ABSTRACT
Objective
to review the current diagnostic and therapeutic landscape of AML in Latin America as a reflection of other low- and middle-income countries and regions of the world. Encompassing both acute promyelocytic and non-promyelocytic disease types.
Methods
We reviewed the literature and study registries concerning epidemiological features of patients with AML/APL treated in Latin America, as well as evaluated diagnostic and genetic stratification and patient fitness assessment challenges, the importance of early mortality and supportive care capacity, intensive and non-intensive chemotherapy alternatives, consolidation, and maintenance strategies including novel agents and hematopoietic stem cell transplantation.
Results
Although most of the current technologies and treatment options are available in the region, a significant fraction of patients have only limited access to them. In addition, mortality in the first weeks from diagnosis is higher in the region compared to developed countries.
Conclusions
Disparities in access to technologies, supportive care capacity, and availability of novel agents and HSCT hinder results in our region, reflecting barriers common to other LMICs. Recent developments in the diagnosis and treatment of this disease must be implemented through education, collaborative clinical research, and advocacy to improve outcomes.
Introduction
Acute myeloid leukemia (AML), a neoplasm derived from the malignant transformation and expansion of myeloid precursors, is common around the world. Across the age spectrum, older adults are the most frequent group affected, with a median age at diagnosis of 65 years in North America and Europe [Citation1–3]. Epidemiological differences in Latin-America (LA) compared to high-income countries (HICs) have been reported. For instance, a younger age at diagnosis ranging from 40–47 years due to our overall younger population, and a higher prevalence of acute promyelocytic leukemia (APL) in comparison to non-APL subtypes [Citation4–6]. Furthermore, differences in access to treatment alternatives for this disease changes the way AML should be approached according to geographic regions and socioeconomic contexts. In this review, we will summarize the diagnosis and treatment of AML through the lens of hematologists working in LA as a reflection of other low and middle-income countries (LMICs) and disadvantaged populations in HICs and focus on specific challenges and their potential solutions faced in these regions of the world.
Diagnosis
The diagnosis of AML is challenging considering the need for specialized diagnostic tools. Including flow cytometry, cytogenetic and molecular assessments which are required to properly classify, risk-stratify, and use targeted therapies [Citation7]. Current recommendations include the use of flow cytometry to confirm the diagnosis and establish the immunophenotype with at least 8 colors which are not always available in LA. The Brazilian Group of Flow Cytometry recognized this limitation and considering that most laboratories were working with 4-colour immunophenotyping, published locally adapted recommendations [Citation8]. For the molecular classification of AML, the European LeukemiaNet(ELN) 2017 guidelines recommended the analysis of at least 8 genes in addition to cytogenetics for a comprehensive assessment [Citation7]. In the 2022 update, another 8havebeen incorporated and required to establish the diagnosis with 20 additional genes recommended to be tested [Citation9]. While in HICs the availability of comprehensive genetic testing through high throughput sequencing has been implemented faster than the evidence to apply it, in LA a different reality is palpable. In Mexico, even conventional cytogenetic assessment has been difficult to standardize. In 3 different AML cohorts from 2010 to 2018, a successful karyotype was analyzed in 46–61% of cases [Citation10–12]. In the largest registry study in the public setting, karyotype data was available in 69% at baseline, withFLT3 and NPM1 status available only in 12% and 8% of cases, respectively [Citation4]. Lack of education, training, availability, and coverage limit the conduct of these expensive tests, which caneven surpass the costs of treatment. This issue raises the question of what therapy should we recommend a patient that has not been appropriately studied according to current standards? Today for any given AML patient in LA that is most likely the case. Locally performed analyzes, training and accessible reagents are urgently needed to improve our diagnostic capacity, avoid over and under-treatment and optimize existing resources. Despite these barriers, available evidence suggest most abnormalities are of a similar incidence in our population than others, including core binding factor [Citation13], FLT3and NPM1 mutations [Citation14–16].
Intensive therapy
Remission induction, supportive measures, and early mortality
The aim of an intensive induction regimen is to eliminate the large burden of leukemic blasts and to re-establish normal hematopoiesis. Most patients with AML in LA will be candidates for this treatment strategy. Traditionally 7 days of cytarabine plus 3 days of daunorubicin (7 + 3) have been an international standard for over 4 decades and remains so in most of LA [Citation17]. This strategy is successful in achieving complete responses (CR) in 40–60% of older adults and 60–80% of younger patients varying according to genetic risk categories. Higher doses of daunorubicin (90 mg/m2 for 3 days) have shown to improve survival outcomes in comparison to 45 mg/m2 up to 65 years [Citation18,Citation19], whereas the dose of 60 mg/m2 is also commonly used and potentially of equivalent benefit and efficacy, similarly to idarubicin at 12 mg/m2 [Citation20,Citation21]. An addition of a third agent to 7 + 3 or the use higher doses of cytarabine have been compared but not consistently proven to improve survival on all patients [Citation22,Citation23], although the latter are still favored for patients with core binding factor AML including inv16, t(16;16) and t(8;21) with some groups advocating for the use of fludarabine, higher doses of cytrabaine, and filgrastim (FLAG)in this population [Citation24,Citation25]. Moreso than adjustments in 7 + 3, historical improvement in outcomes in intensively treated AML patients in HICs have occurred due to a steady improvement in the delivery of supportive care [Citation26–28]. Administered at the time with the highest disease burden, intensive chemotherapy comes with significant risks; severe neutropenia and thrombocytopenia are nearly universal, with transfusion dependence and a high incidence of chemotherapy-induced mucositis. Early mortality, ranges between 3 and 6% in modern clinical trials [Citation20,Citation24,Citation26]. However, when we look at the implementation of induction chemotherapy in LMICs, increased rates of induction mortality are common and reported to be15% in India up to 41% among older adults in Brazil, also higher than historical rates outside of the clinical trial setting in HICs [Citation28–31]. White blood cell counts as a measure of disease burden correlate with induction mortality [Citation32], particularly patients with hyperleukocytosis 100 × 109/L have early death rates that can range from 30 to 50% in patients with leukostasis [Citation32–35]. Therefore, establishing urgent treatment measures for patients with highly elevated white blood cell counts is imperative. Interventions reported include tumor lysis prophylaxis cytoreduction with hydroxyurea, an early initiation of chemotherapy and leukoapheresis for patients with leukostasis. There are no data to support leukapheresis over other measures and this procedure is complex, expensive, and less accessible than rapid pharmacologic treatment [Citation36]. Delaying treatment until genomic data is available is a new trend which should be taken with caution, and it is important to note that the use of cytoreduction does not necessarily limit the acquisition of genomic data [Citation37]. Chemotherapy shortages due to poor governmental leadership and administration is a complex issue faced in several countries in our region [Citation38,Citation39] which complicates patient management at the bedside and forces us to use be creative and substitute drugs for what is available with a negative impact in outcomes. Other non-modifiable patient-related factors associated with early mortality include age and functional status, comorbidities, cytogenetic risk, insurance access, socioeconomic status, and social support [Citation27,Citation29,Citation33].
