ABSTRACT
Objectives
Multiple myeloma(MM) is a malignant plasma cell disease. Maintenance treatment is beneficial to prolong survival time in patients with MM. Ixazomib was approved for the treatment of relapsed or refractory MM in combination with lenalidomide and dexamethasone. Here, we carried out a meta-analysis to determine the efficacy and safety of ixazomib maintenance therapy.
Methods
Several databases were searched including PubMed, Web of Science, Embase, the Cochrane Library, etc. The last search dated back to July, 2020. Three clinical trials with a total of 1440 participants with newly diagnosed MM were included.
Results and conclusion
The pooled HR of progression-free survival (PFS) was 0.69 (95% CI = 0.59–0.79), which suggested ixazomib maintenance therapy could prolong PFS remarkably. In addition, ixazomib was effective in deepening remission (RR = 1.57, 95% CI = 1.26–1.96). But it could not significantly prolong PFS in cytogenetic high-risk patients (HR = 0.74, 95% CI = 0.47–1.00). In terms of adverse reactions, our analysis revealed higher incidences of grade 3–4 thrombocytopenia (RR = 7.47, 95% CI = 2.06–27.06), neuropathy (RR = 1.48, 95% CI = 1.14–1.92), grade 3–4 infections (RR = 1.77, 95% CI = 1.21–2.59) and gastrointestinal disorders (RR = 1.48, 95% CI = 1.32–1.66). There was no significant correlation between the use of ixazomib and grade 3–4 neutropenia (RR = 1.46, 95% CI = 0.77–2.78, p = 0.25) or the occurrence of new primary malignant tumor (RR = 0.88, 95% CI = 0.53–1.46, p = 0.62). Additionally, more RCTs are needed for better choice of treatment regimen.
1. Introduction
Multiple myeloma (MM), a malignant plasma cell disease, accounts for approximately 10% of all hematologic malignancies and is more common in elderly. The median age at diagnosis is about 65 years, with only 12% of them younger than 65 years old [Citation1]. While the application of multiple therapeutic regimens such as high-dose chemotherapy, autologous stem cell transplantation (ASCT), proteasome inhibitors (PIs), immunomodulators (IMiDs), and monoclonal antibodies have increased the remission rate of MM [Citation2], it remains an incurable disease due to high incidence of relapse.
Maintenance treatment following ASCT or remission is beneficial to eliminate the minimal residual disease (MRD) further and prolong survival in patients with MM [Citation3,Citation4]. At present, the common drugs in maintenance treatment include glucocorticoid, interferon, IMiD, PI, etc. Long-term use of the first two is often limited by severe adverse reactions such as infection, mood change, myalgia and hematological toxicity [Citation5]. IMiDs mainly act on myeloma cells and bone marrow hematopoietic microenvironment, thus playing a dual role of killing tumor cells and immune regulation. Maintenance therapy based on thalidomide, lenalidomide and other IMiDs can significantly prolong the progression-free survival (PFS) of patients, while the improvement of the overall survival (OS) is controversial [Citation6,Citation7]. Additionally, IMiDs have several limitations, including the toxic risk of peripheral neuropathy, phlebothrombosis, shorter OS in patients with recurrence and the increased risk of developing second primary malignancies. Myeloma cells naturally synthesize more proteins to meet the needs of rapid growth and secretion, so that PIs are more specific for them. Bortezomib is the first PI approved by the US Food and Drug Administration (FDA). By reducing the expression levels of IGF, TNF-α, and IL-6, it can promote the apoptosis of myeloma cells and reduce their resistance to the glucocorticoid, thus avoiding the occurrence of the second tumor [Citation8]. Nevertheless, bortezomib is not well suited for long-term use given the risk of peripheral neuropathy and need for parenteral administration which brings a lot of inconvenience to MM patients.
