ABSTRACT
Background
Prognostic nutritional index has been found to be related to the clinical outcomes of patients with cancer. However, its role in myelodysplastic syndromes (MDS) patients is unclear. We aimed to assess the value of nutritional status in predicting the prognosis of MDS patients.
Methods
Totally 121 MDS patients were analyzed retrospectively. The prognostic nutritional index (PNI) was used to assess nutritional status of the patients. The bio-informatics tool X-tile was used to define the threshold, and accordingly patients were divided into PNIlow and PNIhigh groups, the characteristics were compared between two groups.
Results
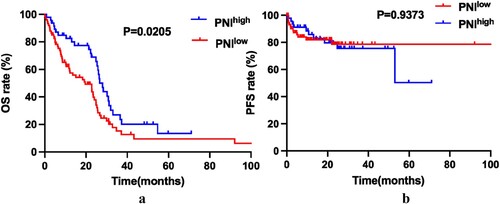
The PNIhigh was associated with better OS (Overall Survival) than PNIlow in MDS patients (Median OS, 28.03 months versus 19.63 months, P = 0.0205). But there were no statistical differences in PFS (Progression-Free-Survival) between the two groups (P = 0.9373). The univariable and multivariable COX proportional hazard analysis adjusted for age, gender, platelet count, HB level and IPSS-R scores, and the results showed that PNI is a useful index in the evaluation of the OS of MDS (HR 0.588, 95%CI 0.374–0.926, P = 0.024).
Conclusion
PNI would be a simple and immediately available tool for predicting the prognosis of MDS.
1. Introduction
Myelodysplastic syndrome (MDS) is a very heterogeneous group of clonal myeloid neoplasms characterized by ineffective hematopoiesis and the risk of progressing to acute myeloid leukemia (AML) [Citation1,Citation2]. Even with the rapid evolution of new active therapies for MDS in recent decades, only a small proportion of patients benefited from these strategies, the overall outcome was very poor [Citation3,Citation4]. Treatment of MDS is risk-adapted according to the therapeutic goals and the precise risk stratification. The International Prognostic Scoring System (IPSS) [Citation5] and Revised IPSS (IPSS-R) [Citation6] are the most commonly used prognostic models and have stratified prognosis of patients with MDS, but these systems have some limitations. Factor such as the role of the patient’s nutritional status is not included.
Nutritional status has been found to be related to the clinical outcomes of cancer patients [Citation7,Citation8]. Regarding hematologic malignancies, recent studies have suggested that nutritional status is a potential parameter affecting the prognosis of acute leukemia, diffuse large B-cell lymphoma, multiple myeloma [Citation9–12]. However, the role of nutritional status on the prognosis of MDS is unclear.
The prognostic nutritional index (PNI) is based on two parameters, the concentration of serum albumin and the total lymphocytes, and computed with the following formula: 10 × serum albumin (g/dl) + 0.005 × total lymphocyte count (per mm3) in peripheral blood. Although it was initially aimed at predicting immunogenic status and risks before gastrointestinal surgery, it has been found in recent years that it has a very close relationship with the prognosis of many cancers such as esophageal cancer, lung cancer, gastric cancer [Citation13–16]. However, the role of PNI in MDS is still unclear.
In this study, we retrospectively evaluated the relationship between baseline PNI scores and clinical outcomes of MDS patients.
2. Materials and methods
2.1. Study design and patient selection
Totally 121 MDS patients who were diagnosed between March 2010 and January 2021 in the Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University were enrolled. This study was approved by the Institutional Review Board of the Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University and carried out in accordance with the Helsinki Declaration. All the patients were anonymous. Informed consent was waived because of the retrospective design of the data collection.
The inclusion criteria: (a) Diagnosed with MDS according to 2008 and 2016 World Health Organization (WHO) definitions; (b) Complete blood samples were approved for experimental analysis; c) Detailed clinical data were available.
The exclusion criteria: (a) Age < 18 years; (b) Incomplete patient information.
2.2. Nutrition assessing scores over time
The PNI was calculated based on the ALB and ALC and computed with the following formula: 10 × serum albumin (g/dl) + 0.005 × total lymphocyte count (per mm3) in peripheral blood. Whole-blood samples collected into tubes were used for measurement. The bio-informatics tool X-tile was used to analyze the nutritional score threshold, and accordingly subjects were classified as PNI low or PNI high cohorts.
