ABSTRACT
Objective
The clinical manifestations of acute arrest of hemopoiesis (AAH) are very similar with severe aplastic anemia (SAA). Currently there are no clear diagnostic criteria to distinguish AAH from SAA. Differentiation of AAH from SAA is challenging in the routine clinical practice. This study aimed to analyze the clinical and laboratory features between AAH and SAA patients.
Patients and methods
We performed a retrospective study with cohort of 425 suspected patients who were hospitalized to the First Affiliated Hospital of Zhengzhou University from 1 January 2019 to 31 December 2020. We identified 11 AAH patients and 49 SAA patients to investigate the differentiation diagnostic features.
Results
Clinical and laboratory examinations of 11 patients with AAH met the diagnostic criteria of SAA, and hematopoietic recovery occurred within a median time of 12 (4–21) days. The median time for neutrophils to recover above 1 × 109/L and platelet to recover above 50 × 109/L in all patients with AAH was 5 (3–8) days and 8 (1–13) days, respectively. Compared with the control group SAA, the 11 AAH patients were older, with a median age of 53 (21–69) years old, and their first symptom is usually fever.
Conclusions
The spontaneous remission of AAH was rapid in most patients, and relapses were rarely observed. With supportive treatment, the AAH patients would show significant improvement on blood routine about a week, otherwise the patients should be treated as early as possible with the SAA regimen.
1. Introduction
Pancytopenia is a common clinical manifestation with different diseases in hematology. It can be seen in a variety of diseases, such as aplastic anemia (AA), myelodysplastic syndrome (MDS), acute leukemia, myelofibrosis, and acute arrest of hemopoiesis (AAH) [Citation1–4]. Differentiation diagnosis and identification sometimes are difficult. AAH, or aplastic crisis or hemolytic crisis, has a very low clinical incidence, accounting for only a small proportion of pancytopenia [Citation5–7]. There are very few studies on acute hematopoietic arrest. Currently, there is no unified nomenclature and diagnostic criteria. The initial clinical presentation and laboratory examination of AAH are very similar with true severe aplastic anemia (SAA) [Citation8–11]. The patients with AAH could be easily misdiagnosed as SAA or VSAA and would receive allogeneic hematopoietic stem cell transplantation or ATG/ALG combined with cyclosporine for strong immunosuppressive therapy, which could cause unnecessary expensive treatment costs and great risks [Citation12–16]. On the other hand, some doctors delay the best opportunity to treat SAA by waiting too long time to identify AAH. Whether the treatment of SAA should be delayed to exclude AAH still remains as an open question to be answered. However, there is no clear distinguishing standard for AAH at present, which cannot be effectively differentiated from SAA in the early stage. To this end, we retrospectively reviewed the clinical and laboratory characteristics of 11 patients with AAH whose clinical manifestations were similar to SAA in our hospital from 1 January 2019 to 31 December 2020 and compared with 49 cases newly diagnosed SAA from 1 January 2019 to 31 December 2019, to explore the prospective reference indicators for early diagnosis of AAH.
2. Materials and methods
2.1. Study population
We performed a retrospective study of 425 suspected patients, with initial diagnosis as aplastic anemia, and identified 11 AAH patients with clinical manifestations similar to SAA. The AAH patients were admitted to our hospital from 1 January 2019 to 31 December 2020. Forty-nine newly diagnosed SAA patients in ward 3 of hematology department were enrolled as control group from 1 January 2019 to 31 December 2019. The exclusion criterion was the diagnosis of an additional hematological malignancy other than AAH or SAA. Patients with congenital SAA, such as Fanconi anemia, as well as patients younger than 14 years old were excluded. Patients with AAH whose peripheral blood level did not achieve SAA or with a definite secondary drug treatment were also excluded. This study was approved by the Ethical Committee of our hospital. Written informed consent was obtained from all patients or their legal guardians.