Ixazomib (Ninlaro®) is a highly selective reversible protease inhibitor approved by FDA for treatment of relapsed or refractory multiple myeloma (RRMM) in combination with lenalidomide and dexamethasone (IRd). In TOURMALINE-MM1, a global phase III clinical study, ixazomib showed therapeutic superiority over placebo regimens for all individuals including high-risk patients [Citation9]. Patients resistant to bortezomib often showed responses to ixazomib [Citation10]. As the first oral dosage form of PI, ixazomib plays an important and positive role in improving the convenience of medication and helping patients return to social life. Currently, several clinical trials have been conducted with some results on ixazomib maintenance. In order to determine the efficacy and safety, it is necessary to systematically integrate the existing research results, namely, to study its impact on survival outcomes of MM patients by systematic evaluation and meta-analysis, and to provide guidance for the selection of clinical maintenance treatment options.
2. Materials and methods
2.1. Data sources and search strategy
This meta-analysis adhered to the guidelines provided by the PRISMA report. A search for clinical trials was conducted by two independent investigators using the following electronic databases: PubMed, Web of Science, Embase, the Cochrane Library and proceedings from major international meetings in hematology and oncology. The search was limited to publications in English. To identify that eligible studies were not omitted, we manually checked the reference lists of published reviews and International Clinical Trials Registry Platform. The last search dated back to July, 2020.
2.2. Study selection and endpoints
The clinical trials included in this article must meet the following criteria: (1) the study population was comprised of patients with newly diagnosed or previously treated MM; (2) the study had an intervention group: ixazomib or ixazomib-dexamethasone maintenance therapy and a control group: placebo or placebo-dexamethasone or no maintenance therapy; (3) they were phase Ⅲ RCTs or phase Ⅱ studies; (4) at least one or more of the primary or secondary outcomes were reported including PFS, OS, remission deepening and safety. We defined remission deepening as patients with very good partial remission (VGPR) and partial remission(PR) getting better remission. The work was carried out by two independent researchers. If there were any differences, they can be discussed and settled through negotiation.
2.3. Data collection process
Two independent reviewers collected data from studies, respectively, including general information and basic features, characteristics of the object of study, interventions and the results. Survival data were directly extracted from the text, table(s), or figure(s) of the publications or were estimated from Kaplan–Meier curve(s) where applicable.
2.4. Assessment of risk of bias
The risk of bias was assessed for included randomized controlled trials according to the criteria and tools defined in the Cochrane Handbook for systematic review of interventions [Citation11]. The evaluation results were ‘low risk’, ‘unclear’, and ‘high risk’. Studies were evaluated in the following ways: random sequence generation, blindness, allocation concealment, data integrity, reporting selectivity, etc.
2.5. Statistical analysis
Stata14.0 was used to perform all calculations related to meta-analysis. Efficacy was assessed with hazard ratio (HR) with 95% confidence interval (95% CI) of PFS and OS outcomes for the ixazomib maintenance arm over the non-ixazomib arm. When HR was not available for a given study, it was estimated using methods described by Tierney et al [Citation12]. The risk ratio (RR) with 95% confidence interval was used to describe the effect size of outcomes about remission deepening and adverse effects. Before quantitative integration of studies, it was necessary to judge whether all the included studies are homogeneous by conducting heterogeneity test. We used I2 index. Heterogeneity was statistically significant when I2 was greater than 50%. Due to the limited number of trials included, no formal assessment of publication bias has been conducted.
3. Results
3.1. Trial selection
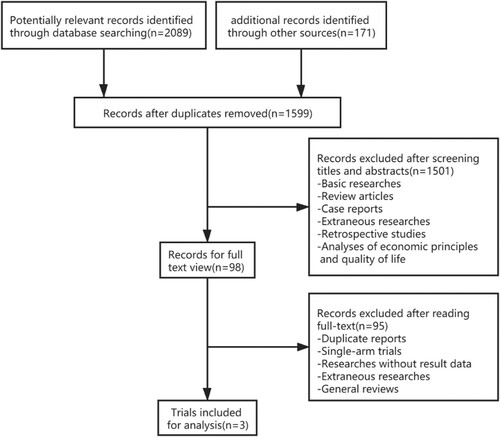
Details of the search and selection process are illustrated in . A total of 2260 potentially relevant records were preliminarily retrieved, including 661 duplicates. After screening titles and abstracts, 1501 records were excluded which included basic researches, case reports, extraneous researches, etc. Ultimately, there were only three trials incorporated into analysis after reading the full text [Citation13–15]. No discrepancies were displayed between the two researchers with regard to study accreditation.
