ABSTRACT
Objectives:
Amyloid light-chain (AL) amyloidosis is a rare disease characterized by amyloid fibril deposits made up of toxic light chains causing progressive organ dysfunction and death. Recent studies suggest that hematologic response may be an important prognostic indicator of overall survival (OS) in AL amyloidosis. The aim of this study was to evaluate the trial-level association between hematologic complete response (CR) or very good partial response or better (≥ VGPR) and OS in newly diagnosed patients.
Methods:
Studies were identified via systematic literature review. Pooled effect estimates were generated by a random-effects model.
Results:
Nine observational studies reporting hematologic CR or ≥VGPR and OS hazard ratios (HRs) were included in the meta-analysis. Achieving hematologic CR was associated with improved OS (HR, 0.21; 95% confidence interval [CI] 0.13–0.34). Achieving ≥ VGPR was also associated with improved OS (HR 0.21; 95% CI 0.17–0.26). Results of a sensitivity analysis excluding one outlier study revealed no heterogeneity and a better overall HR estimate. Potential limitations of this meta-analysis include the small number of eligible studies (consistent with the rarity of the disease) and inconsistencies in reporting of results.
Conclusions:
Overall, our findings support the use of deep hematologic response (CR or ≥VGPR) as a clinical trial endpoint in newly diagnosed AL amyloidosis. This study provides evidence that early hematologic response is a strong patient-level surrogate for long-term OS in patients with AL amyloidosis receiving frontline therapy. Structured data collection of depth of response in future trials will further strengthen these observations.
Introduction
Systemic amyloid light-chain (AL) amyloidosis is a rare, life-threatening disease with reported incidence rates ranging from 3 to 12 cases per million person-years [Citation1–3], although under-reporting is likely. In patients with AL amyloidosis, plasma cell clones produce toxic light chains that misfold and accumulate as amyloid deposits throughout the body, causing progressive organ dysfunction [Citation1,Citation4,Citation5]. The heart and kidneys are the most commonly affected organs, impacting 70–80% and 50–60% of patients, respectively [Citation1]. Without prompt treatment to minimize exposure to toxic light chains, damage to these organs can become irreversible [Citation3]. The prognosis for patients with AL amyloidosis is typically poor, likely due in part to the lengthy period between symptom onset and diagnosis. According to one patient survey, about one-third of patients waited ≥12 months and about two-thirds were evaluated by ≥3 physicians before receiving a correct diagnosis [Citation6]. Real-world studies also have shown that over one-third of patients die within the first year following diagnosis [Citation7], and survival outcomes are especially poor among patients with advanced cardiac involvement at the time of diagnosis [Citation8–12].
Autologous stem cell transplant (ASCT) is effective in patients with AL amyloidosis; however, only about 20% of patients are eligible for this therapy [Citation13], and <50% who undergo ASCT achieve hematologic complete response (CR) [Citation14–18]. Historically, anti-plasma cell therapies approved for the treatment of multiple myeloma were used off-label for AL amyloidosis, with bortezomib, cyclophosphamide, and dexamethasone (VCd) used as the standard of care in many countries. Based on the results of the phase 3 ANDROMEDA trial [Citation19], the combination of the anti-CD38 monoclonal antibody daratumumab plus VCd (D-VCd) recently became the first approved treatment for patients with AL amyloidosis.
In light of the evolving treatment landscape, as well as the substantial proportion of patients who die from their disease within a year of diagnosis, there is a need to identify predictors of improved survival that can be assessed without the long-term follow-up required to reach median overall survival (OS) in a clinical trial setting. There is a large body of evidence showing improved survival outcomes with increasing depth of hematologic response to treatment [Citation9,Citation20,Citation16,Citation21–25], suggesting that hematologic response may be an important prognostic indicator of OS in AL amyloidosis. We conducted a meta-analysis to evaluate the trial-level association between deep hematologic response (either hematologic CR or very good partial response or better [≥VGPR]) and OS in patients with newly diagnosed AL amyloidosis who received pharmacologic treatment for their disease.