2.2. Diagnostic criteria
The diagnosis of SAA was referred to the Camitta's criteria [Citation17,Citation18]. The specific diagnostic criteria are as follows: Marrow cellularity <25% (or 25–50% with <30% residual hematopoietic cells), plus at least 2 of: (1) neutrophils <0.5 × 109/L, (2) platelets <20 × 109/L, and (3) reticulocyte count <20 × 109/L. SAA patients with neutrophils <0.2 × 109/L would be diagnosed as very severe AA (VSAA). Patients meeting the diagnostic criteria for SAA who did not receive allogeneic hematopoietic stem cell transplantation, androgen, or any form of immunosuppressive therapy and had hematopoietic recovery within 1 month of onset were diagnosed as AAH.
2.3. The standard of hematopoietic recovery
In addition to the standards recommended by the European Blood and Bone Marrow Transplantation SAA Working Group [Citation19], reticulocyte marker was added. The specific criteria are as follows: (1) free from blood transfusion; (2) complete response defined as an absolute neutrophil count >1 × 109/L, a hemoglobin level >100 g/L, and a platelet count of >100 × 109/L (all three criteria had to be met), and (3) The count of reticulocytes >20 × 109/L within two consecutive tests.
2.4. Statistics
Comparisons between the two groups AAH and SAA were analyzed by the one-way analysis of variance and the chi-square test. P < .05 was considered statistically significant. Statistical analyses were performed with SPSS 20 software.
3. Results
3.1. Basic characteristics of patients in AAH and SAA
To explore the difference between AAH and SAA, we performed a retrospective analysis. Eleven AAH patients and 49 newly diagnosed SAA from our hospital were enrolled in our study. The 11 patients with AAH included 8 men (72.7%) and 3 women (27.3%), with a median age of 53 (21–69) years. Except for one patient who was 21 years old, the other 10 patients were all older than 43 years old. All 11 patients met the SAA criteria, of which 8 patients (72.7%) met the VSAA criteria. The general clinical data of all 11 patients are shown in . In the control group, there were 49 patients with SAA, with a median age of 37 (15–75) years, including 29 men (59.2%) and 20 women (40.8%). The average ages of AAH patients were older than that of SAA patients. There was statistically significant difference in onset ages between the two groups, but the gender composition has no statistically significant difference (). One patient in the AAH group refused chromosome and related genetic testing. The chromosomal and MDS-related mutations of the remaining 10 patients were negative. All 49 SAA patients were tested for chromosomal and MDS-related gene mutations. All patients had normal chromosomes. Only one patient had a clonal gene mutation at DNMT3A (p. R882C) and the allele variation frequency was 2.36%.
Table 1. The general clinical data of patients with AAH
Table 2. The comparison of AAH and SAA on general clinical data
3.2. Initial symptoms and comorbidities of patients in AAH and SAA
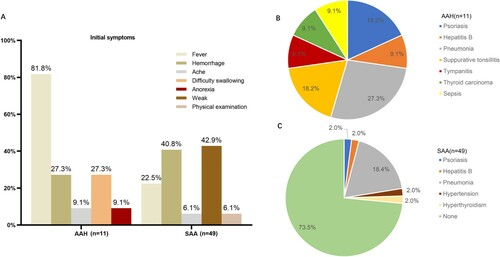
All the AAH patients were associated with other systemic diseases, including one papillary thyroid carcinoma, one chronic viral hepatitis B, two psoriasis, one sepsis, one tympanitis, and two acute suppurative tonsillitis. Fever was the first symptom in nine cases (81.8%), and hemorrhage was the first symptom in three cases (27.3%). However, 36 cases (73.5%) of SAA patients did not have other systemic diseases; 21 cases (42.3%) with fatigue as the first symptom; 20 cases (40.1%) with bleeding as the first symptom, and 11 cases (22.4%) with fever as the first symptom (). The proportion of fever as the first symptom of AAH patients was higher than that of SAA group with statistically significant difference (). Compared with AAH, the majority of SAA patients were not associated with other systemic diseases, and it was statistically significant ().