2.3. Statistical analyses
All analyses were performed with the Statistical Package (SPSS 26.0 Inc., Chicago, Illinois) and GraphPad Prism 6 (GraphPad Software, CA, USA). Differences of categorical variables between groups were made by Mann–Whitney U-test or Chi-Squared test. OS/PFS were estimated by adopting Kaplan–Meier method and survival curves were compared by the log-rank test. The X-tile (Version 3.6.1, Yale University, New Haven, CT, USA) software was conducted to evaluate the optimal cutoff PNI scores. PFS, primary endpoint, was defined as the duration from the first treatment to progression of MDS, death of any cause, or the end of clinical follow-up. OS, secondary endpoint, was defined as the duration from the first treatment to all-cause death or the end of follow-up. Hazard ratio (HR) and 95% confidence interval (CI) were calculated. The significant variables with P < 0.1 defined in univariate survival analyses (by log-rank test) were included for the multivariable analyses to validate the prognostic value of PNI. P-value less than 0.05 (2-tailed) indicated the statistical significance.
3. Results
3.1. The cutoff points of PNI
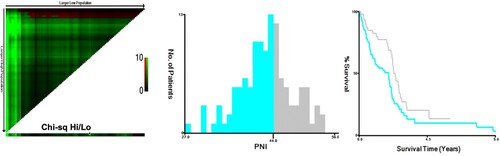
X-tile analyses of overall survival were performed using patients’ data to determine the optimal cutoff values for PNI. Patients’ information of OS was input to X-tile software with the number of PNI, and the optimal cutoff points for the PNI were analyzed by the computer program. The optimal cutoff values highlighted by the black circles in left panels are shown in histograms of the entire cohort (middle panels), and Kaplan–Meier plots are displayed in right panels (). The optimal cutoff value for PNI was 44, which divided the patients into two groups: ‘PNI <44’ group and ‘PNI ≥44’ group.
3.2. Patient characteristics
Totally 121 patients with newly diagnosed MDS were included in the analysis. Median follow-up time was 48.27 months (range, 21.50–59.83 months). Patients’ characteristics were summarized in . The median age was 65(59–72) years and 83(68.5%) were male. Subjects were classified as PNIlow (PNI < 44; N = 74) and PNIhigh (PNI ≥44; N = 47) cohorts. The distribution of characteristics such as WHO subtype, Bone marrow blast count, WBC count, ANC count, PLT count, IPSS-R subtypes and type of therapy were not significantly different between the two groups. There are statistical differences between age, gender and Hb level between groups.
Table 1. Characteristics of 121 subjects with MDS.
3.3. Association between PNI scores and clinical outcomes
Kaplan–Meier survival curves were performed to compare the prognosis between PNIlow and PNIhigh groups by Log rank test. The data showed that PNIhigh is associated with better OS compared to PNIlow patients (median OS, 28.03 months versus 19.63 months, P = 0.0205. (a)). Although there is a similar tendency in PFS between the two groups, but with no statistically significant difference (P = 0.9373. (b)). To determine whether the different risk groups (defined by IPSS-R risk group) will affect the nutrition score on the prognosis of MDS, subgroup analysis was done in lower risk groups (including very low risk and low risk), moderate risk group (including INT) and higher risk groups (including high and very high risk). Patients with lower risk were considered to have better prognosis. The results showed that the effect of PNI on the outcome is mainly on those moderate-risk patients (Figures S1–S3).
3.4. Univariable and multivariable analysis for OS
Univariable analyses were performed to investigate the prognostic factors affecting disease progression and death (). In univariable analyses, PNI (HR 0.588, 95%CI 0.374–0.926, P = 0.022), PLT count (HR 0.997, 95%CI 0.994–1.000, P = 0.028), and IPSS-R scores (HR 1.339, 95%CI 1.188–1.508, P < 0.001) were associated with OS. In the multivariable analysis, we grouped patients according to Hb level, PLT count, IPSS-R scores, and calculated the HRs of the PNIhigh and PNIlow groups. The results showed that in most subgroups, a low PNI could be an independent risk factor for a poor prognosis (HR 0.024, 95%CI 0.358–0.931, P = 0.024).