Material and methods
Literature search
The studies included in the current meta-analysis were identified via one of the 4 literature searches conducted as part of a systematic literature review (SLR) in February 2021, with an update in November 2021. The full details of the SLR have been published elsewhere [Citation26]. In brief, we searched the databases of MEDLINE, EMBASE, and the Cochrane Controlled Register of Trials to identify all randomized controlled trials (RCTs) and observational studies in patients with newly diagnosed AL amyloidosis. This search was supplemented by a gray literature search of ClinicalTrials.gov, hand-searching of abstracts from the European Hematology Association and American Society of Hematology, and a manual review of the reference lists of the SLRs identified by the initial search. Eligible publications were English-language articles from August 2005 onwards or conference abstracts from 2019 or later reporting RCTs or observational studies of newly diagnosed patients with AL amyloidosis who initiated first-line treatment. Studies that included only patients who received ASCT were excluded.
Two reviewers independently reviewed all full-text articles. Information from full-text articles was extracted by one reviewer and validated by a second reviewer. Extracted data were: first author, publication year, study design, number of treatment arms, and regimens used in each treatment arm, as well as study and population characteristics, treatment characteristics, baseline characteristics, efficacy outcomes, and safety outcomes.
A risk of bias assessment was performed using the National Institute for Health and Care Excellence clinical effectiveness quality assessment checklist [Citation27] or Newcastle-Ottawa Quality Assessment Scale [Citation28] as appropriate.
Feasibility assessment
A feasibility assessment of the results of the literature search was conducted to identify studies for inclusion in the final meta-analysis. Reporting of OS hazard ratios (HRs) stratified by hematologic response (either CR vs < CR or ≥ VGPR vs < VGPR) was required for inclusion in the meta-analysis. If HRs were not reported, survival curves were digitized, and individual survival and censoring times were reconstructed using an algorithm by Guyot [Citation29]. For studies that included Kaplan-Meier estimates of OS without the number of patients at risk at each time point, a similar methodology was used. Here, the Kaplan-Meier was reconstructed manually by approximating the censoring and event per drop in the Kaplan-Meier curve, resulting in an approximated number at risk. After that, the algorithm by Guyot [Citation29] was applied to reconstruct pseudo-individual patient-level data.
Studies that included outcomes after initial pharmacological treatment plus ASCT were excluded from the analysis (unless the proportion of patients receiving ASCT was <10%), because such patients have better outcomes that could bias the results of our analysis.
Meta-analysis
To combine the results from multiple studies and to obtain a pooled effect estimate, a meta-analysis was performed by fitting random-effects (RE) and fixed-effects models. Given the heterogeneity between the included studies in patient populations, duration of follow-up for OS, timing of assessment for response, and the first-line treatment regimens that patients were on, the RE model was fitted for the base-case analysis. For each comparison, the proportion of variation in estimates of treatment effect due to heterogeneity between studies (I2 statistic [Citation30]) was calculated.
In the case of outlying studies, a sensitivity analysis was performed. If the sensitivity analysis was not sufficient to explain the heterogeneity level, and the included studies could be divided into subgroups based on potential factors (e.g. age, gender, definition of response, disease severity) associated with the prognostic utility of surrogate endpoint status for OS, a meta-regression was warranted.
Forest plots were used to graphically illustrate the estimates from each study and the overall estimate from the meta-analysis. A P-value < 0.05 was considered statistically significant. The weight presented in the forest plots was based on the number of patients included in each study.
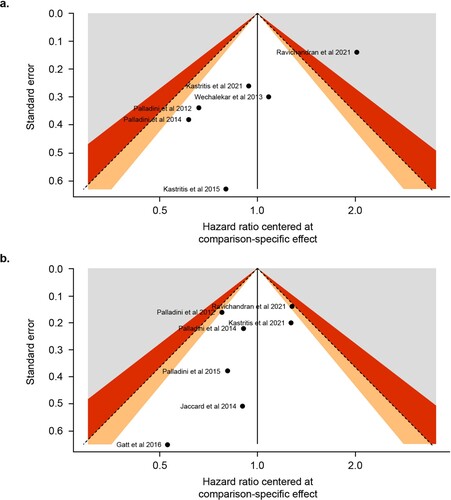
Funnel plots were used to spot outlier studies and to assess bias in the outcomes of the meta-analyses. The y-axis corresponds to the standard error of the included studies. Studies with a larger number of patients have lower standard error and tend to lay on the top of the plot. The x-axis corresponds to the effect estimate from each study or to the comparison-specific effect. In the case that the number of the included studies in the meta-analysis was ≥10, a funnel plot asymmetry test as proposed by Egger et al. [Citation31] was performed. A significant test result (P < 0.05) would imply that there is a sign of asymmetry in the included studies, which could be a result of true heterogeneity, reporting bias, or due to chance [Citation32].