Infections are the most common cause of early death for AML patients with bacterial and invasive fungal infections common culprits. While fluoroquinolones are recommended, widespread resistance begs the question of whether we should continue to use antibacterial prophylaxis at all [Citation40]. New problems that have risen include the high rates of gram-negative carbapenem-resistant organisms in other regions of the globe [Citation29]. Early de-escalation after resolution of fever for 48–72 h can reduce antibiotic-related adverse events and selection for drug-resistant organisms without compromising outcomes [Citation41]. Central venous lines should be handled by trained nurses and access to cultures should be immediate. Using recommended mold-active agents such as posaconazole and isavuconazole can be expensive and inaccessible, and one must rely on earlier generation azoles such as voriconazole or itraconazole which can be limited by toxicities, absorption issues and pharmacologic interactions [Citation42].
Transfusion support is key and the availability of plateletphereses can be challenging, as less than half of the blood supply in LA comes from altruistic donors [Citation43]. Optimization of blood products through patient blood management programs is highly important [Citation44]. Avoiding sibling and family directed donation becomes even more relevant, as the generation of anti-HLA donor-specific antibodies can become a barrier for haploidentical hematopoietic stem cell transplantation (HSCT) [Citation45]. Thus, characteristics of the treatment center itself are of key importance and can improve or limit the capacity to deliver supportive care; induction mortality is inversely correlated with center specialization and experience [Citation27,Citation30,Citation46]. ICU capacity and access, nursing, and quality management, establishment of outreach programs for timely referral to leukemia centers, and a multidisciplinary treatment team with continued education are all relevant factors that should be increasingly fostered and developed in our region [Citation46]. Implementation of practices known to be successful is challenging but can likely save more lives worldwide than any diagnostic study or novel agent addition to the current standard management [Citation41]. Furthermore, novel induction strategies such as early discharge and outpatient follow-up can decrease exposures, costs, and improve quality of life [Citation47]. This strategy has been adopted successfully in the context of allogeneic HSCT in Mexico even in centers without a conventional transplant unit [Citation48].
Novel agents in intensive therapy
Several additions to 7 + 3 have demonstrated to improve outcomes. Midostaurin is a multikinase inhibitor that targets the FLT3pathway and was studied in the randomized, placebo controlled RATIFY CALGB 10603 trial where 717 patients <60 years were included. Patients in the experimental arm received midostaurin 50 mg PO BID on days 8–21 of induction and consolidation; a 7% improvement in 4-year OS and event-free survival (EFS) were obtained in comparison to placebo [Citation49]. It is recommended that midostaurin is started no later day 8, but this can be challenging where there is no access to FLT3 testing in-house. Midostaurin is associated with increased rates of anemia, rash, nausea, and QTc interval prolongation. It is available in several countries in LA (). Sorafenib is another first-generation multi-kinaseFLT3 inhibitor that has been studied in phase 3 trials for AML. The SORMAIN study planned to accrue 200 patients but was terminated early due to poor enrollment; 83 patients with FLT3-ITD AML in CR after transplant were randomized to sorafenib maintenance at 200–400 mg BID for up to 2 years and compared to. placebo. In this study, the primary endpoint of relapse-free survival (RFS) favored the sorafenib arm with a 31.7% improvement at 2 years translating to an OS benefit of 24.3%, with similar results reproduced in an open-label trial [Citation50]. Sorafenib is the FLT3 inhibitor most widely available in LA () due to its original indication in hepatocellular, renal, and thyroid cell carcinomas, but its real-world use in AML in our region is unknown. It is limited by gastrointestinal adverse events, fatigue, and infections. The use of the second-generation FLT3 inhibitor quizartinib, a second-generation FLT3 inhibitor selective to the ITD mutation has been added to 7 + 3 and compared to placebo in the QuANTUM-First trial which included 539 patients 18–75 years. In this study an OS and RFS benefit has been reported with the use of 40 mg per day on days 8–21 for up to 3 years, although it is yet to be approved for this indication [Citation51]. The use of other second-generation FLT3 inhibitorsgilteritinib and crenolanib in combination with chemotherapy vs. midostaurin and in maintenance therapy are under study. Novel agents that have been incorporated to intensive therapy in AML but remain unavailable in most countries in Latin America including gemtuzumabozogamicin, quizartinib and CPX-351 are summarized in .
Figure 1. Availability of novel therapies for acute myeloid leukemia across selected countries in Latin America. Dark-shaded areas refer to partial availability. Light shaded areas refer to universal availability. White areas refer to unavailability.
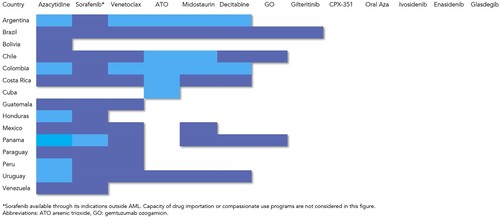
Table 1. Novel agents approved for the treatment of AML that are unavailable in Latin America.
Consolidation, maintenance, and long-term outcomes in LA
For adults, higher doses of single agent cytarabine for consolidation remain the standard [Citation62]. There is debate regarding the exact dose and schedule, however doses of 2–3 g/m2 twice daily can be quite toxic, leading to severe neutropenia and transfusion dependence, and with a risk of significant organ toxicities, thus it is usually limited for patients younger than 60. Intermediate doses of cytarabine (1–1.5 g/m2) seem similarly effective and less toxic and can be a preferred strategy across the age spectrum, being our standard, particularly when supportive care capacity is limited by lack of resources which has also recently been favored in the 2022 ELN update as well [Citation7,Citation9,Citation24,Citation63]. This lower intensity strategy may also improve the rate of treatment abandonment that occurs after achievement of hematopoietic recovery, as financial constraints and a lower educational level can limit patients’ and families’ understanding of their disease and the importance of post-remission care [Citation64]. Retrospective and registry studies report a consistent decrease of approximately 10–30% in the 5-year probability of OS in LA vs. HICs during the last decade, ranging from 22 to 55% in young adults [Citation10,Citation65,Citation66]. Similarly, across risk categories worse outcomes have been observed for favorable risk patients with 5-year OS rates ranging from 60 to 40% and intermediate-risk of 40–20% suggesting there is room for improvement in these subgroups [Citation4,Citation67]. The COVID19 pandemic further impaired our capacity to deliver care and increased the risk of mortality from infection due to prolonged exposure to the medical environment [Citation68,Citation69]. The addition of more chemotherapeutic agents and subcutaneous azacytidine (Aza) to consolidation has not shown to be effective in improving survival [Citation24,Citation70–72]. Oral azacytidine has shown survival benefit in a randomized trial in patients that are ineligible for HSCT, but it is still unavailable in LA. Its use after transplantation is under study (, )
Non-intensive therapy
For patients who are not considered candidates for intensive therapy after the comprehensive evaluation of comorbidities, functional, social, and geriatric assessments, the prior standard was hypomethylating agents continued until progression which achieve a median OS of 10 months [Citation73]. There are not many studies that focus on this population in LA. However, most patients in our region have been treated either with low dose cytarabine (LDAC) and best supportive care achieving median OS of 6–8 months and <3 months, respectively, although the latter is no longer considered an appropriate therapy for AML [Citation74]. In Mexico, most older adults receive LDAC in the public setting with a 30-day mortality of 31.4% and median OS of only 1 month [Citation4,Citation75]. On the other hand, several recent drug approvals for AML in HICs are focused on this patient group. In you can see and adapted algorithm from current NCCN guidelines [Citation76].