Figure 1. The comparison of AAH and SAA on initial symptoms and comorbidities. (A) In the AAH group, the first symptoms mainly included fever (81.8%), bleeding (27.3%), ache (9.1%), difficulty swallowing (27.3%), and anorexia (9.1%), while in the SAA group, the first symptoms were mainly fatigue (42.9%), bleeding (40.8%), fever (22.5%), ache (6.1%), and found in physical examination (6.1%). (B) Patients in the AAH group (n = 11) had complicated diseases including pneumonia (n = 3, 27.3%), psoriasis (n = 2, 18.2%), acute suppurative tonsillitis (n = 2, 18.2%), hepatitis B (n = 1, 9.1%), tympanitis (n = 1, 9.1%), thyroid carcinoma (n = 1, 9.1%), and serious sepsis (n = 1, 9.1%). (C) Patients in the SAA group (n = 49) had complicated diseases including pneumonia (n = 9, 18.4%), psoriasis (n = 1, 2.0%), hepatitis B (n = 1, 2.0%), tympanitis (n = 1, 9.1%), hyperthyroidism (n = 1, 2.0%), hypertension (n = 1, 9.1%), but most of the SAA patients (n = 36, 73.5%) did not have other systemic diseases.
3.3. The comparison of blood routine examination between AAH and SAA groups
Routine blood examination at initial diagnosis revealed a reduction in hemoglobin levels of 92 (42–105) g/L and 70(23–99) g/L in the AAH and SAA groups, respectively. The anemia was more severe in the SAA group with statistically significance. White blood cell (WBC) levels were decreased in both AAH and SAA groups, with specific values of 0.60 (0.20–3.49) × 109/L and 1.60 (0.20–4.18) × 109/L, respectively. The WBC counts were lower in the AAH group, but the degree of neutrophil deficiency was not statistically significant between the two groups. More interestingly, lymphocyte counts in the SAA group were statistically significance higher than those in the AAH group. Platelet levels were decreased in both AAH and SAA groups, with specific values of 27 (1–124) × 109/L and 9 (1–64) × 109/L, respectively. Platelet counts were lower in the SAA group than that in the AAH group. The reticulocyte counts were decreased in both groups without statistically significant difference. However, both the AAH and SAA groups had extremely low reticulocyte counts (13.7 × 109/L and 12.1 × 109/L, respectively). The detailed results of blood routine examination are shown in .
Table 3. The comparison of AAH and SAA on blood routine examination
3.4. The comparison of the iron metabolism indexes between AAH and SAA groups
The iron metabolism was measured in 7 patients in AAH and 42 patients in SAA. There was no increase on serum iron in the AAH group, but there were 26 patients (61.9%) with elevated serum iron in the SAA group. The serum iron-specific values of AAH and SAA groups were 16.0 ± 2.6 and 38.4 ± 1.4 μmol/L, respectively. The difference between the two groups was statistically significant. Ferritin levels were increased in both AAH and SAA groups, with specific values of 1343.8 ± 459.4 and 1029.3 ± 124.6 ng/mL, respectively. There was no significant difference between the two groups. Both the total iron binding ability and unsaturated iron binding ability of the two groups were decreased, and the difference between the two groups was statistically significant. However, the decrease in total iron binding ability was more obvious in the AAH group, whereas the decrease in unsaturated iron binding ability was more significant in the SAA group. The specific comparison of iron metabolism indexes between the two groups is shown in .
Table 4. The comparison of AAH and SAA on the iron metabolism indexes
3.5. The comparison of the biochemical indexes between the AAH and SAA groups
All patients in the two groups were tested for biochemical-related indicators. There was an increase in serum creatinine level in one elderly patient with serious sepsis in the AAH group, but no increase in other patients. Serum creatinine level did not increase in the SAA group. The specific values of albumin in AAH and SAA groups were 32.2 ± 1.9 and 38.9 ± 1.0 g/L, respectively. The albumin level was reduced in eight patients (72.7%) in the AAH group compared with nine patients (18.4%) in the SAA group. Compared with the SAA group, the albumin level decreased much more in the AAH group with statistically significant difference. The specific levels of serum lactate dehydrogenase in the AAH and SAA groups were 161.9 ± 28.4 and 203.6 ± 21.8U/L, respectively, and there was no difference between the two groups. The specific comparison of biochemical indexes between the two groups is shown in .