The meta-analysis and sensitivity analysis were run with the use of R studio packages ‘meta’ and ‘netmeta.’
Results
The SLR identified 66 full-text publications (11 RCTs and 55 observational studies, Online Resource 1) that were screened for outcomes (OS stratified by hematologic response with Kaplan-Meier estimates and/or HRs) and intervention (pharmacologic treatment) of interest. Nine publications, all of which reported results of observational studies, were included in the meta-analysis [Citation9,Citation12,Citation20,Citation22,Citation33–37]. A flow diagram illustrating study selection for the meta-analysis is shown in . The characteristics of the 9 included studies are summarized in . Two of the studies reported OS stratified by hematologic CR vs < CR, 3 reported OS stratified by ≥VGPR vs <VGPR, and 4 reported OS stratified by both levels of response. Details regarding the response assessments in the included studies are reported in .
Figure 1. Flow diagram of study selection. ASH, American Society of Hematology; EHA, European Hematology Association; MA, meta-analysis; NMA, network meta-analysis; OS, overall survival; SLR, systematic literature review.
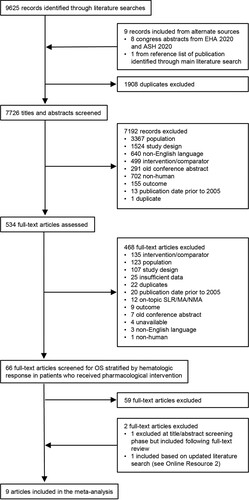
Table 1. Study and population characteristics of the studies included in the meta-analysis.
Table 2. Response assessments in the studies included in the meta-analysis.
Analysis using an RE model showed that achieving hematologic CR was associated with significantly improved OS (HR 0.21; 95% CI 0.13–0.34; P < 0.0001). These results are illustrated in a. The I2 statistic for this comparison was 75.4%, indicating moderate to substantial heterogeneity between the 6 included studies. One study (ALchemy, by Ravichandran et al [Citation37]) exceeded the boundaries of the funnel plot (a). An asymmetry test was not performed because the number of included studies was <10, meaning that the power of the test would be low.
Figure 2. Forest plot of the HRs for the risk of mortality between (a) hematologic CR vs <CR and (b) ≥VGPR vs <VGPR.
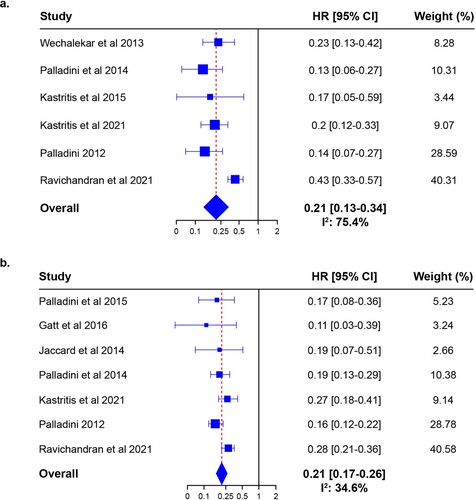
Achieving ≥ VGPR was also associated with significantly improved OS (HR 0.21; 95% CI 0.17–0.26; P < 0.0001). These results are shown in b. The I2 statistic was 34.6%, reflecting a not important to moderate heterogeneity between the studies included in the analysis. No study exceeded the boundaries of the funnel plot (b).
A sensitivity analysis was performed to evaluate whether achieving CR vs VGPR would have a different impact on survival. Four studies were identified that reported data in which it was possible to make this comparison. The HR estimates for CR vs VGPR in these studies were 0.24 (95%CI: 0.10–0.59) [Citation35], 0.32 (95%CI: 0.16–0.67) [Citation33], 0.34 (95%CI: 0.18–0.62) [Citation22] and 0.72 (95%CI: 0.53–0.98) [Citation37], suggesting that patients achieving CR have better OS compared to those achieving VGPR.
