol. 2016;104(2):168–174.
- Cheng Y, Xu Z, Zhang Y, et al. First-line choice for severe aplastic anemia in children: transplantation from a haploidentical donor vs immunosuppressive therapy. Clin Transplant. 2018;32(2). doi:https://doi.org/10.1111/ctr.13179 [Epub 2018 Jan 3].
- Ye L, Zhang F, Kojima S. Current insights into the treatments of severe aplastic anemia in China. Int J Hematol. 2020;112(3):292–299.
- Yoshida N, Kobayashi R, Yabe H, et al. First-line treatment for severe aplastic anemia in children: bone marrow transplantation from a matched family donor versus immunosuppressive therapy. Haematologica. 2014;99(12):1784–1791.
- Tichelli A, Socie G, Henry-amar M, et al. Effectiveness of immunosuppressive therapy in older patients with aplastic anemia. European group for blood and marrow transplantation severe aplastic anaemia working party. Ann Intern Med. 1999;130(3):193–201.
- Bacigalupo A. Guidelines for the treatment of severe aplastic anemia. Working Party on Severe Aplastic Anemia (WPSAA) of the European group of Bone Marrow Transplantation (EBMT). Haematologica. 1994;79(5):438–444.
- Marsh JC, Ball SE, Darbyshire P, et al. Guidelines for the diagnosis and management of acquired aplastic anaemia. Br J Haematol. 2003;123(5):782–801.
- Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129(11):1428–1436.
- Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after bone marrow transplantation for acquired aplastic anemia using HLA-matched sibling donors. Haematologica. 2010;95(12):2119–2125.
- Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94(9):1312–1315.
- Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French society of bone marrow transplantation and cell therapies and the EBMT severe aplastic anemia working party. Haematologica. 2016;101(7):884–890.
- Ades L, Mary JY, Robin M, et al. Long-term outcome after bone marrow transplantation for severe aplastic anemia. Blood. 2004;103(7):2490–2497.
- Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367(1):11–19.
- Lengline E, Drenou B, Peterlin P, et al. Nationwide survey on the use of eltrombopag in patients with severe aplastic anemia: a report on behalf of the French reference center for aplastic anemia. Haematologica. 2018;103(2):212–220.
- Cheng H, Wang X, Zhou D, et al. Eltrombopag combined with cyclosporine may have an effect on very severe aplastic anemia. Ann Hematol. 2019;98(8):2009–2011.
- Bartoszko J, Panzarella T, Lau A, et al. Effect of red blood cell transfusion dependence on the natural history of myeloproliferative neoplasm-associated myelofibrosis. Clin Lymphoma Myeloma Leuk. 2015;15(11):e151–e156.
- Braga Lemos M, Rodrigues SR, Schroeder T, et al. Association between red blood cell transfusion dependence and burden in patients with myelodysplastic syndromes: a systematic literature review and meta-analysis. Eur J Haematol. 2021;107(1):3–23.
- Marsh J, Schrezenmeier H, Marin P, et al. Prospective randomized multicenter study comparing cyclosporin alone versus the combination of antithymocyte globulin and cyclosporin for treatment of patients with nonsevere aplastic anemia: a report from the European Blood and Marrow Transplant (EBMT) severe aplastic anaemia working party. Blood. 1999;93(7):2191–2195.