Figure 2. Adapted treatment algorithm for patients with acute myeloid leukemia who judged not to be candidates for intensive chemotherapy.
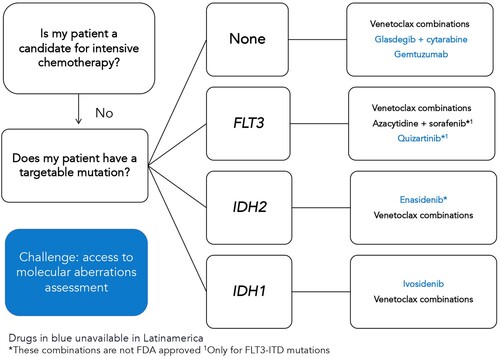
Venetoclax-based combinations were the first change in the standard non-intensive treatment for AML after the publication of two randomized phase 3 trials combining the BLC2 inhibitor with cytarabine and azacytidine. With the cytarabine combination, the initial OS benefit was not significant until longer follow-up revealed a median of 8.4 vs. 4.1 months when compared to placebo [Citation77]. In contrast, the combination with azacytidine was associated with a clear OS benefit (14.7 vs. 9.6 months) and became the standard of care in many countries [Citation78]. A retrospective experience on the use of venetoclax combinations included 50 patients from Mexico and Peru was recently published. Interestingly, venetoclax-based combinations were used in both, newly diagnosed and relapsed/refractory patients, combined with cytarabine in 40% of cases, and frequently in lower doses due to its combination with azoles, reflecting a need for lower-cost regimens [Citation79]. CR/CRi rates were 78.6% in newly diagnosed patients with a median OS of 9.6 months, shorter than the phase 3 trial but improved over prior regional experiences showing this therapy can be implemented in LA [Citation79]. This drug combination is particularly effective in patients with IDH2 and NPM1 mutations with 2-year OS exceeding 70% [Citation80]. On the other hand, responses for FLT3 mutated patients are usually not durable, lasting less than a year [Citation80]. The use of FLT3 inhibitors in addition to venetoclax and hypomethylating agents is under study. Other drugs recently available in high-income countries that have yet to reach most of LA are IDH inhibitors and glasdegib ().
Interestingly, some countries in LA participated in the study that led to the approval of ivosidenib(6 in Brazil and 2 in Mexico) but in our region the approval of a drug is not immediately associated to increased uptake and access for most of the population and is frequently limited to patients with private insurance, leading to local disparities [Citation59]. ().
Hematopoietic stem cell transplantation
HSCT remains one of the most potent antileukemic treatments available and is a treatment goal for all fit patients with intermediate and high-risk disease, as well as those with positive MRD and relapsed/refractory disease [Citation81,Citation82]. Unfortunately, most transplant candidates in our region still do not have access to this potentially life-saving therapy [Citation4]. The limitations and barriers for HSCT faced in our region are discussed in another review in this series, but several important developments in AML relevant to our region should be noted. The availability of reduced intensity conditioning regimens (RIC) has allowed for more patients to receive allogeneic transplantation; age should no longer be considered a limit for undergoing the procedure and arbitrary age limits to access HSCT programs should be eliminated. This is more relevant now as the probability to achieve remission and hematopoietic recovery is improved with venetoclax-based regimens. Patients who were not previously considered fit for intensive therapy may be considered candidates for transplant and achieve favorable outcomes [Citation83]. In the latest CIBMTR analysis including 1,321 patients ≥60 years in CR1 showed comparable OS at 3 years of 49.4% in ages 60–64, 42.3% in 65–60 and 44.7% in those ≥70 years [Citation84].
A historical paradigm in AML was to administer a myeloablative conditioning regimen (MAC) if possible, based on predicted tolerability, similarly to the decision to receive intensive therapy due to an increased risk of relapse with RIC regimens, but this has been challenged by a recent study in the era of MRD. The phase III BMT CTN 0901 trial randomized patients with AML and myelodysplastic syndrome in CR to MAC or RIC; pre-transplant bone marrow samples for MRD with high throughput sequencing were taken and analyzed post-hoc in 190 patients. In this study, MRD-positive patients had a 3-year cumulative incidence of relapse (CIR) and survival favoring MAC (67% vs. 19% and 61% vs. 43%, respectively). In contrast, MRD-negative patients had similar 3-year OS regardless of conditioning intensity (MAC 56% vs. RIC 63%) balanced due to a lower transplant-related mortality (MAC 27% vs. RIC 9%) [Citation85]. This study allows us to conclude that RIC is a reasonable approach even in patients eligible for MAC if MRD is negative, with less toxicity and similar outcomes. This is highly relevant for our region as MAC demands high quality supportive care capacity, whereas RIC regimens can be implemented as outpatients and performed even without a conventional HSCT unit [Citation48].
Intensive chemotherapy promptly followed by a RIC regimen and cell infusion without the need for cytopenia recovery (sequential or augmented conditioning) has been studied to reduce the risk of relapse and improve on efficacy. This strategy, performed most frequently with fludarabineamsacrine, cytarabine and busulfan (FLAMSA-Bu), was studied in 244 patients with high-risk AML or MDS in a randomized fashion and compared to RIC in the FIGARO trial. No differences in 2-year OS (58.8% vs. 60.9%) or CIR (26.7% vs. 29.5%) were revealed [Citation86]. In this study, up to approximately 50% of patients with pre-transplant MRD by flow cytometry did not relapse. Therefore, although pre-transplant MRD is a poor prognostic factor it should not discourage transplantation or become an impediment for referral to a transplant center. For patients who are not in CR, an alternative double-alkylator strategy with the addition of thiotepa to busulfan and fludarabine has not proven successful [Citation87]. Taken together, these data suggest that, as conditioning regimens go, we can go lower, but we probably should not go higher.
The availability of haploidentical donors in Latin America through post-transplant cyclophosphamide has greatly increased the number of procedures that can be performed in our region as most countries in our region do not have an unrelated donor registry and face great bureaucratic and economic challenges with international unrelated donors [Citation88]. So far, results with the haploidentical donor strategy have shown to be superior to umbilical cord blood grafts in a randomized trial and comparable to unrelated donors in single-arm studies with both bone marrow or peripheral blood and across conditioning regimen intensities [Citation89,Citation90]. The Beijing protocol based on anti-thymocyte globulin, and a primed bone marrow graft has also shown comparable results to matched sibling grafts [Citation91]. In contrast with matched donors, the presence of donor-specific antibodies is a limiting barrier, and viral reactivations are more common than with calcineurin inhibitor and methotrexate prophylaxis. In our context, we recommend considering first-line allogeneic transplants regardless of a matched or haploidentical donor for patients without proper risk stratification, and those with high-risk and intermediate-risk disease to be performed in specialized and experienced centers.