Table 2. Univariate and multivariate analysis for OS.
4. Discussion
PNI was an effective tool to evaluate the nutritional conditions and predict the prognosis of some cancer [Citation17]. Previous studies showed that nutritional status at disease diagnosis was associated with the prognosis of patients with some hematologic malignancies. However, whether nutritional status has impact on the outcome of MDS is exclusive.
In this study, we examined the relationship between PNI and clinical outcome in 121 MDS patients. We found that PNI-low was associated with shorter OS, suggesting that the PNI could be used to identify patients who are likely to experience an unfavorable clinical outcome. There is seemingly similar tendency in Kaplan–Meier curves of PFS, however, the difference was not significant, maybe due to the small sample sizes and bias in the study.
Univariable and multivariable analyses were also performed to investigate the prognostic factors affecting disease progression and death. Results indicated that PNI is a prognostic item for MDS patients. Although the underline mechanism of action and how low PNI indicate true undernutrition or cachexia are still unclear, it becomes a consensus that PNI is a useful tool, which can be quickly and accurately detected in peripheral blood test at diagnosis. Moreover, the baseline Hb level seemingly has a prognostic impact in MDS. While various studies have evaluated the prognostic significance of Hb level in cancers, such as cervical cancer [Citation18], chronic myeloid leukemia [Citation19], lung cancer [Citation20] and colorectal cancer [Citation21].
Studies have shown that the nutritional status has prognostic value in different pathological types of hematological malignancies. Mozas et al. analyzed clinical characteristics and outcomes according to the PNI of 351 grading 1–3 A follicular lymphoma (FL) patients, and found that the PNI can identify a small subset of FL patients with shorter survival, and it could be an aid to improve the nutritional status of patients prior to treatment initiation [Citation22]. An analysis of total 990 DLBCL patients was performed to examine factors associated with survival. The results showed that PNI can accurately stratify the prognosis of DLBCL [Citation11]. Recent analyses in myeloid diseases have come to similar conclusions. All the findings above were consistent with the results of this study, which showed that MDS patients with low PNI scores had a worse prognosis.
The reasons why nutritional status influences prognosis of cancer is poorly understood. There are some possible explanations for it. Substantial studies have shown that malnutrition in cancer is associated with poor prognosis, poor Quality of Life, lower activity level, increased treatment-related side effects and toxicity, and reduced tumor response to therapy [Citation23,Citation24]. The PNI is a component indicator. Parameters of them could explain the mechanism by which the two indicators predict the survival of patients with cancer. ALB, as a component of systemic inflammation, is correlated with cancer-related inflammation and tumor progression. The production of ALB could be affected by hepatocytes due to the inflammatory cytokines released by the tumor cells [Citation25]. Lymphocytes play an important role in anti-tumor immunity, and they were regarded as biomarkers to predict survival in several hematologic malignancies [Citation26,Citation27].
There are some limitations in this study. First, as in most retrospective analyses, selection bias in collection cannot be completely avoided. Second, we could not find the significant correlation between the type of therapy and the survival, which may be because of substandard treatment and small sample size. Third, we ignored the influence of disease state and race on nutritional status, because the presented data were all collected at the time of initial diagnosis and at a single center.
5. Conclusions
In conclusion, to the best of our knowledge, this is the first study to comprehensively assess the relationship between PNI and prognosis of MDS patients.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived because of the retrospective design of the data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Karantanos T, DeZern AE. Biology and clinical management of hypoplastic MDS: MDS as a bone marrow failure syndrome. Best Pract Res Clin Haematol. 2021;34(2):101280.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405.
- Stubbins RJ, Karsan A. Differentiation therapy for myeloid malignancies: beyond cytotoxicity. Blood Cancer J. 2021;11(12):193.
- Xu K, Hansen E. Novel agents for myelodysplastic syndromes. J Oncol Pharm Pract. 2021;27(8):1982–1992.
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088.
- Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–2465.
- Sun W, Li G, Zhang J, et al. The role of nutritional assessment for predicting radiotherapy-induced adverse events in patients with gastric cancer. Br J Radiol. 2021;95:20201004.