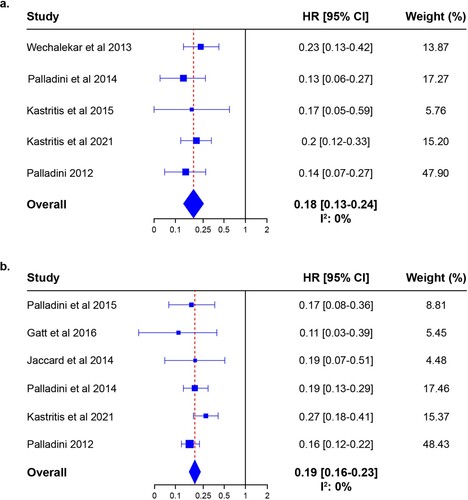
To assess the impact of the ALchemy study [Citation37] on the pooled HR estimate, a sensitivity analysis was conducted in which that study was excluded. When the 5 remaining studies were analyzed, the pooled HR estimate for CR vs < CR was 0.18 (95% CI 0.13–0.24; P < 0.0001), there was no heterogeneity between the included studies (I2: 0%), and no study exceeded the boundaries of the funnel plot. These results are shown in a and Online Resource 3a. The HR estimate for ≥VGPR vs <VGPR when the ALchemy study was excluded was 0.19 (95% CI 0.16–0.23; p < 0.0001), there was no heterogeneity (I2: 0%) between studies, and no study exceeded the boundaries of the funnel plot (b and Online Resource 3b).
Discussion
We conducted a meta-analysis to evaluate the association between hematologic response (either CR or ≥VGPR) and OS in patients with newly diagnosed AL amyloidosis with the aim of determining the prognostic value of hematologic response. A total of 9 published observational studies reporting OS stratified by hematologic response were included. The results of the meta-analysis showed that achieving hematologic CR and ≥VGPR were both associated with a significantly reduced risk of death, meaning that deep hematologic responses were associated with improved OS in patients with AL amyloidosis. In particular, patients achieving CR showed better OS than those who achieved VGPR, suggesting that achieving CR has prognostic value.
In ALchemy, the HR for the comparison of CR vs <CR was 0.43 [Citation37], which is higher than that reported in the other studies included in the meta-analysis (range, 0.13–0.23). This difference is likely due to the definition of hematologic CR used in the study. Patients with a normal free light chain (FLC) ratio were classified as CR, regardless of their FLC levels; as a result, the difference between CR and VGPR was less than expected. When patients were further classified based on their FLC levels, separation between the cohorts increased. We performed a sensitivity analysis in which ALchemy was excluded. This resulted in no heterogeneity and a pooled HR estimate of 0.18, reflecting a stronger association than the HR for the base-case analysis (0.21).
In many patients with AL amyloidosis, rapid and deep hematologic response can lead to at least partial reversal of organ damage, resulting in improvements in patients’ quality of life and prolonged survival [Citation3,Citation5,Citation22,Citation38]. It has been shown that the deeper the hematologic response, the better the survival outcomes; however, timing of response is important and even a brief delay in achieving a deep response (eg, at 3 months instead of at 1 month) can have negative clinical consequences [Citation22]. Without prompt reduction of the toxic light chains that are the precursor to amyloid deposits, organ damage may progress to the point where it is no longer reversible [Citation39,Citation40]. Therefore, the goal of treatment is generally understood to be the most rapid achievement possible of a deep hematologic response [Citation3,Citation5,Citation22,Citation38]; whether a response of ≥VGPR is adequate remains to be fully elucidated [Citation22]. Only two papers identified in this meta-analysis contained information on the impact of time to response on OS or improved organ function, prohibiting detailed analysis in this report. Both of these studies reported that achieving an early deep response was associated with better organ response and survival outcomes than achieving deep responses later [Citation22,Citation37]. The results of the current meta-analysis, which demonstrate a significant association between deep hematologic response and reduced risk of death, are supportive of this treatment paradigm.
