ABSTRACT
Objective
To evaluate the prognostic value of t(4; 14) translocation for newly diagnosed multiple myeloma (MM) patients in the novel agent era.
Methods
We retrospectively analyzed 606 newly diagnosed MM patients treated with novel agents. The propensity score matching technique was used to reduce the bias between groups.
Results
Among 606 patients, t(4; 14) was observed in 108 (17.8%) patients, among which 79 (73.1%) were accompanied by 1q21 gain and/or del 17p. Median overall survival (OS) (56.2 vs. 87.3 months) and progression-free survival (PFS) (25.7 vs. 37.6 months) were significantly shorter in patients with t(4;14) compared with patients without cytogenetic abnormalities. Univariate Cox proportional hazards regression analysis showed that the t(4;14) was not associated with shorter OS (p = 0.666) and PFS (p = 0.164). The multivariable analysis also showed t(4;14) was not a poor prognostic factor for OS and PFS of patients with newly diagnosed MM (p > 0.05). After balancing the distribution of factors between patients with and without t(4;14) by the propensity score matching technique, patients with t(4;14) had similar OS (57.6 vs. 56.5 months, p = 0.964) and PFS (26.5 vs. 28.1 months, p = 0.740) with the patients without t(4;14).
Conclusions
These results demonstrated that t(4; 14) alone may be not a poor prognostic factor patients with newly diagnosed MM in the novel agent era.
Introduction
Multiple myeloma (MM) is a malignant hematological disease originating from monoclonal plasma cells, mainly manifested as hypercalcemia, renal insufficiency, anemia and bone lesions [Citation1,Citation2]. MM is a highly heterogeneous disease with significant discrepancies in survival, ranging from a few months to more than ten years. So it is very important to clarify the prognostic factors of MM patients and design personalized treatment to improve the outcome of patients. Cytogenetic abnormalities represent the biological characteristics of myeloma cells and play an important role in the prognosis of multiple myeloma patients. Fluorescence in situ hybridization (FISH) is a common method to detect cytogenetic abnormalities in MM patients. IGH gene locates at 14q32 and several cytogenetic abnormalities based on 14q32 translocations are identified which have special prognostic significance for MM patients. These translocations mainly include t(4;14)(p16;q32), t(14;16)(q32;q23), t(11;14)(q13;q32), and t(14;20)(q32;q12). In prior reports, t(4;14) translocation could be detected by FISH in 10–30% patients with newly diagnosed MM [Citation3–6]. Several studies found that the t(4;14) was a poor prognostic factor for the outcome of MM patients [Citation7–16]. However, some studies suggested that t(4;14) was not associated with the prognosis of MM patients [Citation3,Citation5,Citation17–19]. At present, the prognostic value of t(4; 14) in newly diagnosed MM patients is still controversial.
To evaluate the prognostic value of t(4;14) in newly diagnosed MM patients in the novel agent era, we conducted this retrospective analysis of 606 newly diagnosed MM patients in Beijing Chaoyang Hospital, Capital Medical University and found that t(4; 14) alone was not significantly associated with overall survival (OS) and progression-free survival (PFS) of patients with newly diagnosed MM in the era of novel agents.
Methods
Patients
We screened newly diagnosed MM patients who received initial treatment in Beijing Chaoyang Hospital, Capital Medical University from 1 January 2010 to 1 January 2022. A total of 606 patients had available FISH results before MM treatment and received novel agent therapy and thus were included in this study. We recorded the baseline data and conducted regular follow up with the electronic medical record system. All patients were diagnosed according to the MM criteria of the International Myeloma Working Group (IMWG) [Citation2] and followed up until 1 June 2022. FISH was used to detect cytogenetic abnormalities in myeloma cells prior to initial treatment. Before the detection of FISH, bone marrow plasma cells of patients were purified by anti-CD138 + magnetic beads (Miltenyi technology, Bergisch Gladbach, Germany). Then DNA probes were used to analyze aberrations in chromosomal regions 1q21, 17p13, 14q32(IGH),16q23(MAF), 4p16.3(FGFR3), 11q13(CCND1). A total of 200 interphase nuclei were analyzed. The technical thresholds of cytogenetic abnormalities for 1q21 gain, del17p13, t(14;16), t(4;14), and t(11;14) were set as 20%, 20%, 10%, 10% and 10%, respectively. The baseline data were collected including age, sex, hemoglobin (HGB), serum creatinine (SCr), corrected serum calcium (CsCa), lactate dehydrogenase (LDH) and types of MM. Approval for this study was obtained from the medical ethics committee at Beijing Chaoyang Hospital.