3.2. Study characteristics
Three clinical trials with a total of 1440 participants with newly diagnosed multiple myeloma (NDMM) were included in the present meta-analysis. One literature published in 2019 examined the safety and efficacy of ixazomib maintenance therapy following ASCT within 12 months of diagnosis [Citation13]. A total of 656 patients who had achieved at least a partial response (PR) were randomly assigned to receive either ixazomib or a placebo maintenance in a 3:2 ratio. The median follow-up time was 30.9 months for the experimental group and 31.3 months for the control group. The other two papers were published in 2020, and they were both for patients with NDMM not undergoing ASCT [Citation14,Citation15]. 464 patients were assigned to the ixazomib group and 320 to the placebo group. The median age is 73 years. No OS data were obtained in any of the above studies because of insufficient events. The characteristics of the three trials are summarized in .
Table 1. Characteristics of studies included in the meta-analysis.
All included studies were assessed for risk of bias according to the Cochrane collaboration’s assessment tool. The results are shown in . All analyses in these three papers were performed according to the intention to treat (ITT) principle.
3.3. Efficacy
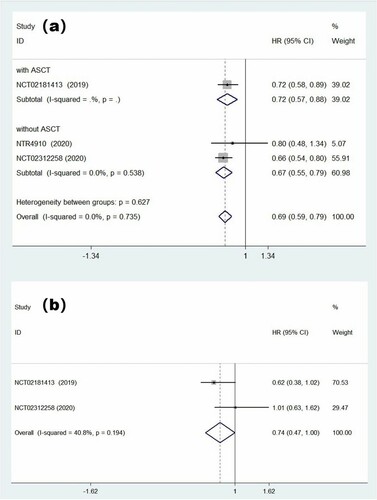
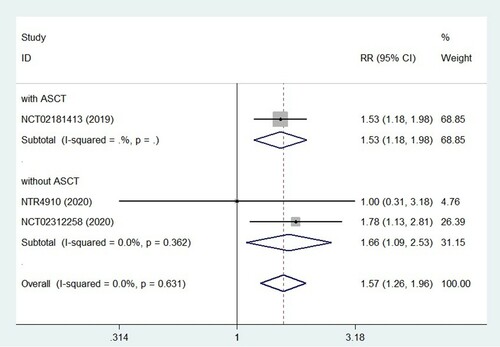
We tried to combine effect quantities by using the fixed effects model at first. The pooled HR of PFS was 0.69 (95% CI = 0.59–0.79; (a)), and the results showed that there was no statistically significant heterogeneity among trials (p = 0.74, I2 = 0.0%). It suggested that ixazomib maintenance therapy, a protective factor in patients with multiple myeloma, could prolong PFS remarkably. However, in our analysis of MM patients with high cytogenetic risk, we found that ixazomib maintenance therapy did not provide a significant PFS benefit (HR = 0.74, 95% CI = 0.47–1.00). The forest graph and detailed results are shown in (b). In addition, ixazomib maintenance was effective in deepening remission (RR = 1.57, 95% CI = 1.26–1.96; ), which can make patients with VGPR or PR achieve better efficacy.