Regarding autologous transplantation, a randomized study by the HOVON-SAKK group revealed a reduced relapse rate but higher non-relapse mortality resulting in similar OS vs. a third cycle of consolidation chemotherapy in intermediate-risk patients [Citation92]. Concerns regarding the presence of residual disease and the availability of alternative donors have led to declining rates of autologous grafts [Citation93]. However, in LA autologous transplants could be efficacious and cost-effective compared to repeated doses of consolidation chemotherapy as it can lead to a reduced exposure to severe neutropenia, transfusions, and hospital admissions. This strategy has been studied in the prospective GIMEMA AML1310risk-adapted trial in which patients with favorable and MRD-negative intermediate-risk patients were allocated to autologous HSCT after a single consolidation, achieving two-year OS of 74% and 79% respectively, and disease-free survival of 61% in both groups [Citation94]. In APL, HSCT is a strategy that remains a standard for consolidating relapsed disease, although based on retrospective, uncontrolled studies. It should be avoided in the first line altogether given the high probability for cure even with a regimen that does not include arsenic trioxide (ATO) [Citation7]. In this context, autologous transplantation is viewed more favorably as non-randomized comparisons have shown superior EFS and OS to allogeneic transplants in large registry studies [Citation95–97] with allo-HSCT recommended only for patients who fail to achieve a second molecular remission.
Relapsed/refractory AML
Patients with relapsed or refractory AML have dismal survival. Second-line chemotherapy regimens such as FLAG IDA, HAM or MEC followed by allogeneic hematopoietic stem cell transplantation are still recommended [Citation81]. We have had success treating patients with venetoclax-based combinations in relapsed/refractory patients with a 45.5% CR/Cri rate which agrees with other experiences worldwide as no RCT is available in this context [Citation79]. Patients who were not considered candidates for intensive therapy and have relapsed or are refractory to either Aza, LDAC or venetoclax-based combinations fair the worst, with supportive care often the only remaining alternative, although this has recently changed in HICs. The use of FLT3 inhibitor monotherapy has been compared with chemotherapy in two randomized phase 3 trials in patients with the ITD mutation. Quizartinib was evaluated in the randomized QUANTUM-R trial and compared with second-line intensive and non-intensive strategies documenting an improvement in median OS of 6.2 months vs. 4.7 months, albeit this drug was not granted approval in the United States and Europe due to concerns with the quality of the data [Citation98]. Similarly, gilteritinib, has been studied in the phase 3 trial ADMIRAL trialat 120 mg vs. a mixed choice of intensive and non-intensive second-line strategies where it achieved a 3.7-month median OS advantage [Citation99]. Gilteritinib is currently only available in Brazil () and although sorafenib is available off-label there are only phase 2 studies to support its use in post-transplantation relapse [Citation100]. Lastly both ivosidenib and enasidenib are approved in the US for relapsed/refractory AML based on phase 2 studies with ORR of 41.6% and 40.3%, respectively, with median OS of 9months and neither are yet available in LA [Citation101,Citation102].
Acute promyelocytic leukemia
APL is a distinct subtype of AML characterized by gene rearrangements involving the Retinoic Acid Receptor α (RARA) locus on chromosome 17 in 98% of cases with the PML gene; bcr1 breakpoints are more common in our population than non-Latino patients [Citation103,Citation104] with variant translocations of similar frequency than in Europe and the US [Citation105].
Diagnosis and early management
Death within the first hours or days from the diagnosis is more frequent in APL than in other subtypes of acute leukemia [Citation106,Citation107] and, therefore patients suspected to have APL should be managed as a medical emergency [Citation108]. The most frequent cause of death is bleeding which is associated with an APL-associated coagulopathy, and even before the genetic confirmation of the diagnosis, all trans retinoic acid (ATRA) and measures to counteract the coagulopathy should be initiated immediately based solely on the clinical suspicion of APL and the review of the peripheral blood smear [Citation108]. The identification of the APL-specific genetic lesion is mandatory and in LA most laboratories investigate initially for the PML/RARA rearrangement which can be detected by fluorescence in situ hybridization(FISH), reverse transcriptase polymerase chain reaction (RT–PCR; or real-time quantitative PCR [RQ-PCR]) [Citation108]. The analysis of PML nuclear staining in leukemic cells using anti-PML monoclonal antibodies can be a surrogate for genetic diagnosis and has been successfully used in the ICAL study as a rapid method diagnosis [Citation106]. Conventional karyotyping is an important diagnostic method but due to its long turnaround time, it is used for confirmation of results obtained by techniques of molecular biology, to identify variant translocations and additional abnormalities. Several additional genetic abnormalities have been shown to affect the outcome of APL patients and our group has proposed an integrative score (ISAPL) based on FLT3-ITD mutational status, ΔNp73/TAp73 expression ratio, ID1, BAALC, ERG and KMT2E gene expression levels [Citation109]. The ISAPL was developed in patients treated with ATRA and chemotherapy and there is data suggestive that the presence of genetic mutations other than PML/RARA inpatients receiving arsenic trioxide (ATO) plus ATRA do not change the outcome.
APL coagulopathy is characterized by consumptive coagulation as well as activation of primary and secondary fibrinolysis due to multiple mechanisms which are the basis for the high incidence of intracerebral and pulmonary hemorrhages reported as the most frequent causes of death, both prior to and shortly after treatment initiation [Citation110,Citation111]. The ELN recommendations for supportive measures to counteract the coagulopathy are the administration of transfusions of fibrinogen and/or cryoprecipitate, platelets, and fresh-frozen plasma immediately upon suspicion of the diagnosis, to maintain the fibrinogen concentration above 100–150 mg/dL, the platelet count above 30 × 109/L to50 × 109/L, and the international normalized ratio (INR)below1.5 [Citation108].
APL treatment
Studies combining ATRA and chemotherapy have reported complete remission rates of 90–95% and rates of long-term survival in >80% of newly diagnosed APL cases [Citation112,Citation113]. The most common protocols used in LA that are based on the use of ATRA, anthracycline, and cytarabine in a risk-stratified therapy following the experience of the PETHEMA group [Citation106,Citation114–116]. The comparison of the PETHEMA/HOVONLPA2005 and the ICAPL2006 studies which used idarubicin and daunorubicin, respectively, showed that the 2 drugs were associated with similar rates of primary resistance, molecular persistence of disease, and molecular and hematological relapse rates [Citation106,Citation114]. In the IC-APL2006, complete hematological remission was achieved in 85% and 15% of patients died during induction. After a median follow-up of 28 months, the 2-year CIR, OS, and DFS were 4.5%, 80%, and 91%, respectively, [Citation106,Citation114]. Lower ATRA doses of 25 mg/m2 in combination with chemotherapy have been studied based on similar pharmacodynamic data to the standard 45 mg/m2 dose achieving similar initial results, this strategy can be useful if resources are limited, although concerns for long-term relapses exist [Citation117–119]. Despite the dramatic progress achieved in frontline therapy of APL with ATRA plus anthracycline-based regimens, relapses still occur in approximately 20% of patients. Moreover, these regimens are associated with significant toxicities due to severe myelosuppression frequently associated with life-threatening infections and potentially serious late effects including the development of secondary myelodysplastic syndrome and AML.