Table 5. The comparison of AAH and SAA on the biochemical indexes
3.6. Hematopoiesis recovery in patients of AAH
One of the 11 patients with AAH refused to undergo iliac bone marrow aspiration for cytology test at initial diagnosis. Among the remaining 10 patients, only 1 (10%) had low degree of myeloid hyperplasia, while the remaining 9 (90%) had extremely low degree of myeloid hyperplasia. All the SAA patients underwent iliac bone marrow aspiration for cytology test, of which 8 patients (16.3%) had active myeloid hyperplasia, 34 patients (69.4%) had hypoplasia, and only 7 patients (14.3%) had extremely hypoplasia. The eight patients with active myelodysplasia were all very young, ranging in ages from 15 to 21 years. Compared with the SAA group, the degree of myeloid hyperplasia in the AAH group was much lower, which was statistically significant (). Hematological complete remission was achieved in all 11 patients with AAH. The median time from the onset to recovery of hematopoietic function in AAH patients was 12 (4–21) days, without recurrence, and the longest follow-up time was 25 months. One elderly patient with severe sepsis had the longest recovery time of hematopoietic function, which was 21 days. The recovery of hematopoietic function in the other 10 patients was <15 days (). Further analysis revealed that the median times for neutrophils to recover to above 0.5 × 109/L and above 1 × 109/L in patients were 4 (2–7) and 5 (3–8) days, respectively. The median times for platelet values to return to above 20 × 109/L and above 50 × 109/L in patients were 6 (4–7) days and 8 (1–13) days, respectively. The reticulocyte recovery time to >20 × 109/L was much longer, with a median time of 12 (4–21) days. The specific recovery times are shown in .
Table 6. The hematopoiesis recovery time of patients with AAH
4. Discussion
AA is a bone marrow hematopoietic failure syndrome characterized by pancytopenia, especially with SAA [Citation20–22]. Without timely and effective treatment, patients often develop serious infection, bleeding and even death [Citation23]. Spontaneous remission of SAA is extremely rare [Citation11]. Patients with spontaneous remission of pancytopenia were retrospectively diagnosed as AAH [Citation6,Citation24]. The clinical manifestations, blood routine, and bone marrow examinations of the AAH cases reported in our study were very similar to SAA. The misdiagnosis of AAH could put patients at unnecessary treatment-related risk. On the other hand, the best opportunity to treat SAA is sometimes missed by waiting too long time to identify AAH. It is important to distinguish these AAH patients from SAA patients in the early stage. Therefore, we included 11 cases of newly diagnosed AAH patients who had clinical manifestations and laboratory results consistent with the diagnosis of SAA and 49 newly diagnosed true SAA patients. And we analyzed the differences in clinical manifestations and laboratory test results between AAH and SAA, to search for clinical indicators for early differential diagnosis between AAH and SAA.
Compared with SAA patients, our data showed that the newly diagnosed AAH patients who met the SAA diagnosis were older, with a median age of 53 (21–69) years. All AAH patients were accompanied with other systemic diseases, mainly with infections of different systems, including pulmonary infection, suppurative tonsillitis, tympanitis, etc. With effective anti-infective therapy, the infection of AAH patients was well cured and blood routine quickly returned to normal. Patients with SAA, however, tended to be younger, with a median age of 37 (15–75) years and often had idiopathic onset without other systemic diseases. The primary symptoms of SAA were fatigue and bleeding. The AAH had a rapid onset and could progress to critical conditions quickly. The primary symptom of AAH was fever. However, after effective symptomatic supportive treatment, all patients with AAH could spontaneously achieve remission, with a median time of 12 (4–21) days. Only one elderly patient with severe sepsis had the longest recovery time of hematopoietic function, which was 21 days. The recovery of hematopoietic function in the other 10 patients was within 15 days. The median times for neutrophils to recover above 1 × 109/L and platelet to recover above 50 × 109/L in all patients with AAH were 5 (3–8) and 8 (1–13) days, respectively. These data suggest that patients of true AAH will show significant recovery of hematopoietic function in about a week. We do not need to wait a month to rule out AAH and that treatment can be started as early as possible for true SAA. Previous studies have reported that the spontaneous remission time of AAH was slightly longer than ours, with a median time of 18 (8–48) days [Citation24]. This may be related to the progress of current symptomatic supportive drugs, especially the progress of anti-infective drugs. Because patients with AAH often have severe infection, and hematopoiesis recovery is faster after infection under control.