3.4. Safety
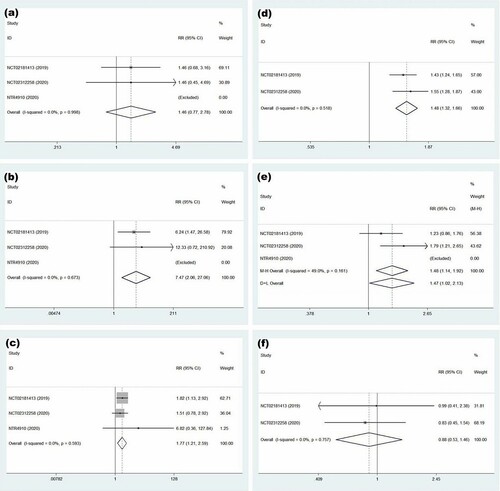
A meta-analysis was performed by calculating the pooled RR to clear adverse events of ixazomib maintenance therapy. In terms of hematological toxicity, the results showed that its increased risk of grade 3–4 neutropenia did not achieve statistical significance (RR = 1.46, 95% CI = 0.77–2.78, p = 0.25; (a)). But, maintenance treatment with ixazomib increased the risk of grade 3–4 thrombocytopenia by approximately 7-fold (RR = 7.47, 95% CI = 2.06–27.06; (b)). When it came to non-hematological toxicity, our analysis revealed higher incidences of grade 3–4 infections (RR = 1.77, 95% CI = 1.21–2.59; (c)) and gastrointestinal disorders (RR = 1.48, 95% CI = 1.32–1.66; (d)). Because of insufficient data, two clinical trials (NCT02181413, NCT02312258) were used to analyze the association of ixazomib with neuropathy and the new primary malignant tumor. The incidence of neuropathy in the ixazomib maintenance group was higher than that in the placebo group and the difference was statistically significant (RR = 1.48, 95% CI = 1.14–1.92, p = 0.004). These trials had moderate heterogeneity when fixed effects model was executed (I-squared = 49%). The forest graph is shown in (e). There was no clear association between ixazomib with the new primary malignant tumor (RR = 0.88; 95% CI = 0.53–1.46, p = 0.623; (f)).
Figure 5. Forest plot of safety for treatment with ixazomib group versus placebo. (a) Forest plot of grade 3–4 neutropenia. (b) Forest plot of grade 3–4 thrombocytopenia. (c) Forest plot of grade 3–4 infections. (d) Forest plot of gastrointestinal disorders. (e) Forest plot of neuropathy. (f) Forest plot of the new primary malignant tumor.
4. Discussion
While multiple myeloma is still an incurable malignancy, the goal of its treatment is to maximize the survival time and quality of life of the patients. A network meta-analysis cross-compared several maintenance medication regimens, including lenalidomide, thalidomide, bortezomib, and interferon [Citation16]. The results showed that lenalidomide – prednisone and lenalidomide monotherapy had the most significant effect on prolonged PFS, and lenalidomide was proved to be the best choice in the analysis of OS. In the subgroup analysis, lenalidomide based maintenance therapy yielded greater benefits for patients with good prognosis and standard risk of chromosomal abnormalities, while bortezomib was recommended for patients with poor prognosis (ISS Ⅲ). Lenalidomide was recommended for maintenance therapy in the NCCN guidelines (category 1) [Citation17]. However, the side effects of lenalidomide and bortezomib need to be considered and monitored. Philip L. McCarthy et al. compared lenalidomide with placebo maintenance therapy at a median follow-up time of 79.5 months, and the incidence of the second primary malignancy was about 10% in the lenalidomide group before the disease progressed [Citation18]. In an open, randomized, multicenter phase 3 trial, 1509 MM patients who were suitable for ASCT and 1223 patients not eligible for ASCT were randomly divided into two groups, respectively, and received induction and maintenance treatment regimens including lenalidomide or thalidomide. The lenalidomide group had a higher 3-year cumulative incidence in the second malignancy, but whether it was caused by drug exposure or the effect of multiple factors such as age and occasion of the group remained open to discussion [Citation19]. The use of bortezomib is limited by the following three factors. First, the emergence of drug resistance. Bortezomib had been applied to the treatment of RRMM in a study, and the result showed that almost all patients had progressive disease [Citation20]. Even when bortezomib was used as a single drug in newly diagnosed patients, 52 percent had no partial or better response [Citation21]. Second, the occurrence of adverse events. A meta-analysis published in Bioscience Reports in 2017 showed that bortezomib maintenance therapy significantly increased the risk of neutropenia [Citation22]. In another study on peripheral neuropathy in MM and lymphoma patients associated with intravenous bortezomib, 34 clinical trials involving 6,492 patients were included in the statistical analysis. The relative risk of bortezomib group was increased compared with the placebo group [Citation23]. Third, the limitation of application method. Bortezomib can only be given intravenously or subcutaneously.