Results from two phases III studies comparing the efficacy and safety of ATRA plus ATO versus ATRA plus chemotherapy have led to ATRA-ATO becoming the new standard of care for treating patients with low/intermediate-risk APL (WBC ≤ 10x 109/l) [Citation108]. However, ATO is not available in most of LA () due to the prohibitive costs and the requirement of intravenous administration in a hospital setting, a challenge in countries with limited infrastructures. There are two most used treatment regimens with ATRA-ATO for patients with low/intermediate-risk APL, based on the APL 0406 trial (GIMEMA-SAL-AMLSG) [Citation120,Citation121] and the AML17 trial (UK NCRI) [Citation122,Citation123] studies. Both use a similar total dose of ATO; however, they have differences in the duration of treatment and the distribution of the dose of ATO during therapy. In the APL 0406, ATO was administered at a lower dose daily, while in the AML17 it was used at a higher dose administered 2 or 3 days per week. APL 0406is a phase 3, randomized, multicenter, non-inferiority study comparing ATRA-chemotherapy versus ATRA-ATO in low-intermediate-risk APL patients. After a median follow-up of 40.6 months, EFS, CIR, and 50-month OS for patients in the ATRA-ATO versus ATRA-chemotherapy arms were 97.3% vs 80%, 1.9% vs 13.9% and 99.2% vs 92.6%, respectively with significant differences [Citation120,Citation121]. ATRA-ATO had less neutropenia and prolonged thrombocytopenia and fewer episodes of febrile neutropenia. Adverse effects of the ATRA-ATO combination consisted mainly of frequent increase in liver enzymes, QTc interval prolongation, and hyperleukocytosis [Citation120,Citation121]. The AML17 trial showed that the combination of ATO-ATRA was highly effective in all risk APL patients with a high cure rate and less relapse than AIDA treatment [Citation122,Citation123]. This study confirms the applicability of this approach in low/intermediate-risk patients and suggested the feasibility of minimizing chemotherapy in high-risk patients, using 1–2 doses of GO [Citation122,Citation123].
For high-risk patients, the use of ATRA-ATO plus chemotherapy has not yet been shown to be superior to conventional treatment with ATRA plus chemotherapy in a randomized trial, although studies with historical control, such as APML4 [Citation124], have shown a benefit in overall survival. A large, randomized trial involving most European cooperative groups (APOLLO trial, NCT02688140) is being conducted in this population (high-risk APL) comparing ATRA + ATO + 2 doses of idarubicin in induction versus ATRA + chemotherapy. Regardless of which first-line treatment is selected, CNS prophylaxis should be restricted to patients with WBC counts≥10 × 109/L at presentation, or to those who have had a CNS hemorrhage. Regarding measurable residual disease (MRD) assessment, the most important MRD endpoint in APL is PCR negativity for PML-RARA at the end of consolidation [Citation108]. For patients with low/intermediate-risk APL, MRD monitoring is only recommended after completion of consolidation and may be discontinued once bone marrow MRD-negativity is achieved. For high-risk APL, MRD is recommended to be assessed by qPCR from BM every 3 months for 24 months, starting at the end of treatment or from peripheral blood every 4–6 weeks during follow-up. However, the availability of MRD assessment at the recommended frequency in LA is quite heterogeneous [Citation125], even in the private system in some countries. Conversion of PML-RARA by PCR from undetectable to detectable, and/or a ≥ 1 log10 increase in high-risk patients with previously stable PML/RARA levels should be regarded as imminent disease relapse in APL and must be confirmed in a second sample [Citation108]. Two independent retrospective studies demonstrated that early intervention of patients with molecular relapse provides a better outcome than treatment in hematologic relapse alone. Salvage therapy for molecular persistence after consolidation, molecular relapse, or hematologic relapse should be chosen considering the first-line treatment used previously and the duration of the first relapse. For patients relapsing after ATRA + chemotherapy is that they should be managed with ATRA + ATO–based approaches and patients relapsing after ATRA + ATO should be managed with ATRA + chemotherapy. A potential exception for crossing over to a different treatment of relapsed patients may be considered for those with late relapse. Patients who reach the second CR should receive intensification with HSCT as discussed. For patients in whom HSCT is not feasible, available options include repeated cycles of ATO with or without ATRA with or without chemotherapy [Citation108].
Future directions
Adapting existing and upcoming therapies for AML in our region should have its own development pathway different to that of HICs and be adapted to our reality. Establishment of collaborative efforts led by Latin American investigators with cooperative groups in HICs can help implement existing technologies and decision-making through clinical networking, exemplified by the experience of the ICAL [Citation126]. Opportunities for studying alternative dosing and scheduling of expensive drugs in clinical trials can increase access and decrease costs while improving outcomes. Similarly, alternative frameworks of care through early discharge and outpatient strategies can decrease the burden of AML on our health systems. Haploidentical transplants should continue to grow in regions where unrelated donors are not feasible. Timely reference, clinician education and advocacy for access to effective therapies through grassroots efforts including all stakeholders are critical to improving our limited treatment arsenal, which should be guided by cost-efficacy analyzes and critical thinking.
Conclusions
AML represents a great challenge to all stakeholders in LA. Disparities in access to technologies, supportive care capacity, and availability of novel agents and HSCT hinder results in our region, reflecting barriers common to other LMICs. Recent developments in the diagnosis and treatment of this disease must be implemented through education, collaborative clinical research, and advocacy to improve outcomes.
Acknowledgements
The authors would like to thank our Latin-American colleagues for their inputs into drug availability in their countries: Argentina: Marcelo Lastrebner, Gregorio Jaimovich; Brazil: André Días-Américo; Bolivia: Rosio Baena; Chile: FransicoBarriga, Patricio Rojas and BlazLesina; Colombia: Amado Karduss and Javier Fonseca, Costa Rica: Tomás Alfaro; Cuba: Calixto Hernández; Guatemala: Fabiola Valvert; Honduras: Jose Angel Sánchez; Panamá: Ninotchka Mendoza; Paraguay: Cristóbal Frutos; Perú: Alfredo Wong; Uruguay: Sebastián Galeano, Sofia Grille and Carolina Oliver; Venezuela: Laura Saavedra; Andres Gómez-De León: honoraria Abbvie, Astellas; Roberta Demichelis-Gomez: honoraria and advisory board Abbvie, Astellas, TEVA, AMGEN, Gilead. David Gómez-Almaguer: Bristol Myers Squibb honoraria and advisory, Abbvie advisory board, honoraria. Novartis: honoraria, advisory board. Abel da Costa Neto: Honoraria AstraZeneca, Pfizer, Janssen, Takeda and Libbs. Advisory Board: AstraZeneca. Eduardo Rego: Honoraria and Advisory Board Abbvie, Pfizer and Astellas. Research grant: Astellas.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Xie Y, Davies SM, Xiang Y, et al. Trends in leukemia incidence and survival in the United States (1973–1998). Cancer. 2003;97(9):2229–2235.
- Sasaki K, Ravandi F, Kadia TM, et al. De novo acute myeloid leukemia: A population-based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results (SEER) database, 1980 to 2017. Cancer. 2021;127(12):2049–2061.
- Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105(11):1684–1692.
- Demichelis-Gomez R, Zapata-Canto N, Leyto-Cruz F, et al. Acute myeloid leukemia in Mexico: The specific challenges of a developing country. results from a multicenter national registry. Clin Lymphoma Myeloma Leuk. 2019;20:e295–e303.
- Gomez-Almaguer D, Marcos-Ramirez ER, Montano-Figueroa EH, et al. Acute leukemia characteristics are different around the world: the Mexican perspective. Clin Lymphoma Myeloma Leuk. 2017;17(1):46–51.