This meta-analysis also makes a strong case for the use of deep hematologic response as a patient-level surrogate for OS in studies exploring the effectiveness of anti-clonal therapies. The use of OS as a clinical trial endpoint in patients with Mayo stage I and II requires long-term follow-up, which poses a unique set of challenges for AL amyloidosis. There has been a trend toward improved survival outcomes among patients with AL amyloidosis in recent years. For example, among patients diagnosed at the Mayo Clinic between 1977–1986 and 1987–1996, median OS was 1.2 years, compared with 1.5 years among those diagnosed between 1997 and 2006 [Citation41]. More recently, the EMN23 study group reported increases in median OS from 3.6 years for patients who initiated treatment between 2004–2010 to 4.2 years for those who started treatment between 2011–2018 [Citation42]. This latter, larger improvement is likely due in part to more widespread use of bortezomib-based treatment regimens in the management of patients with AL amyloidosis. On the other hand, the subset of patients with the most severe cardiac involvement (Mayo stage IIIB) at the time of diagnosis continue to have an extremely poor prognosis (median OS of just 3.5 months in those who initiated treatment post 2010), although even these patients can benefit from a deep hematologic response [Citation42]. Taken together with the small AL amyloidosis patient population, these factors suggest that a trial in stage I and II patients with the primary endpoint of OS would take many years to complete, resulting in delays in the availability of potentially active therapies in a rare and devastating disease. Prompt treatment initiation is critical in AL amyloidosis, and prolonged waiting for OS data from clinical trials to inform treatment decision-making is not ideal. In the clinical trial setting, there is a need for reliable surrogate endpoints for OS that can be evaluated without long-term follow-up. The present meta-analysis demonstrates that hematologic response is a strong patient-level surrogate for OS. Additional work and data from more RCTs are needed to evaluate hematologic response as a trial-level surrogate for OS in newly diagnosed patients with AL amyloidosis.
Our results in patients treated with pharmacologic therapy supplement published data demonstrating a relationship between hematologic response and survival outcomes in patients with AL amyloidosis who received ASCT. In a retrospective analysis of 80 patients who underwent ASCT at Boston University Medical Center between 1994 and 1997, the rate of hematologic CR among evaluable patients was 51% [Citation24]. More than 10 years following ASCT, median OS was still not reached in patients with hematologic CR versus 50 months in those without hematologic CR (P < 0.001). Another retrospective study evaluated 282 patients who received ASCT at the Mayo Clinic [Citation25]. In that study, Gertz and coauthors found that median OS was only reached by the 81 patients (28.7%) who failed to achieve at least partial response (≥ PR). In a landmark analysis of 213 patients who survived 6 months after ASCT, median OS was not reached in patients who achieved CR (n = 86, 40.4%) or PR (n = 91, 42.7%) but was 40.1 months in those without a response (n = 36, 16.9%). This difference was statistically significant (P = 0.001). In a subset of 151 patients with cardiac involvement, response rates were similar to those in the overall study population and survival was better in responders (≥ PR) than in non-responders. Hematologic response was a prognostic indicator of OS in both univariate and multivariate analyses. D’Souza et al reported on a subset of patients from the Center for International Blood and Marrow Transplant Research database who underwent ASCT between 1995 and 2012 and had available FLC results at baseline and day 100 post transplant [Citation16]. Of the 104 patients included, 13 (12%) achieved CR, 65 (63%) had VGPR, 15 (14%) had PR, and 11 (11%) had no response at day 100. The 5-year OS rate increased with increasing depth of hematologic response with no difference between CR and VGPR or between PR and no response. Meanwhile, OS was significantly better with ≥VGPR vs PR/NR (P = 0.02). Long-term outcomes were reported for a sample of 334 patients who underwent ASCT between 1994 and 2017 at either Boston University Medical Center or Memorial Sloan Kettering Cancer Center [Citation21]. Of 252 assessable patients, 174 (69.0%) had ≥PR and 78 (31.0%) had no hematologic response. Median OS increased with increasing depth of response, ranging from 13.4 years among patients with hematologic CR to 1.6 years for non-responders (P < 0.0001).