As the first reversible PI in the oral dosage form, ixazomib promotes downregulation of NFκβ pathway, thus inhibits the growth of myeloma cells and reduces drug resistance [Citation24,Citation25]. In a meta-analysis of safety and efficacy, ixazomib-based chemotherapy showed positive efficacy, especially in three-drug regimens, for both RRMM and NDMM [Citation26]. The positive results led to further clinical trials incorporating ixazomib into maintenance therapy. Currently, there was no quantitative analysis on its efficacy and safety, so we conducted this meta-analysis to provide some reference for the selection of clinical medication regimen. We searched major databases and clinical trial registration websites, and only 3 RCTs were included.
NCT02181413 was a double-blind, randomized, placebo-controlled phase 3 trial which began in 2014. Patients must have underwent standard of care induction therapy including PIs or IMiDs, followed by single ASCT without consolidation therapy [Citation13]. NCT02312258 was also a multicenter clinical trial. It began in September 2015 with a primary completion date of August 12, 2019. Patients had completed 6–12 months of initial therapy without ASCT before being enrolled in this study, and there were no restrictions on the choice of drugs for treatment [Citation15]. NTR4910, a randomized phase 2 trial, was divided into two procedures. Patients ineligible for ASCT received induction therapy with ixazomib, thalidomide and dexamethasone (ITd), then they were randomly divided into two groups for maintenance therapy [Citation14]. Because the latter two clinical trials looked at patients who were not eligible for transplantation, the median age of patients was significantly older than in NCT02181413. In addition, there were differences among three studies in terms of treatment background, especially induction therapy regimen.
A total of 1440 patients were randomly assigned to the ixazomib maintenance group and the placebo group. The results revealed that ixazomib had a significant effect on prolongation of PFS compared with placebo, and it was effective in deepening remission. Nevertheless, the role of ixazomib maintenance therapy in prolonging PFS of cytogenetic high-risk patients was not satisfactory. When it came to an OS benefit, trials were still under observation and insufficient data were available at this time. In terms of safety, there was no evidence that the use of ixazomib was associated with grade 3–4 neutropenia or secondary tumorigenesis. But it increased the risk of grade 3–4 thrombocytopenia markedly (RR = 7.47; 95% CI = 2.06–27.06). Patients need to monitor platelet levels during medication.
In our meta-analysis, high-risk cytogenetic abnormalities were traditionally defined as t(4; 14), t(14; 16) or del(17p). Results indicated that ixazomib maintenance therapy had no significant protective effect in prolongation of PFS in high-risk NDMM patients, which is slightly different from previous studies. In TOURMALINE-MM1, IRd significantly extended median PFS in high-risk cytogenetic patients of RRMM compared with placebo-Rd (HR = 0.54, 95% CI = 0.32–0.92). But the results varied among subgroups of patients with individual high-risk cytogenetic abnormalities. In the del(17p) and t(4;14) subgroups, the respective HRs were 0.60 (95% CI = 0.29–1.24) and 0.65 (95% CI = 0.25–1.66) [Citation9,Citation27]. Therefore, our results may be related to specific cytogenetic typing. Due to the lack of relevant data, subgroup analysis based on individual differences cannot be performed to obtain quantitative results. Limitations on the number of studies may also play a role. In recent years, MRD has received more and more attention in the efficacy evaluation of MM. In a meta-analysis of association of MRD with survival outcomes, patients with negative MRD tended to have better PFS (HR = 0.41, 95% CI = 0.36–0.48) and OS (HR = 0.57, 95% CI = 0.46–0.71). The results were similar in patients who had achieved a complete response [Citation28]. In addition, the detection of MRD was expected to realize the identification and stratification of patients with different prognosis to guide clinical decision-making [Citation29]. Therefore, it is important to determine whether ixazomib maintenance therapy can change the MRD status of patients with MM. Among the clinical trials included in our study, only NCT02181413 published relevant data with inapparent statistical difference. But the PFS of MRD-positive patients was significantly prolonged with ixazomib [Citation13]. Patients of NCT02181413 and NCT02312258 were treated with 26 cycles of maintenance therapy. The proportions of discontinuation of ixazomib due to adverse drug reactions in the two clinical trails were 7% and 12.9% respectively, indicating the possibility of ixazomib as a long-term maintenance drug. In NTR4910, according to the data provided to date, the maximum known number of ixazomib maintenance treatment cycles was 34. But it provided limited and incomplete data on whether long-term use of ixazomib could improve survival outcomes. Furthermore, the median PFS was less than or similar to maintenance time in all three clinical trials. Prolonged maintenance therapy may have little effect on median PFS. More results need to be analyzed and individualized treatment needs to be developed based on tolerance to adverse reactions.