- Capra M, Vilella L, Pereira WV, et al. Estimated number of cases, regional distribution and survival of patients diagnosed with acute myeloid leukemia between 1996 and 2000 in Rio Grande do Sul, Brazil. Leuk Lymphoma. 2007;48(12):2381–2386.
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Ikoma MR, Sandes AF, Thiago LS, et al. First proposed panels on acute leukemia for four-color immunophenotyping by flow cytometry from the Brazilian group of flow cytometry-GBCFLUX. Cytometry B Clin Cytom. 2015;88(3):194–203.
- Dohner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 ELN recommendations from an International Expert Panel. Blood. 2022;140:1345–1377.
- Jaime-Perez JC, Padilla-Medina JR, Fernandez LT, et al. Outcomes of adolescents and young adults with acute myeloid leukemia treated in a Single Latin American Center. Clin Lymphoma Myeloma Leuk. 2018;18(4):286–292.
- Buitron-Santiago NAOL, Rosas López A, Aguayo González A, et al. Acute myeloid leukemia in adults: experience at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán from 2003 to 2008. Rev Invest Clin. 2010;62(2):100–108.
- Alvarado-Ibarra MGALMZV, Ortiz Zepeda M, Espitia Rios E, et al. Frontline treatment of acute myeloid leukemia in adults long-term results in a Mexican medical center. Cancer Therapy & Oncology. 2018;10(5):1–9.
- Ruiz-Delgado GJ, Macias-Gallardo J, Lutz-Presno J, et al. Core binding factor acute myeloid leukemia (CBF-AML) in Mexico: a single institution experience. Rev Invest Clin. 2011;63(1):25–30.
- Vela-Ojeda J, Cardenas PV, Garcia-Ruiz Esparza MA, et al. FLT3-ITD and CD135 over-expression are frequent findings of poor survival in adult patients with acute leukemias. Arch Med Res. 2021;52(2):217–223.
- Cuervo-Sierra J, Jaime-Perez JC, Martinez-Hernandez RA, et al. Prevalence and clinical significance of FLT3 mutation status in acute myeloid leukemia patients: A multicenter study. Arch Med Res. 2016;47(3):172–179.
- Cruz NG, Ribeiro AF, Gloria AB, et al. Characterization of NPM1, FLT3, and IDH1 mutations in adult patients with acute myeloid leukemia: a Brazilian cohort study. Leuk Lymphoma. 2016;57(12):2901–2904.
- Yates JW, Wallace HJ, Jr., Ellison RR, et al. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep. 1973;57(4):485–488.
- Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361(13):1249–1259.
- Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361(13):1235–1248.
- Burnett AK, Russell NH, Hills RK, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood. 2015;125(25):3878–3885.
- Teuffel O, Leibundgut K, Lehrnbecher T, et al. Anthracyclines during induction therapy in acute myeloid leukaemia: a systematic review and meta-analysis. Br J Haematol. 2013;161(2):192–203.
- Kern W, Estey EH. High-dose cytosine arabinoside in the treatment of acute myeloid leukemia: review of three randomized trials. Cancer. 2006;107(1):116–124.
- Lowenberg B, Pabst T, Vellenga E, et al. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011;364(11):1027–1036.
- Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol. 2013;31(27):3360–3368.
- Borthakur G, Kantarjian H, Wang X, et al. Treatment of core-binding-factor in acute myelogenous leukemia with fludarabine, cytarabine, and granulocyte colony-stimulating factor results in improved event-free survival. Cancer. 2008;113(11):3181–3185.
- Othus M, Kantarjian H, Petersdorf S, et al. Declining rates of treatment-related mortality in patients with newly diagnosed AML given ‘intense’ induction regimens: a report from SWOG and MD Anderson. Leukemia. 2014;28(2):289–292.
- Ho G, Jonas BA, Li Q, et al. Early mortality and complications in hospitalized adult californians with acute myeloid leukaemia. Br J Haematol. 2017;177(5):791–799.
- Percival ME, Tao L, Medeiros BC, et al. Improvements in the early death rate among 9380 patients with acute myeloid leukemia after initial therapy: A SEER database analysis. Cancer. 2015;121(12):2004–2012.
- Chauhan P, Gupta A, Gopinathan M, et al. Real-world challenges in the management of acute myeloid leukemia: a single-center experience from north India. Ann Hematol. 2022;101(6):1261–1273.
- Zeidan AM, Podoltsev NA, Wang X, et al. Patterns of care and clinical outcomes with cytarabine-anthracycline induction chemotherapy for AML patients in the United States. Blood Adv. 2020;4(8):1615–1623.
- Mendes FR, da Silva WF, da Costa Bandeira de Melo R, et al. Predictive factors associated with induction-related death in acute myeloid leukemia in a resource-constrained setting. Ann Hematol. 2022;101(1):147–154.
- Greenwood MJ, Seftel MD, Richardson C, et al. Leukocyte count as a predictor of death during remission induction in acute myeloid leukemia. Leuk Lymphoma. 2006;47(7):1245–1252.
- Hug V, Keating M, McCredie K, et al. Clinical course and response to treatment of patients with acute myelogenous leukemia presenting with a high leukocyte count. Cancer. 1983;52(5):773–779.
- Ventura GJ, Hester JP, Smith TL, et al. Acute myeloblastic leukemia with hyperleukocytosis: risk factors for early mortality in induction. Am J Hematol. 1988;27(1):34–37.
- Lester TJ, Johnson JW, Cuttner J. Pulmonary leukostasis as the single worst prognostic factor in patients with acute myelocytic leukemia and hyperleukocytosis. Am J Med. 1985;79(1):43–48.
- Bewersdorf JP, Giri S, Tallman MS, et al. Leukapheresis for the management of hyperleukocytosis in acute myeloid leukemia-A systematic review and meta-analysis. Transfusion. 2020;60(10):2360–2369.
- Kim K, Konopleva M, DiNardo CD, et al. Urgent cytoreduction for newly diagnosed acute myeloid leukemia patients allows acquisition of pretreatment genomic data and enrollment on investigational clinical trials. Am J Hematol. 2022;97:885–894.
- Burki TK. Ongoing drugs shortage in Venezuela and effects on cancer care. Lancet Oncol. 2017;18(5):578.
- Das M. Shortage of cancer drugs in Mexico. Lancet Oncol. 2021;22(9):1216.
- Taplitz RA, Kennedy EB, Bow EJ, et al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J Clin Oncol. 2018;36(30):3043–3054.
- Aguilar-Guisado M, Espigado I, Martin-Pena A, et al. Optimisation of empirical antimicrobial therapy in patients with haematological malignancies and febrile neutropenia (How long study): an open-label, randomised, controlled phase 4 trial. Lancet Haematol. 2017;4(12):e573–ee83.
- Logan C, Koura D, Taplitz R. Updates in infection risk and management in acute leukemia. Hematol Am Soc Hematol Educ Program. 2020;2020(1):135–139.
- Organization PAH. Latin America and the Caribbean country report [June 7th 2022]. Available from: https://www.paho.org/en/topics/blood/transfusion-blood-supply-latin-america-and-caribbean-2020.