The EMN23 study, mentioned above, was a retrospective, multicenter study that enrolled 4480 patients at 13 sites in 10 European countries. Included patients were treated for AL amyloidosis between 2004 and 2018; therefore, the study provides data from the period before and after the introduction of VCd in routine clinical practice. EMN23 is also notable for its inclusion of patients with severe cardiac involvement (stage IIIb). Although a 2021 congress abstract reporting data from EMN23 [Citation43] was identified by the clinical evidence literature search of the SLR, it was decided to exclude the study from the current meta-analysis due to a potential overlap with the patient populations of other studies included in the meta-analysis (eg, ALchemy [Citation37]). The results reported in the EMN23 abstract were consistent with our findings: deeper hematologic responses at 3 months were significantly associated with better survival, with HR (95% CI) of 0.19 (0.14–0.25) for CR vs <CR and 0.19 (0.15–0.22) for ≥VGPR vs <VGPR.
Our study does have limitations. First, there were no RCTs that fulfilled the eligibility criteria for inclusion in this meta-analysis. Second, the 9 observational studies included in the meta-analysis used a number of different treatment regimens and information on salvage therapies offered to patients or receipt of solid organ transplantation were sparse in the studies analyzed. The duration of follow-up varied across studies and was short in some studies; however, this did not appear to be a driver of heterogeneity. There was also variability in the time points at which hematologic response was assessed and in the definitions of hematologic CR used across the studies, although consistent results irrespective of the definition of hematologic CR have been reported elsewhere [Citation44]. All included studies reported that normal FLC ratio was part of their definition of hematologic CR; however, it is possible that this ratio was disregarded if the involved FLC was less than the uninvolved FLC or reached very low values. Not all included studies had published HR values, so some HRs were estimated from digitized survival curves. Details on the cause of death were not recorded consistently across the studies and are not included in this analysis. In addition, while the majority of newly diagnosed patients are ineligible for an upfront transplant [Citation13], the exclusion of ASCT-based studies from this meta-analysis did hinder the evaluation of the impact of ASCT on survival. Future research might shed light on the prognostic value of hematologic response in this patient population. Finally, it is possible that the patient populations of some of these observational studies may have overlapped, although we believe we have mitigated the greatest potential overlap by excluding the EMN23 study.
Despite the differences across the studies included in the meta-analysis, the association between improving depth of hematologic response and OS was largely consistent across studies. This association supports the use of treatments that confer a rapid and deep hematologic response in patients with AL amyloidosis. Furthermore, the patient-level association between deep hematologic response (CR or ≥VGPR) and OS in this meta-analysis suggests there is value in further exploring trial-level surrogacy of this endpoint. A trial-level association would support the use of deep hematologic response as an endpoint in studies of patients with AL amyloidosis, potentially accelerating the timeframe for novel agents and regimens to become available to these patients for whom prompt treatment initiation is critical.
Supplemental Material
Download MS Word (292.2 KB)Acknowledgments
The authors thank Diana Tran of Eversana for her review of the SLR methods and flow diagram. Medical writing support was provided by Corey Eagan, MPH, of Eloquent Scientific Solutions, and was funded by Janssen Global Services, LLC.
Disclosure statement
EK serves in a consulting or advisory role for Amgen, Genesis Pharma, Janssen, Pfizer, and Takeda; received honoraria from Amgen, Genesis Pharma, Janssen, Pfizer, and Takeda; and received research funding from Amgen and Janssen. AM, LG, FK, and AV are employees of Cytel and were compensated by Janssen for conducting the study. JV, EA, AL, and SC are employees of Janssen. ADW serves in a consulting or advisory role for Caelum and Janssen; received honoraria from Celgene, Janssen, and Takeda; and travel, accommodations, and expenses from Takeda.
Data availability
The data underlying this article are sourced from the public domain and are available in the articles cited throughout.
Additional information
Funding
References
- Muchtar E, Dispenzieri A, Magen H, et al. Systemic amyloidosis from A (AA) to T (ATTR): a review. J Intern Med. 2021;289:268–292. doi:10.1111/joim.13169
- Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59.