Response and outcome measures
All patients received induction regimens containing at least one novel agent (bortezomib, thalidomide, lenalidomide), including bortezomib based regimens, immunomodulatory (IMIDs) based regimens or bortezomib combined with IMIDs regimens. Responses of patients receiving autologous stem cell transplant (ASCT) were evaluated 3 months after ASCT, and maintenance therapy was performed in the non-progressive patients. When the efficacy reached a plateau, the non-ASCT patients received maintenance therapy. Bortezomib combined with lenalidomide was recommended for high-risk patients. Most patients received lenalidomide or thalidomide monotherapy, while a small number received bortezomib or ixazomib monotherapy. All patients received maintenance therapy until intolerance or disease progression. Patient responses were evaluated according to the IMWG criteria [Citation20], including strict complete response (sCR), complete response (CR), very good partial response (VGPR), partial response (PR), stable disease (SD), and progressive disease (PD). The primary outcomes were progression-free survival (PFS) and overall survival (OS). PFS was defined as from the time of diagnosis to disease progression or death, and OS was defined as from the time of diagnosis to death from any cause or last follow-up date. Patients who cannot be followed up were censored at the last follow-up date.
Statistical analysis
SPSS 23.0 software was used for statistical analysis. The Chi-square test was used to test categorical variables. Kaplan-Meier method was used for survival analysis, and the log-rank test was used to compare the differences between groups. The Cox proportional hazards regression analysis was used to assess prognostic factors and results were reported as hazard ratios (HRs) with 95% confidence intervals (95% CIs). In order to balance the distribution of factors between patients with and without t(4;14), the propensity score matching technique was used to match to reduce the deviation between the two groups. Patients with and without t(4;14) were matched at 1:1 with a caliper value of 0.05. A p value <0.05 was considered statistically significant.
Results
Patient characteristics
A total of 606 patients with newly diagnosed MM were enrolled. Clinical characteristics of patients were summarized in . Cytogenetic abnormalities were found in 448 (73.9%) of 606 patients, of which 312 (51.5%) with 1q21 gain, 59 (9.7%) with del 17p, 22 (3.6%) with t(14; 16), 108 (17.8%) with t(4; 14) and 145 (23.9%) with t(11; 14) (). Among 606 patients, 172 (28.4%) had at least 2 cytogenetic abnormalities. The median age was 61 (30–87) years, and the male-to-female ratio was 1.24 (336/270). Of all 606 patients, IgG-type (49.2%) myeloma was the most common type, and 306 patients (50.5%) were at stage III of the International Scoring System (ISS). All patients were treated with novel agent combination induction therapy, including 319 patients (52.6%) who received bortezomib based combination therapy, 49 patients (8.1%) who received immunomodulatory (IMiD) based combination therapy, and 238 patients (39.3%) who received bortezomib and IMiD based combination therapy. After induction therapy, 180 (29.7%) patients received ASCT. Patients were divided into three groups according to cytogenetic abnormalities for further analysis: patients with no cytogenetic abnormalities, patients with t (4:14), and patients with other cytogenetic abnormalities. As shown in , there were statistically significant differences among three groups in sex, monoclonal protein type, hemoglobin, 1q21 gain, del 17p, t(14;16) and t(11;14).