Through comparison of examination results, we found that the degree of lymphocyte decrease in the AAH group was higher than that in SAA group with statistically significant. The degree of anemia and thrombocytopenia in the SAA group was higher than that in the AAH group with statistically significant. Moreover, although the SAA group had granulocyte deficiency, the absolute number of lymphocytes was not significantly reduced, which was consistent with the characteristics of the increased lymphocyte proportion in SAA patients. In the aspect of iron metabolism, the comparison between the two groups indicated that SAA patients had higher serum iron value and significantly decreased unsaturated iron binding force. In the AAH group, the total iron binding force was reduced. Ferritin was increased in both groups without statistical significance. However, patients in the AAH group were often complicated with acute and severe infection, and fever was often the first symptom. Ferritin could be regarded as the increase in inflammatory protein, so only the ferritin is elevated in the AAH group. In the SAA group, both the increase in serum iron and ferritin and the decrease in the binding force of unsaturated iron indicate the disorder of iron metabolism. The albumin level in the AAH group was significantly lower than that in the SAA group. In addition, the decrease in the albumin level in the AAH group was reported in the literature [Citation11,Citation24], which was in consistent with our results. However, the exact pathogenesis mechanism of AAH is still unclear and needs further exploration. The patients with AAH may be seriously ill at the onset, often accompanied by high fever, which could cause the patients into the malnutrition status.
To date, the diagnosis of AAH has been retrospective and lacks reliable prospective diagnostic indicators. Lee et al. [Citation11] believed that the presence of infection, drug therapy, and serum albumin lower than 34 g/L at the time of AA diagnosis were independent predictors of spontaneous response. According to our study, the diagnosis of these AA may be in doubt and should be classified as AAH. The study of Yan et al. [Citation24] suggested that serum albumin and iron decreased in patients with AAH. We demonstrated 11 cases of AAH clinically manifesting as SAA. The clinical features of AAH were summarized as follows: (1) rapid onset, short history, and fever as the first symptom; (2) even lower lymphocytopenia; (3) bone marrow hyperplasia is extremely low; (4) the serum albumin and iron was much lower; (5) after conventional symptomatic supportive treatment, the hematopoietic function would show significant recovery in about a week. Therefore, we believe that patients with the aforementioned possible spontaneous remission factors, or even a definite tendency to increase blood routine parameters in a short period of time, can be appropriately delayed hematopoietic stem cell transplantation or combined with strong immunosuppressive therapy. But for those patients without the spontaneous factors, or without rising tendency of routine blood-related index in about a week, the SAA treatment should start as soon as possible, rather than watch 1 month or longer. Early treatment of the SAA can avoid serious infection and repeated transfusions. Because our patient cohort size is small, more robust clinical trials should be carried out for further exploration.
5. Conclusions
The spontaneous remission of AAH was rapid in most patients, and relapses were rarely observed. With supportive treatment, the AAH patients would show significant improvement on blood routine in about a week; otherwise, the patients should be treated as early as possible with the SAA regimen to improve therapeutic effect, reduce the risk of infection and repeated transfusions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Gnanaraj J, Parnes A, Francis CW, et al. Approach to pancytopenia: diagnostic algorithm for clinical hematologists. Blood Rev. 2018;32(5):361–367.
- Savage SA, Viard M, O'HUigin C, et al. Genome-wide association study identifies HLA-DPB1 as a significant risk factor for severe aplastic anemia. Am J Hum Genet. 2020;106(2):264–271.
