ABSTRACT
Objectives
There is no meta-analysis about the effects of pegfilgrastim on the occurrence of febrile neutropenia (FN) in pediatric/adolescent cancer patients. The study explored the efficacy of prophylactic pegfilgrastim in preventing FN in children/adolescents with cancer.
Methods
PubMed, Embase, and the Cochrane Library were searched for studies published before April 7, 2020. The primary outcome was the rate of FN. Effect size (ES) and odds ratio (OR) with 95% confidence intervals (CIs) were used to evaluate the outcome. The ES represented the rate of FN, and the STATA ‘metaprop’ command was used to synthesize the rate.
Results
Eight studies were included, comprising 167 patients and 550 courses of treatment. There was no difference between pegfilgrastim and filgrastim for the rate of FN in children receiving chemotherapy (OR = 0.68, 95% CI: 0.20–2.23, P = 0.520). In patients receiving pegfilgrastim, the rate of FN was 25.6% (95% CI: 14.9%−36.3%), the rate of grade 4 FN was 38.3% (95% CI: 19.2%−59.5%), the rate of severe neutropenia (SN) was 40.5% (95% CI: 35.1%−46.1%), and the rate of treatment delays due to FN was 4.8% (95% CI: 0.8%−11.3%).
Discussion
The number of studies that could be included was small; therefore, a specific type of cancer or a specific treatment could be studied. Heterogeneity was high.
Conclusion
There was no difference between pegfilgrastim and filgrastim for the rate of FN. The use of pegfilgrastim was still associated with rates of FN, grade 4 FN, severe neutropenia, and treatment delays due to FN in pediatric cancer patients.
Introduction
Severe neutropenia (SN) is defined as a current or anticipated absolute neutrophil count (ANC) < 500 cells/mm3, while febrile neutropenia (FN) is defined as a single fever (38.3°C) or sustained elevated temperature (38°C) in a patient with a current or anticipated ANC of <500 cells/mm3 [Citation1–3]. FN most commonly occurs in patients receiving cytotoxic chemotherapy and affects 10%−50% of patients with solid tumour malignancies and >80% of patients with hematologic malignancies. The complications are delayed treatments, dose reduction, and increased risk of infection [Citation1–3]. Most infections are believed to arise from the patient’s endogenous flora, with identified sites including bacteremia in 20%−35% and respiratory, urinary, gastrointestinal (GI), or skin infections in 20%−30% [Citation1–3]. High-risk FN is defined as anticipated prolonged (>7 days duration) and profound neutropenia (ANC ≤100 cells/mm3 following cytotoxic chemotherapy) and is associated with significant comorbid conditions, including hypotension, pneumonia, new-onset abdominal pain, or neurologic changes [Citation3–5].
The current guidelines recommend granulocyte colony-stimulating factors (G-CSF) for primary prophylaxis of chemotherapy-induced FN [Citation3,Citation6]. G-CSF can also be used in the management of invasive fungal infections [Citation7,Citation8]. Most available G-CSF treatments are short-acting, but new long-acting agents (e.g. pegfilgrastim and lipegfilgrastim) are being made available [Citation9,Citation10]. Of note, short-acting G-CSF can exert their effect within 24–48 h, while long-acting G-CSF can take up to 1–2 weeks [Citation9,Citation10]. Prophylaxis with G-CSF has been shown to reduce the incidence of FN and improve cancer treatment outcomes [Citation11,Citation12]. Unfortunately, the use of G-CSF in clinical practice is suboptimal [Citation13–16]. Additional studies indicating the usefulness of G-CSF might improve this outcome.
Pegfilgrastim had a favourable efficacy and safety profile in clinical trials [Citation17–21]. Meta-analyses showed that G-CSF in adult patients receiving chemotherapy is effective and safe, including pegfilgrastim and biosimilars [Citation22], that it reduced the occurrence of FN compared with filgrastim [Citation23], and that long-acting G-CSF had better outcomes compared with short-acting ones [Citation24]. Using filgrastim or pegfilgrastim vs. placebo improved the overall survival (OS) of patients receiving chemotherapy [Citation25].
Nevertheless, there is only one guideline for pediatric and adolescent cancer patients regarding the management of FN [Citation4], and there is no meta-analysis about the effects of pegfilgrastim on the occurrence of FN in pediatric and adolescent cancer patients. In addition, there is insufficient evidence to suggest that short- or long-acting G-CSF can increase the survival of pediatric patients in FN. Moreover, G-CSF might have an economical cost and some potential risks, such as the risk of inducing acute myeloid leukemia or myelodysplastic syndromes [Citation26]. Therefore, this meta-analysis aimed to explore the efficacy of prophylactic pegfilgrastim in preventing FN in children/adolescents with cancer compared with G-CSF or no treatment/placebo.
Methods
Literature review
This meta-analysis was performed and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines [Citation27]. Since no original clinical raw data was collected or used, ethical approval was not requested for this meta-analysis.
PubMed, Embase, and the Cochrane Library were searched for studies published before April 7, 2020, using the MeSH terms ‘child’, ‘pediatrics’, ‘adolescent’, and ‘pegfilgrastim’, combined with relevant key words.