Figure 1. Incidence of cytogenetic abnormalities detected by FISH in new diagnosed multiple myeloma patients. Cytogenetic abnormalities were detected in 448 (73.9%) of 606 patients, including 312 (51.5%) 1q21 gain, 59 (9.7%) del 17p, 22 (3.6%) t (14; 16),108 (17.8%) t(4; 14) and 145 (23.9%) t(11; 14).172 (28.4%) patients had at least 2 cytogenetic abnormalities.
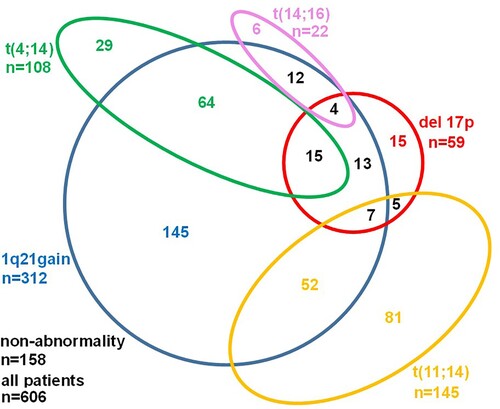
Table 1. Baseline clinical and biological characteristics of MM patients.
Multivariate analysis for survival
Univariate analysis found nine factors associated with OS and they were age >65 years old, HGB ≥ 100 g/L, LDH > 250 U/L, SCr >2 mg/dL, CsCa >2.75 mmol/L, 1q21 gain, del (17p13), ASCT and ISS stage. Multivariate analysis was performed for t(14; 16), t(4; 14), t(11; 14) and these nine covariates. Results showed that t(4; 14) was not an independent prognostic factor for OS (HR = 1.077, 95%CI: 0.769–1.507, p = 0.666) and PFS (HR = 1.209, 95%CI: 0.925–1.581, p = 0.164) of patients with newly diagnosed MM ( and ). The multivariate analysis also confirmed that t(4:14) was not associated with poor survival of patients with newly diagnosed MM treated with novel agents (p > 0.05, and ).
Table 2. Cox analysis (univariate and multivariate) of prognostic factors for OS.
Table 3. Cox analysis (univariate and multivariate) of prognostic factors for PFS.
Matched pairs of patients
The translocation t(4; 14) was observed in 108 (17.8%) patients, among which 79 (73.1%) were accompanied by 1q21 gain or del 17p. In order to balance the distribution of factors between patients with and without t(4;14), the propensity score matching technique was used to match to reduce the deviation between the two groups. Among 606 patients, patients with and without t(4;14) were matched for age, MM subtype, ISS stage, HGB, SCr, CsCa, LDH, 1q21 gain, del(17p13), t(14; 16), t(11; 14) and ASCT. A total of 208 patients were identified by propensity score matching technique, with 104 patients in each group. It was shown that there was no significantly difference in matched patients with and without t(4;14) with respect to these characteristics ().
Table 4. Baseline characteristics between matched patients with and without t(4;14).
Response analysis
All patients were monitored for the best response after ASCT and consolidation therapy. Among the 266 patients including t(4;14) and non-abnormality, 242(91.0%) patients achieved at least PR, of which 93 (35.0%) achieved sCR, 28 (10.5%) achieved CR, 64 (24.1%) achieved VGPR, and 57 (21.4%) achieved PR. Patients with t(4;14) had similar response rates to the patients without cytogenetic abnormalities (p = 0.897, ). Among the 208 matched patients with and without t(4;14), 188 (90.4%) patients achieved at least PR. Sixty-four patients (30.8%) achieved sCR, 26 (12.5%) CR, 50 (24.0%) VGPR, and 48 (23.1%) PR. Patients with and without t(4;14) had similar response rates (p = 0.721, ).
Table 5. Best response rate of MM patients.