- Yokomizo-Nakano T, Kubota S, Bai J, et al. Overexpression of RUNX3 represses RUNX1 to drive transformation of myelodysplastic syndrome. Cancer Res. 2020;80(12):2523–2536.
- Tanaka TN, Bejar R. MDS overlap disorders and diagnostic boundaries. Blood. 2019;133(10):1086–1095.
- Fang BZ, He GS, Zhou HX, et al. Three times spontaneous remission of severe aplastic anemia following granulocyte transfusion from related donors: a case report and literature review. Chin Med Sci J. 2013;28(1):58–60.
- Bi L, Li J, Lu Z, et al. Acute arrest of hematopoiesis induced by infection with Staphylococcus epidermidis following total knee arthroplasty: a case report and literature review. Exp Ther Med. 2016;11(3):957–960.
- Koduri PR, Joshi S, Vanajakshi S. Aplastic crisis in a woman with autoimmune hemolytic anemia. Indian J Hematol Blood Transfus. 2020;36(2):422–423.
- Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172(2):187–207.
- Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128(3):337–347.
- Mende M, Sockel K. Parvovirus B19 infection. N Engl J Med. 2018;379(24):2361.
- Lee JH, Lee JH, Shin YR, et al. Spontaneous remission of aplastic anemia: a retrospective analysis. Haematologica. 2001;86(9):928–933.
- Xu ZL, Huang XJ. Haploidentical stem cell transplantation for aplastic anemia: the current advances and future challenges. Bone Marrow Transplant. 2021;56(4):779–785.
- Shin SH, Park SS, Yoon JH, et al. Comparison of HLA-matched sibling and unrelated donor transplantation in adult patients with acquired severe aplastic anemia. Bone Marrow Transplant. 2020;55(8):1570–1579.
- Park SS, Min GJ, Park S, et al. Comparable outcomes between unrelated and haploidentical stem cell transplantation in adult patients with severe aplastic anemia. Transplantation. 2020; 105(5):1097–1105.
- Liu L, Zhang Y, Jiao W, et al. Comparison of efficacy and health-related quality of life of first-line haploidentical hematopoietic stem cell transplantation with unrelated cord blood infusion and first-line immunosuppressive therapy for acquired severe aplastic anemia. Leukemia. 2020;34(12):3359–3369.
- Tichelli A, Peffault de Latour R, Passweg J, et al. Long-term outcome of a randomized controlled study in patients with newly diagnosed severe aplastic anemia treated with antithymocyte globuline, cyclosporine, with or without G-CSF: a Severe Aplastic Anemia Working Party Trial from the European Group of Blood and Marrow transplantation. Haematologica. 2019;105(5):1223–1231.
- Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376(16):1540–1550.
- Hosokawa K, Yamazaki H, Tanabe M, et al. High-dose romiplostim accelerates hematologic recovery in patients with aplastic anemia refractory to eltrombopag. Leukemia. 2021;15((Suppl.1)):1–2.
- Tichelli A, de Latour RP, Passweg J, et al. Long-term outcome of a randomized controlled study in patients with newly diagnosed severe aplastic anemia treated with antithymocyte globulin and cyclosporine, with or without granulocyte colony-stimulating factor: a Severe Aplastic Anemia Working Party Trial from the European Group of Blood and Marrow Transplantation. Haematologica. 2020;105(5):1223–1231.
- Young NS. Aplastic anemia. N Engl J Med. 2018;379(17):1643–1656.
- Young DJ, Dunbar CE. Immunosuppression and growth factors for severe aplastic anemia: new data for old questions. Haematologica. 2020;105(5):1170–1171.
- Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018;124(21):4192–4201.
- Bacigalupo A, Oneto R, Schrezenmeier H, et al. First line treatment of aplastic anemia with thymoglobuline in Europe and Asia: outcome of 955 patients treated 2001–2012. Am J Hematol. 2019;94(1):165.
- Yan ZS, Zhang L, Wang HJ, et al. [Acute arrest of hemopoiesis mimics aplastic anemia: 23 cases report]. Zhonghua Xue Ye Xue Za Zhi. 2007;28(11):750–753.