- Jaime-Perez JC, Garcia-Salas G, Turrubiates-Hernandez GA, et al. An audit of platelet transfusion indications in acute leukaemia patients: six-year experience at an Academic Centre. Blood Transfus. 2021;19(1):37–44.
- Ciurea SO, Al Malki MM, Kongtim P, et al. The European Society for Blood and Marrow Transplantation (EBMT) consensus recommendations for donor selection in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. 2020;55(1):12–24.
- Bhatt VR, Shostrom V, Giri S, et al. Early mortality and overall survival of acute myeloid leukemia based on facility type. Am J Hematol. 2017;92(8):764–771.
- Halpern AB, Walter RB, Estey EH. Outpatient induction and consolidation care strategies in acute myeloid leukemia. Curr Opin Hematol. 2019;26(2):65–70.
- Gomez-Almaguer D, Gomez-De Leon A, Colunga-Pedraza PR, et al. Outpatient allogeneic hematopoietic stem-cell transplantation: a review. Ther Adv Hematol. 2022;13:20406207221080739.
- Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–464.
- Burchert A, Bug G, Fritz LV, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia With FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol. 2020;38(26):2993–3002.
- Erba H MP, Vrhovac R, Patkowska E, et al. Quizartinib prolonged survival vs. placebo plus intensive induction and consolidation therapy followed by single-agent continuation in patients aged 18-75 years with newly diagnosed FLT3-ITD+ AML. European Hematology Association Congress; Viena. Austria: EHA Library. 2022: S100.
- Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29(4):369–377.
- Castaigne S, Pautas C, Terre C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379(9825):1508–1516.
- Cortes JE, de Lima M, Dombret H, et al. Prevention, recognition, and management of adverse events associated with gemtuzumab ozogamicin use in acute myeloid leukemia. J Hematol Oncol. 2020;13(1):137.
- Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986–996.
- Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121(24):4854–4860.
- Lancet JE, Uy GL, Newell LF, et al. CPX-351 versus 7 + 3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021;8(7):e481–ee91.
- Wei AH, Dohner H, Pocock C, et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526–2537.
- Montesinos P, Recher C, Vives S, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386(16):1519–1531.
- Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379–389.
- DiNardo CD, Schuh AC, Stein EM, et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021;22(11):1597–1608.
- Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331(14):896–903.
- Lowenberg B. Sense and nonsense of high-dose cytarabine for acute myeloid leukemia. Blood. 2013;121(1):26–28.
- Fagundes EM, Rocha V, Gloria AB, et al. De novo acute myeloid leukemia in adults younger than 60 years of age: socioeconomic aspects and treatment results in a Brazilian university center. Leuk Lymphoma. 2006;47(8):1557–1564.
- Ssenyonga N, Stiller C, Nakata K, et al. Worldwide trends in population-based survival for children, adolescents, and young adults diagnosed with leukaemia, by subtype, during 2000-14 (CONCORD-3): analysis of individual data from 258 cancer registries in 61 countries. The Lancet Child & Adolescent Health. 2022;6(6):409–431.
- Jaime-Perez JC, Brito-Ramirez AS, Pinzon-Uresti MA, et al. Characteristics and clinical evolution of patients with acute myeloblastic leukemia in northeast Mexico: an eight-year experience at a university hospital. Acta Haematol. 2014;132(2):144–151.
- Benicio MTL, Ribeiro AFT, Americo AD, et al. Evaluation of the European LeukemiaNet recommendations for predicting outcomes of patients with acute myeloid leukemia treated in low- and middle-income countries (LMIC): A Brazilian experience. Leuk Res. 2017;60:109–114.
- Fagundes EM, Neto NN, Caldas LM, et al. Mortality by COVID-19 in adults with acute myeloid leukemia: a survey with hematologists in Brazil. Ann Hematol. 2022;101(4):923–925.
- Demichelis-Gomez R, Alvarado-Ibarra M, Vasquez-Chavez J, et al. Treating acute leukemia during the COVID-19 pandemic in an environment with limited resources: A multicenter experience in four Latin American countries. JCO Glob Oncol. 2021;7:577–584.
- Thomas X, Elhamri M, Raffoux E, et al. Comparison of high-dose cytarabine and timed-sequential chemotherapy as consolidation for younger adults with AML in first remission: the ALFA-9802 study. Blood. 2011;118(7):1754–1762.
- Miyawaki S, Ohtake S, Fujisawa S, et al. A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 study. Blood. 2011;117(8):2366–2372.
- Oran B, de Lima M, Garcia-Manero G, et al. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv. 2020;4(21):5580–5588.
- Miyamoto T, Sanford D, Tomuleasa C, et al. Real-world treatment patterns and clinical outcomes in patients with AML unfit for first-line intensive chemotherapy. Leuk Lymphoma. 2022;63(4):928–938.
- Sekeres MA, Guyatt G, Abel G, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020;4(15):3528–3549.
- Jaime-Perez JC, Ramos-Davila EM, Picon-Galindo E, et al. Outcomes and survival predictors of Latin American older adults with acute myeloid leukemia: data from a single center. Hematol Transfus Cell Ther. 2022;25:S2531-1379(22)00010-4.
- National Comprehensive Cancer Network I. NCCN Clinical Practice Guidlelines in Oncology. Acute Myeloid Leukemia Version 3.0. 2022.
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137–2145.
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629.
- Leon A G-D, Demichelis-Gomez R, Pinedo-Rodriguez A, et al. Venetoclax-based combinations for acute myeloid leukemia: optimizing their use in Latin-America. Hematology. 2022;27(1):249–257.
- DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135(11):791–803.
- Kanate AS, Majhail NS, Savani BN, et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2020;26(7):1247–1256.
- Silla L, Dulley F, Saboya R, et al. Brazilian guidelines on hematopoietic stem cell transplantation in acute myeloid leukemia. Eur J Haematol. 2017;98(2):177–183.
- Pollyea DA, Winters A, McMahon C, et al. Venetoclax and azacitidine followed by allogeneic transplant results in excellent outcomes and may improve outcomes versus maintenance therapy among newly diagnosed AML patients older than 60. Bone Marrow Transplant. 2022;57(2):160–166.
- Maakaron JE, Zhang MJ, Chen K, et al. Age is no barrier for adults undergoing HCT for AML in CR1: contemporary CIBMTR analysis. Bone Marrow Transplant. 2022;57:911–917.
- Hourigan CS, Dillon LW, Gui G, et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia With genomic evidence of residual disease. J Clin Oncol. 2020;38(12):1273–1283.
- Craddock C, Jackson A, Loke J, et al. Augmented reduced-intensity regimen does Not improve postallogeneic transplant outcomes in acute myeloid leukemia. J Clin Oncol. 2021;39(7):768–778.
- Bonifazi F, Pavoni C, Peccatori J, et al. Myeloablative conditioning with thiotepa-busulfan-fludarabine does not improve the outcome of patients transplanted with active leukemia: final results of the GITMO prospective trial GANDALF-01. Bone Marrow Transplant. 2022;57:949–958.
- Correa C, Gonzalez-Ramella O, Baldomero H, et al. Increasing access to hematopoietic cell transplantation in Latin America: results of the 2018 LABMT activity survey and trends since 2012. Bone Marrow Transplant. 2022;57:881–888.