Survival analysis
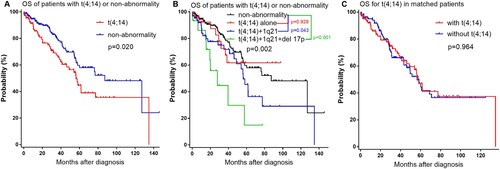
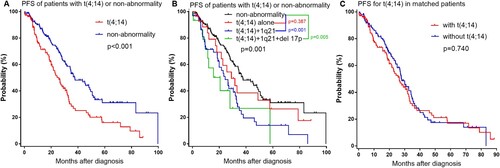
The median follow-up time for all patients was 28.5 (range 0.3–145.9) months. Among the 266 patients including t(4;14) and non-abnormality, patients with t(4;14) had shorter median OS estimated by the Kaplan-Meier method (56.2 months, 95% CI: 46.2–66.2) compared with patients without cytogenetic abnormality (87.3 months, 95% CI: 64.6–110.0) (p = 0.020, (a)). Among 108 patients with t(4;14), 29 (26.9%) had t(4;14) alone, 64 (59.3%) had t(4;14) with 1q21 gain, 15 (13.9%) had both t(4;14) with 1q21 gain and del 17p (). The OS of patients who had t(4;14) with 1q21 gain was shorter than those without cytogenetic abnormalities (56.2 vs.87.3 months, p = 0.043); and the OS of patients who had t(4;14) with 1q21 gain and del 17p was also shorter than non-abnormality patients (27.8 vs.87.3 months, p < 0.001) ((b)). However, patients with t(4;14) alone had similar OS with non-abnormality patients (not reached vs.87.3 months, p = 0.929). In matched patients, patients with t(4;14) also had similar OS with patients without t(4;14) (56.5 vs.57.6 months, p = 0.964) ((c)). Among the 266 patients including t(4;14) and non-abnormality, patients with t(4;14) had shorter PFS compared with patients without cytogenetic abnormality (25.7 vs.37.6 months, p < 0.001) ((a)). The PFS of patients who had t(4;14) with 1q21 gain was shorter than the patients without cytogenetic abnormality (24.0 vs.37.6 months, p < 0.001); and the PFS of patients who had t(4;14) with 1q21 gain and del 17p was also shorter than non-abnormality patients (20.7 vs.37.6 months, p = 0.005) ((b)). However, patients with t(4;14) alone had similar PFS with non-abnormality patients (29.1 vs.37.6 months, p = 0.387). In matched patients, patients with t(4;14) also had similar PFS with patients without t(4;14) (26.5 vs.28.1 months, p = 0.740) ((c)).
Discussion
In this study, we evaluated the prognostic impact of t(4;14) on the outcome of newly diagnosed MM patients. We found that t(4;14) alone had no prognostic value for newly diagnosed MM patients in the novel agent era.
MM is characterized by recurrent chromosomal translocations involving the immunoglobulin heavy-chain locus. These translocations are detectable in 60% of MM patients by FISH. One of these recurrent translocations was t(4;14), which could be identified by FISH in 10–30% of MM patients [Citation3–6]. Many studies have shown that t(4;14) was associated with poor outcomes in MM patients [Citation7–16]. Sato S et al. [Citation6] retrospectively investigated 93 patients with newly diagnosed MM and found that the rate of 3-year OS was lower in t(4;14) patients than those without t(4;14), and considered that t(4;14) MM still had a poor prognosis in the era of novel drugs. Gertz MA et al. [Citation7] analyzed the prognostic value of t(4;14) in 238 patients who received high dose therapy and found that PFS and OS were significantly shorter for patients with t(4;14). A single-center retrospective longitudinal cohort study of newly diagnosed MM patients undergoing ASCT within 12 months from initial diagnosis also considered t(4;14) as a high-risk factor for patients [Citation4]. One study analyzed the data derived from four phase III trials involving patients younger than 66 years treated with novel agent-based induction and ASCT and showed that t(4;14) was related to the risk of early MM progression – related death [Citation8]. Nemec P et al. [Citation9] evaluated prognostic value of t(4;14) in 207 patients with newly diagnosed MM who were treated with high dose therapy followed by ASCT in the CMG2002 clinical trial and reported that t(4;14) was an independent prognostic factors associated with poor OS. Moreau P et al. [Citation10] evaluated the relationship between t(4;14) and survival of 1064 MM patients who were enrolled into three IFM99-02, IFM99-03 and 99-04 therapeutic trials of double intensive therapy and found that OS was shorter than that of patients without