- Fotiou D, Dimopoulos MA, Kastritis E. Systemic AL amyloidosis: current approaches to diagnosis and management. Hemasphere. 2020;4:e454. doi:10.1097/HS9.0000000000000454
- Comenzo RL, Reece D, Palladini G, et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia. 2012;26:2317–2325. doi:10.1038/leu.2012.100
- Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4:38. doi:10.1038/s41572-018-0034-3
- Lousada I, Comenzo RL, Landau H, et al. Light chain amyloidosis: patient experience survey from the Amyloidosis Research Consortium. Adv Ther. 2015;32:920–928. doi:10.1007/s12325-015-0250-0
- Palladini G, Merlini G. What is new in diagnosis and management of light chain amyloidosis? Blood. 2016;128:159–168. doi:10.1182/blood-2016-01-629790
- Chaulagain CP, Comenzo RL. New insights and modern treatment of AL amyloidosis. Curr Hematol Malig Rep. 2013;8:291–298. doi:10.1007/s11899-013-0175-0
- Palladini G, Sachchithanantham S, Milani P, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126:612–615. doi:10.1182/blood-2015-01-620302
- Dittrich T, Kimmich C, Hegenbart U, et al. Prognosis and staging of AL amyloidosis. Acta Haematol. 2020;143:388–400. doi:10.1159/000508287
- Lin HM, Seldin D, Hui AM, et al. The patient’s perspective on the symptom and everyday life impact of AL amyloidosis. Amyloid. 2015;22:244–251. doi:10.3109/13506129.2015.1102131
- Wechalekar AD, Schonland SO, Kastritis E, et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121:3420–3427. doi:10.1182/blood-2012-12-473066
- Gertz MA. Immunoglobulin light chain amyloidosis: 2020 update on diagnosis, prognosis, and treatment. Am J Hematol. 2020;95:848–860. doi:10.1002/ajh.25819
- Sarosiek S, Zheng L, Sloan JM, et al. Comparing measures of hematologic response after high-dose melphalan and stem cell transplantation in AL amyloidosis. Blood Cancer J. 2020;10:88. doi:10.1038/s41408-020-00354-7
- Jaccard A, Moreau P, Leblond V, et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med. 2007;357:1083–1093. doi:10.1056/NEJMoa070484
- D'Souza A, Huang J, Hari P. New light chain amyloid response criteria help risk stratification of patients by day 100 after autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:768–770. doi:10.1016/j.bbmt.2015.12.021
- Minnema MC, Nasserinejad K, Hazenberg B, et al. Bortezomib-based induction followed by stem cell transplantation in light chain amyloidosis: results of the multicenter HOVON 104 trial. Haematologica. 2019;104:2274–2282. doi:10.3324/haematol.2018.213900
- Sanchorawala V, Sun F, Quillen K, et al. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem cell transplantation: 20-year experience. Blood. 2015;126:2345–2347. doi:10.1182/blood-2015-08-662726
- Kastritis E, Palladini G, Minnema MC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385:46–58. doi:10.1056/NEJMoa2028631
- Kastritis E, Roussou M, Gavriatopoulou M, et al. Long-term outcomes of primary systemic light chain (AL) amyloidosis in patients treated upfront with bortezomib or lenalidomide and the importance of risk adapted strategies. Am J Hematol. 2015;90:E60–E65. doi:10.1002/ajh.23936
- Nguyen VP, Landau H, Quillen K, et al. Modified high-dose melphalan and autologous stem cell transplantation for immunoglobulin light chain amyloidosis. Biol Blood Marrow Transplant. 2018;24:1823–1827. doi:10.1016/j.bbmt.2018.06.018
- Kastritis E, Fotiou D, Theodorakakou F, et al. Timing and impact of a deep response in the outcome of patients with systemic light chain (AL) amyloidosis. Amyloid. 2021;28:3–11. doi:10.1080/13506129.2020.1798224
- Cibeira MT, Oriol A, Lahuerta JJ, et al. A phase II trial of lenalidomide, dexamethasone and cyclophosphamide for newly diagnosed patients with systemic immunoglobulin light chain amyloidosis. Br J Haematol. 2015;170:804–813. doi:10.1111/bjh.13500
- Sanchorawala V, Skinner M, Quillen K, et al. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem-cell transplantation. Blood. 2007;110:3561–3563. doi:10.1182/blood-2007-07-099481