- Fuchs EJ, O'Donnell PV, Eapen M, et al. Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: the BMT CTN 1101 trial. Blood. 2021;137(3):420–428.
- Kunacheewa C, Ungprasert P, Phikulsod P, et al. Comparative efficacy and clinical outcomes of haploidentical stem cell transplantation to other stem sources for treatment in acute myeloid leukemia and myelodysplastic syndrome patients: A systematic review and meta-analysis. Cell Transplant. 2020;29:963689720904965.
- Wang Y, Liu QF, Xu LP, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125(25):3956–3962.
- Vellenga E, van Putten W, Ossenkoppele GJ, et al. Autologous peripheral blood stem cell transplantation for acute myeloid leukemia. Blood. 2011;118(23):6037–6042.
- Ferrara F, Picardi A. Is there still a role for autologous stem cell transplantation for the treatment of acute myeloid leukemia? Cancers (Basel. 2019;12(1).
- Venditti A, Piciocchi A, Candoni A, et al. GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood. 2019;134(12):935–945.
- Sanz J, Labopin M, Sanz MA, et al. Hematopoietic stem cell transplantation for adults with relapsed acute promyelocytic leukemia in second complete remission. Bone Marrow Transplant. 2021;56(6):1272–1280.
- de Botton S, Fawaz A, Chevret S, et al. Autologous and allogeneic stem-cell transplantation as salvage treatment of acute promyelocytic leukemia initially treated with all-trans-retinoic acid: a retrospective analysis of the European acute promyelocytic leukemia group. J Clin Oncol. 2005;23(1):120–126.
- Holter Chakrabarty JL, Rubinger M, Le-Rademacher J, et al. Autologous is superior to allogeneic hematopoietic cell transplantation for acute promyelocytic leukemia in second complete remission. Biol Blood Marrow Transplant. 2014;20(7):1021–1025.
- Cortes JE, Khaled S, Martinelli G, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):984–997.
- Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728–1740.
- Antar AI, Otrock ZK, Jabbour E, et al. FLT3 inhibitors in acute myeloid leukemia: ten frequently asked questions. Leukemia. 2020;34(3):682–696.
- DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386–2398.
- Stein EM. Enasidenib, a targeted inhibitor of mutant IDH2 proteins for treatment of relapsed or refractory acute myeloid leukemia. Future Oncol. 2018;14(1):23–40.
- Douer D, Santillana S, Ramezani L, et al. Acute promyelocytic leukaemia in patients originating in Latin America is associated with an increased frequency of the bcr1 subtype of the PML/RARalpha fusion gene. Br J Haematol. 2003;122(4):563–570.
- Ruiz-Arguelles GJ, Garces-Eisele J, Reyes-Nunez V, et al. More on geographic hematology: the breakpoint cluster regions of the PML/RARalpha fusion gene in Mexican mestizo patients with promyelocytic leukemia are different from those in Caucasians. Leuk Lymphoma. 2004;45(7):1365–1368.
- Rohr SS, Pelloso LA, Borgo A, et al. Acute promyelocytic leukemia associated with the PLZF-RARA fusion gene: two additional cases with clinical and laboratorial peculiar presentations. Med Oncol. 2012;29(4):2345–2347.
- Rego EM, Kim HT, Ruiz-Arguelles GJ, et al. Improving acute promyelocytic leukemia (APL) outcome in developing countries through networking, results of the international consortium on APL. Blood. 2013;121(11):1935–1943.
- Silva WFD, Jr., Rosa LID, Marquez GL, et al. Real-life outcomes on acute promyelocytic leukemia in Brazil - early deaths are still a problem. Clin Lymphoma Myeloma Leuk. 2019;19(2):e116–ee22.
- Sanz MA, Fenaux P, Tallman MS, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood. 2019;133(15):1630–1643.
- Lucena-Araujo AR, Coelho-Silva JL, Pereira-Martins DA, et al. Combining gene mutation with gene expression analysis improves outcome prediction in acute promyelocytic leukemia. Blood. 2019;134(12):951–959.
- Kwaan HC, Weiss I, Tallman MS. The role of abnormal hemostasis and fibrinolysis in morbidity and mortality of acute promyelocytic leukemia. Semin Thromb Hemost. 2019;45(6):612–621.
- Menell JS, Cesarman GM, Jacovina AT, et al. Annexin II and bleeding in acute promyelocytic leukemia. N Engl J Med. 1999;340(13):994–1004.
- Ades L, Guerci A, Raffoux E, et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood. 2010;115(9):1690–1696.
- Martinez-Cuadron D, Montesinos P, Vellenga E, et al. Long-term outcome of older patients with newly diagnosed de novo acute promyelocytic leukemia treated with ATRA plus anthracycline-based therapy. Leukemia. 2018;32(1):21–29.
- Sanz MA, Montesinos P, Rayon C, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115(25):5137–5146.
- Zapata-Canto N, Aguilar M, Arana L, et al. Acute promyelocytic leukemia: A long-term retrospective study in Mexico. J Hematol. 2021;10(2):53–63.
- Crespo-Solis E, Contreras-Cisneros J, Demichelis-Gomez R, et al. Survival and treatment response in adults with acute promyelocytic leukemia treated with a modified International Consortium on Acute Promyelocytic Leukemia protocol. Rev Bras Hematol Hemoter. 2016;38(4):285–290.
- Jaime-Perez JC, Gonzalez-Leal XJ, Pinzon-Uresti MA, et al. Is there still a role for low-dose all-transretinoic acid in the treatment of acute promyelocytic leukemia in the arsenic trioxide era? Clin Lymphoma Myeloma Leuk. 2015;15(12):816–819.
- Castaigne S, Lefebvre P, Chomienne C, et al. Effectiveness and pharmacokinetics of low-dose all-trans retinoic acid (25 mg/m2) in acute promyelocytic leukemia. Blood. 1993;82(12):3560–3563.
- Lou Y, Qian W, Meng H, et al. Long-term efficacy of low-dose all-trans retinoic acid plus minimal chemotherapy induction followed by the addition of intravenous arsenic trioxide post-remission therapy in newly diagnosed acute promyelocytic leukaemia. Hematol Oncol. 2014;32(1):40–46.
- Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–121.
- Platzbecker U, Avvisati G, Cicconi L, et al. Improved outcomes With retinoic acid and arsenic trioxide compared With retinoic acid and chemotherapy in Non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. J Clin Oncol. 2017;35(6):605–612.
- Burnett AK, Russell NH, Hills RK, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(13):1295–1305.
- Russell N, Burnett A, Hills R, et al. Attenuated arsenic trioxide plus ATRA therapy for newly diagnosed and relapsed APL: long-term follow-up of the AML17 trial. Blood. 2018;132(13):1452–1454.
- Iland HJ, Bradstock K, Supple SG, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012;120(8):1570–1580; quiz 752.
- Lange AP, Lima AS, Lucena-Araujo AR, et al. The experience of the International Consortium on Acute Promyelocytic Leukemia in monitoring minimal residual disease in acute promyelocytic leukaemia. Br J Haematol. 2018;180(6):915–918.
- Correa de Araujo Koury L, Ganser A, Berliner N, et al. Treating acute promyelocytic leukaemia in Latin America: lessons from the International Consortium on Acute Leukaemia experience. Br J Haematol. 2017;177(6):979–983.