t(4;14). Avet-Loiseau H et al. [Citation11] analyzed 520 newly diagnosed MM patients younger than 66 years of age in the IFM 99-02 or IFM 99-04 trials and found that shorter PFS and OS were associated with t(4;14) in multivariate analyzes. Paiva B et al. [Citation12] reported a prospective analysis of 295 newly diagnosed MM patients uniformly treated in the GEM2000 protocol VBMCP/VBAD induction plus ASCT and identified FISH cytogenetics (any t(4;14), t(14;16), or del(17p)) as an independent prognostic factor for PFS. However, some studies failed to confirm t(4;14) as a poor prognostic factor for newly diagnosed MM patients [Citation3,Citation5,Citation17–19]. One retrospective observational study analyzed 97 patients with newly diagnosed MM who received a single, planned ASCT after treatment with 200 mg/m2 melphalan and found that the t(4;14) group was not significantly different from those of normal karyotype/FISH groups [Citation5]. Jiang N et al. [Citation17] investigated a cohort of 86 light chains only MM patients treated with ASCT and found that there was no significant difference in PFS or OS in patients with or without t(4;14). One study evaluated 409 newly diagnosed multiple myeloma patients received ASCT at Mayo Clinic Rochester and reported that there was no statistical difference in PFS or OS between the full cohort and the t(4;14) group [Citation3]. Another study cannot also confirm the unfavorable impact of t(4;14) on the outcome of newly diagnosed MM patients who were treated with high-dose chemotherapy and ASCT[Citation18]. At present, the prognostic value of t(4;14) in newly diagnosed MM patients remains controversial. Our study found that translocation t(4;14) was observed in 17.8% newly diagnosed MM patients which was similar to previous studies. In this study, patients with t(4;14) had shorter PFS and OS estimated by the Kaplan-Meier method than patients without cytogenetic abnormality. However, univariate and multivariate analysis revealed that t(4;14) was not a poor prognostic factor for PFS and OS of newly diagnosed MM patients. After balancing the distribution of factors between patients with and without t(4;14) by the propensity score matching technique, patients with t(4;14) had similar OS and PFS with the patients without t(4;14). In this study, there were 108 patients with t(4;14), more than 70% of patients had other cytogenetic abnormalities including 1q21 gain and del 17p, while less than 30% of patients had t(4;14) alone. The multivariate analysis and the propensity score matching analysis confirmed that t(4;14) alone had lost its prognostic value for newly diagnosed MM patients in the novel agent era. This may indicate that t(4;14) alone had little prognostic value for survival of patients with newly diagnosed MM, and other cytogenetic abnormalities including 1q21 gain and del 17p enhanced the poor prognostic power of t(4;14). As a result, t(4;14) alone cannot be considered a poor prognostic factor for patients with newly diagnosed MM.
This study had some limitations. One main limitation was its single-center database and retrospective nature. Another limitation was that the treatment of some patients may be transferred to other medical centers, resulting in some data loss. This may interfere with the assessment of the prognostic value of t(4;14). Finally, follow-up of patients is insufficient and further larger population studies are needed to validate the results.
In conclusion, our study demonstrated that t(4;14) lost its prognostic value for newly diagnosed MM patients in the novel agent era. Further studies are needed to confirm its prognostic value in the future.
Ethics approval and consent to participate
This study has been approved by the Medical Ethics Committee of Beijing Chaoyang Hospital. We followed the patients through the electronic medical record system without disturbing the patients in any way or interfering with the treatment of the patients. No informed consent was required because the data are anonymized.