In the previous, there was disagreement about the benefits of maintenance therapy in patients who are not candidates for transplantation. Balitsky 2020 performed a meta-analysis of maintenance therapy in transplant ineligible adults with NDMM, and identified five eligible trials. Compared to observation, maintenance therapy could prolong PFS of patients (HR = 0.48; 95% CI = 0.38–0.62) [Citation30]. It provided an evidence for the use of maintenance therapy in patients ineligible for ASCT. This meta-analysis included an article about ixazomib, which was also enrolled in our study. At the same time, Parrondo 2021 conducted a meta-analysis and systematic review to determine efficacy of PI-based maintenance following ASCT in MM. Three phase III clinical trials were included. The results showed that PFS was significantly improved by the use of PI-based maintenance compared to non PI-based maintenance (HR = 0.77, 95% CI = 0.69–0.86) [Citation31]. The above research results laid foundations for our meta-analysis. Our results, which agree with them to some extent, are further validations of their results. Meanwhile, our study further refines previous studies in the direction of maintenance therapy with ixazomib.
Although we conducted this meta-analysis in accordance with standard procedures, there are still limitations in the results. First, only three clinical trials were included in this analysis and one of them had a small sample size. Sufficient data for consolidation, such as adverse drug reactions, were not available. Second, results of OS have not yet been obtained in studies. It is not clear whether ixazomib will give patients an overall survival benefit. Third, possible publication bias may affect our interpretation. Fourth, due to the limited number of included studies and the limited significance of sensitivity analysis, we did not conduct it.
5. Conclusion
Overall, our meta-analysis demonstrated the positive role of ixazomib in prolonging PFS and deepening remission in newly diagnosed patients with MM. However, monitoring of platelet levels and treatments of bleeding prevention are required during medication. The choice of treatment regimen needs to be combined with the head-to-head results of ixazomib versus other drugs. we are looking forward to more results.
Disclosure statement
No author of this paper has a conflict of interest, including specific financial interests, relationships, and/or affiliations relevant to the subject matter or materials included in this article.
Additional information
Funding
References
- Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33.
- Costa LJ, Brill IK, Omel J, et al. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Adv. 2017 Jan 4;1(4):282–287.
- Rawstron AC, Child JA, de Tute RM, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013 Jul 10;31(20):2540–2547.
- Munshi NC, Avet-Loiseau H, Rawstron AC, et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol. 2017 Jan 1;3(1):28–35.
- Liu H, McCarthy P. New developments in post-transplant maintenance treatment of multiple myeloma. Semin Oncol. 2013 Oct;40(5):602–609.
- Wang Y, Yang F, Shen Y, et al. Maintenance therapy with immunomodulatory drugs in multiple myeloma: a meta-analysis and systematic review. J Natl Cancer Inst. 2015 Nov 18;108(3):djv342.
- McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017 Oct 10;35(29):3279–3289.
- Chauhan D, Anderson KC. Mechanisms of cell death and survival in multiple myeloma (MM): therapeutic implications. Apoptosis. 2003;8(4):337–343.