- Gertz MA, Lacy MQ, Dispenzieri A, et al. Effect of hematologic response on outcome of patients undergoing transplantation for primary amyloidosis: importance of achieving a complete response. Haematologica. 2007;92:1415–1418. doi:10.3324/haematol.11413
- Lee C, Lam A, Kangappaden T, et al. Systematic literature review of evidence in amyloid light-chain amyloidosis. J Comp Eff Res. 2022;11:451–472. doi:10.2217/cer-2021-0261
- National Institute for Health and Care Excellence. (2015). Single technology appraisal: user guide for company evidence submission template [cited 2021 Dec 14]. Available from: https://www.nice.org.uk/process/pmg24/chapter/clinical-effectiveness#quality-assessment-of-the-relevant-clinical-effectiveness-evidence.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [cited 2021 Dec 14]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi:10.1186/1471-2288-12-9
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi:10.1002/sim.1186
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi:10.1136/bmj.315.7109.629
- Sterne JA, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Br Med J. 2011;343:d4002. doi:10.1136/bmj.d4002
- Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30:4541–4549. doi:10.1200/JCO.2011.37.7614
- Jaccard A, Comenzo RL, Hari P, et al. Efficacy of bortezomib, cyclophosphamide and dexamethasone in treatment-naive patients with high-risk cardiac AL amyloidosis (Mayo Clinic stage III). Haematologica. 2014;99:1479–1485. doi:10.3324/haematol.2014.104109
- Palladini G, Milani P, Foli A, et al. Oral melphalan and dexamethasone grants extended survival with minimal toxicity in AL amyloidosis: long-term results of a risk-adapted approach. Haematologica. 2014;99:743–750. doi:10.3324/haematol.2013.095463
- Gatt ME, Hardan I, Chubar E, et al. Outcomes of light-chain amyloidosis patients treated with first-line bortezomib: a collaborative retrospective multicenter assessment. Eur J Haematol. 2016;96:136–143. doi:10.1111/ejh.12558
- Ravichandran S, Cohen OC, Law S, et al. Impact of early response on outcomes in AL amyloidosis following treatment with frontline bortezomib. Blood Cancer J. 2021;11:118. doi:10.1038/s41408-021-00510-7
- Manwani R, Foard D, Mahmood S, et al. Rapid hematologic responses improve outcomes in patients with very advanced (stage IIIb) cardiac immunoglobulin light chain amyloidosis. Haematologica. 2018;103:e165–e168. doi:10.3324/haematol.2017.178095
- Roccatello D, Fenoglio R, Sciascia S, et al. CD38 and anti-CD38 monoclonal antibodies in AL amyloidosis: targeting plasma cells and beyond. Int J Mol Sci. 2020;21:4129. doi:10.3390/ijms21114129
- Gatt ME. Light chain amyloidosis 2014. Int J Hematol. 2014;1:21–29. doi:10.12691/ijhd-1-1A-4.
- Kumar SK, Gertz MA, Lacy MQ, et al. Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin Proc. 2011;86:12–18. doi:10.4065/mcp.2010.0480
- Palladini G, Schönland S, Merlini G, et al. First glimpse on real-world efficacy outcomes for 2000 patients with systemic light chain amyloidosis in Europe: a retrospective observational multicenter study by the European Myeloma Network. Blood. 2020;136:50–51. doi:10.1182/blood-2020-140708
- Palladini G, Schönland S, Merlini G, et al. Systemic light chain amyloidosis across Europe: key outcomes from a retrospective study of 4500 patients. Blood. 2021;138:153. doi:10.1182/blood-2021-152675
- Comenzo R, Kastritis E, Palladini G, et al. Reduction in absolute involved free light chain and difference between involved and uninvolved free light chain is associated with prolonged major organ deterioration progression-free survival in patients with newly diagnosed AL amyloidosis receiving bortezomib, cyclophosphamide, and dexamethasone with or without daratumumab: results from ANDROMEDA. Blood. 2020;136:48–50. doi:10.1182/blood-2020-137582