Disclosure statement
No potential conflict of interest was reported by the author(s ).
Additional information
Funding
References
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–1060.
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548.
- Kaufman GP, Gertz MA, Dispenzieri A, et al. Impact of cytogenetic classification on outcomes following early high-dose therapy in multiple myeloma. Leukemia. 2016;30(3):633–639.
- Byun JM, Kim D, Shin DY, et al. Combination of genetic aberration With international staging system classification for stratification of Asian multiple myeloma patients undergoing autologous stem cell transplantation. In Vivo. 2019;33(2):611–619.
- Takamatsu H, Yamashita T, Kurahashi S, et al. Clinical implications of t(11;14) in patients with multiple myeloma undergoing autologous stem cell transplantation. Biol Blood Marrow Transplant. 2019;25(3):474–479.
- Sato S, Kamata W, Okada S, et al. Clinical and prognostic significance of t(4;14) translocation in multiple myeloma in the era of novel agents. Int J Hematol. 2021;113(2):207–213.
- Gertz MA, Lacy MQ, Dispenzieri A, et al. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32), and −17p13 in myeloma patients treated with high-dose therapy. Blood. 2005;106(8):2837–2840.
- Moreau P, Cavo M, Sonneveld P, et al. Combination of international scoring system 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front-line autologous stem-cell transplantation at high risk of early MM progression-related death. J Clin Oncol. 2014;32(20):2173–2180.
- Nemec P, Zemanova Z, Kuglik P, et al. Complex karyotype and translocation t(4;14) define patients with high-risk newly diagnosed multiple myeloma: results of CMG2002 trial. Leuk Lymphoma. 2012;53(5):920–927.
- Moreau P, Attal M, Garban F, et al. Heterogeneity of t(4;14) in multiple myeloma. Long-term follow-up of 100 cases treated with tandem transplantation in IFM99 trials. Leukemia. 2007;21(9):2020–2024.
- Avet-Loiseau H, Attal M, Campion L, et al. Long-term analysis of the IFM 99 trials for myeloma: cytogenetic abnormalities [t(4;14), del(17p), 1q gains] play a major role in defining long-term survival. J Clin Oncol. 2012;30(16):1949–1952.
- Paiva B, Vidriales MB, Cerveró J, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112(10):4017–4023.
- Cavo M, Terragna C, Renzulli M, et al. Poor outcome with front-line autologous transplantation in t(4;14) multiple myeloma: low complete remission rate and short duration of remission. J Clin Oncol. 2006;24(3):e4–e5.
- Gutiérrez NC, Castellanos MV, Martín ML, et al. Prognostic and biological implications of genetic abnormalities in multiple myeloma undergoing autologous stem cell transplantation: t(4;14) is the most relevant adverse prognostic factor, whereas RB deletion as a unique abnormality is not associated with adverse prognosis. Leukemia. 2007;21(1):143–150.
- Chang H, Sloan S, Li D, et al. The t(4;14) is associated with poor prognosis in myeloma patients undergoing autologous stem cell transplant. Br J Haematol. 2004;125(1):64–68.
- Baysal M, Demirci U, Umit E, et al. Concepts of double hit and triple hit disease in multiple myeloma, entity and prognostic significance. Sci Rep. 2020;10(1):5991–5996.
- Jiang N, Qi C, Trieu Y, et al. Genomic aberrations and survival of patients with light-chain-only multiple myeloma undergoing autologous stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(12):1790–1795.
- Greslikova H, Zaoralova R, Filkova H, et al. Negative prognostic significance of two or more cytogenetic abnormalities in multiple myeloma patients treated with autologous stem cell transplantation. Neoplasma. 2010;57(2):111–117.
- Luo T, Qiang W, Lu J, et al. Development and validation of prognostic implications of chromosome abnormalities algorithm for newly diagnosed multiple myeloma. Blood Sci. 2020;3(3):78–86.
- Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473.