- Moreau P, Masszi T, Grzasko N, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016 Apr 28;374(17):1621–1634.
- Fricker LD. Proteasome inhibitor drugs. Annu Rev Pharmacol Toxicol. 2020;60:457–476.
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007 Jun 7;8:16.
- Dimopoulos MA, Gay F, Schjesvold F, et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2019 Jan 19;393(10168):253–264.
- Zweegman S, Stege CAM, Haukas E, et al. Ixazomib-thalidomide-low dose dexamethasone induction followed by maintenance therapy with ixazomib or placebo in newly diagnosed multiple myeloma patients not eligible for autologous stem cell transplantation; results from the randomized phase II HOVON-126/NMSG 21.13 trial. Haematologica. 2020 Feb 13. DOI:https://doi.org/10.3324/haematol.2019.240374
- Dimopoulos MA, Špička I, Quach H, et al. Ixazomib as postinduction maintenance for patients with newly diagnosed multiple myeloma not undergoing autologous stem cell transplantation: the phase III TOURMALINE-MM4 trial. J Clin Oncol. 2020 Dec 1;38(34):4030–4041.
- Gay F, Jackson G, Rosiñol L, et al. Maintenance treatment and survival in patients with myeloma: a systematic review and network meta-analysis. JAMA Oncol. 2018 Oct 1;4(10):1389–1397.
- Kumar SK, Callander NS, Hillengass J, et al. NCCN guidelines insights: multiple myeloma, version 1.2020. J Natl Compr Canc Netw. 2019 Oct 1;17(10):1154–1165.
- McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017 Oct 10;35(29):3279–3289.
- Jones JR, Cairns DA, Gregory WM, et al. Second malignancies in the context of lenalidomide treatment: an analysis of 2732 myeloma patients enrolled to the Myeloma XI trial. Blood Cancer J. 2016 Dec 9;6(12):e506.
- Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003 Jun 26;348(26):2609–2617.
- Dispenzieri A, Jacobus S, Vesole DH, et al. Primary therapy with single agent bortezomib as induction, maintenance and re-induction in patients with high-risk myeloma: results of the ECOG E2A02 trial. Leukemia. 2010;24(8):1406–1411.
- Sun CY, Li JY, Chu ZB, et al. Efficacy and safety of bortezomib maintenance in patients with newly diagnosed multiple myeloma: a meta-analysis. Biosci Rep. 2017;37(4):BSR20170304.
- Peng L, Ye X, Zhou Y, et al. Meta-analysis of incidence and risk of peripheral neuropathy associated with intravenous bortezomib. Support Care Cancer. 2015;23(9):2813–2824.
- Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4(5):349–360.
- Gilmore TD. Multiple myeloma: lusting for NF-kappaB. Cancer Cell. 2007;12(2):95–97.
- Shafqat M, Jamil F, Shah Z, et al. A systematic review and meta-analysis: efficacy and toxicity profile of ixazomib for treatment of multiple myeloma. Blood. 2018;132(Suppl. 1):5639.
- Avet-Loiseau H, Bahlis NJ, Chng WJ, et al. Ixazomib significantly prolongs progression-free survival in high-risk relapsed/refractory myeloma patients. Blood. 2017;130(24):2610–2618.
- Munshi NC, Avet-Loiseau H, Rawstron AC, et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol. 2017 Jan 1;3(1):28–35.
- Perrot A, Lauwers-Cances V, Corre J, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018 Dec 6;132(23):2456–2464.
- Balitsky AK, Karkar A, McCurdy A, et al. Maintenance therapy in transplant ineligible adults with newly-diagnosed multiple myeloma: a systematic review and meta-analysis. Eur J Haematol. 2020;105(5):626–634.
- Parrondo RD, Reljic T, Iqbal M, et al. Efficacy of proteasome inhibitor-based maintenance following autologous transplantation in multiple myeloma: a systematic review and meta-analysis. Eur J Haematol. 2021;106(1):40–48.