- Xue W, Zhang Y, Wang H, et al. Multicenter study of controlling nutritional status (CONUT) score as a prognostic factor in patients with HIV-related renal cell carcinoma. Front Immunol. 2021;12:778746.
- Sonowal R, Gupta V. Nutritional status in children with acute lymphoblastic leukemia, and its correlation with severe infection. Indian J Cancer. 2021;58(2):190–194.
- Senjo H, Onozawa M, Hidaka D, et al. A novel nutritional index ‘simplified CONUT’ and the disease risk index independently stratify prognosis of elderly patients with acute myeloid leukemia. Sci Rep. 2020;10(1):19400.
- Shen Z, Wang F, He C, et al. The value of prognostic nutritional index (PNI) on newly diagnosed diffuse large B-cell lymphoma patients: a multicenter retrospective study of HHLWG based on propensity score matched analysis. J Inflamm Res. 2021;14:5513–5522.
- Zhou X, Lu Y, Xia J, et al. Association between baseline Controlling Nutritional Status score and clinical outcomes of patients with multiple myeloma. Cancer Biomark. 2021;32(1):65–71.
- Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38–45.
- Okadome K, Baba Y, Yagi T, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271(4):693–700.
- Wang Z, Wang Y, Zhang X, et al. Pretreatment prognostic nutritional index as a prognostic factor in lung cancer: review and meta-analysis. Clin Chim Acta. 2018;486:303–310.
- Abe A, Kurita K, Hayashi H, et al. Correlation between prognostic nutritional index and occlusal status in gastric cancer. Oral Dis. 2020;26(2):465–472.
- Yang L, Yu W, Pan W, et al. A clinical epidemiological analysis of prognostic nutritional index associated with diabetic retinopathy. Diabetes Metab Syndr Obes. 2021;14:839–846.
- Barkati M, Fortin I, Mileshkin L, et al. Hemoglobin level in cervical cancer: a surrogate for an infiltrative phenotype. Int J Gynecol Cancer. 2013;23(4):724–729.
- Liu Z, Shi Y, Yan Z, et al. Impact of anemia on the outcomes of chronic phase chronic myeloid leukemia in TKI era. Hematology. 2020;25(1):181–185.
- Pirker R, Wiesenberger K, Pohl G, et al. Anemia in lung cancer: clinical impact and management. Clin Lung Cancer. 2003;5(2):90–97.
- Kwon YH, Lim HK, Kim MJ, et al. Impacts of anemia and transfusion on oncologic outcomes in patients undergoing surgery for colorectal cancer. Int J Colorectal Dis. 2020;35(7):1311–1320.
- Mozas P, Rivero A, Rivas-Delgado A, et al. The Prognostic Nutritional Index (PNI) is an independent predictor of overall survival in older patients with follicular lymphoma. Leuk Lymphoma. 2021;63(4):903–910.
- Yang F, Li L, Mi Y, et al. Effectiveness of an early, quantified, modified oral feeding protocol on nutritional status and quality of life of patients after minimally invasive esophagectomy: a retrospective controlled study. Nutrition. 2021;94:111540.
- Lere-Chevaleyre A, Bernadach M, Lambert C, et al. Toxicity of induction chemotherapy in head and neck cancer: the central role of skeletal muscle mass. Head Neck. 2021;44(3):681–690.
- Oh JS, Park DJ, Byeon KH, et al. Decrease of preoperative serum albumin-to-globulin ratio as a prognostic indicator after radical cystectomy in patients with urothelial bladder cancer. Urol J. 2021;18(1):66–73.
- Pepedil-Tanrikulu F, Buyukkurt N, Korur A, et al. Significance of lymphocyte count, monocyte count, and lymphocyte-to-monocyte ratio in predicting molecular response in patients with chronic myeloid leukemia: a single-centre experience. Clin Lab. 2020;66(3):190628.
- Cheng Y, Luo Z, Yang S, et al. The ratio of absolute lymphocyte count at interim of therapy to absolute lymphocyte count at diagnosis predicts survival in childhood B-lineage acute lymphoblastic leukemia. Leuk Res. 2015;39(2):144–150.