Inclusion and exclusion criteria
The inclusion criteria included 1) patients: pediatric or adolescent cancer patients (including both solid tumours and liquid cancers) receiving chemotherapy; 2) intervention: pegfilgrastim; 3) control: no restrictions; 4) outcome: febrile neutropenia, neutropenia, or treatment delay; 5) study design: no restrictions.
The exclusion criteria were: 1) conference abstract, case report, meta or review, animal study, and protocol; 2) language not English; 3) full text could not be obtained or no data available.
The primary outcome and any secondary outcomes
The primary outcome was the rate of FN. The secondary outcomes include rates of FN for pegfilgrastim vs. filgrastim, rate of grade 4 FN, rate of SN, and treatment delays.
Data extraction and quality assessment
Potentially relevant publications were screened and evaluated by two reviewers (Weiling Zhang and Yi Zhang) double-blindly, with a third reviewer (Huimin Hu) being requested to solve any disagreement. A structured data collection form was developed. Two researchers (Xia Zhu and Yizhuo Wang) independently extracted the data, including authors, year of publication, country, study design, sample size, age, percentage of males, episodes or courses, disease, dosage, etc.
The randomized controlled trials (RCTs) were evaluated using the Cochrane risk bias tool [Citation28,Citation29]. The observational studies were evaluated using the Newcastle-Ottawa scale (NOS) [Citation30]. The case series studies were evaluated according to the National Institute for Health and Clinical Excellence (NICE) method [Citation31]. The non-randomized trials were evaluated according to the revised and validated version of the methodological index for non-randomized studies (MINORS) [Citation32]. Finally, the crossover studies were evaluated according to Hong Ding’s quality assessment standard [Citation33].
Statistical analysis
All analyses were performed using STATA MP 14.0 (StataCorp, College Station, Texas, USA). Effect size (ES) and odds ratio (OR), and their 95% confidence intervals (CI) were used for analysis. The STATA ‘metaprop’ command was used to pool the rates. Statistical heterogeneity among the studies was calculated using Cochran’s Q-test and the I2 index. An I2 > 50% and Q-test P < 0.10 indicated high heterogeneity, and the random-effects model was used when high heterogeneity was present among studies; otherwise, the fixed-effects model was applied. P-values <0.05 were considered statistically different. Sensitivity analysis was performed to check the stability of the occurrence of FN for pegfilgrastim vs. filgrastim. Potential publication bias, Egger’s test, and Begg’s test were not performed due to the small number of studies included in each analysis [Citation28].
Results
Study selection
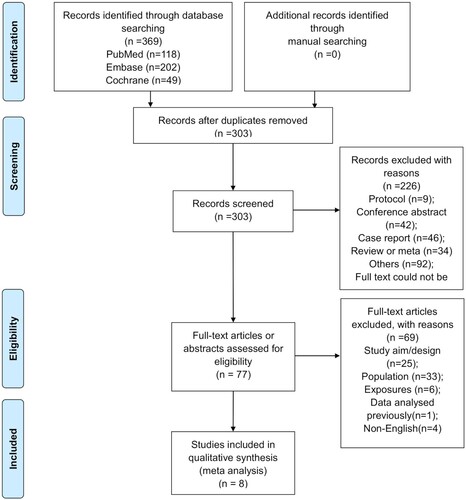
presents the study selection process. A total of 369 records were identified, and 303 were left after removing the duplicates. Then, 226 were excluded after screening, and 77 full-text papers were assessed for eligibility. Among them, 69 were excluded because of study aim/design (n = 25), population (n = 33), exposures (n = 6), data previously analyzed (n = 1), and non-English text (n = 4).
Finally, eight studies were included. There were four prospective studies [Citation34–37] and four retrospective studies (Supplementary Table S1) [Citation38–41]. There were 167 patients and 550 episodes/courses. Four studies had no control group, and four studies used filgrastim as a control. Supplementary Table S2 presents the quality evaluation of the included study.
Rate of FN
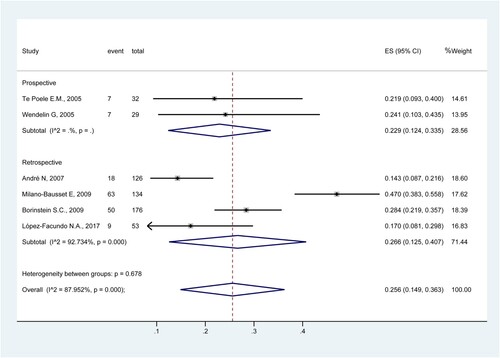
Six studies were included to determine the rate of FN [Citation34,Citation35,Citation38–41]. The results showed that the rate of FN was 25.6% (95%CI: 14.9%−36.3%; I2 = 88.0%) (). The rates were 22.9% (95%CI: 12.4%−33.5%) in prospective studies [Citation34,Citation35] and 26.6% (95%CI: 12.5%−40.7%) in retrospective studies [Citation38–41].
Association between FN and pegfilgrastim vs. filgrastim
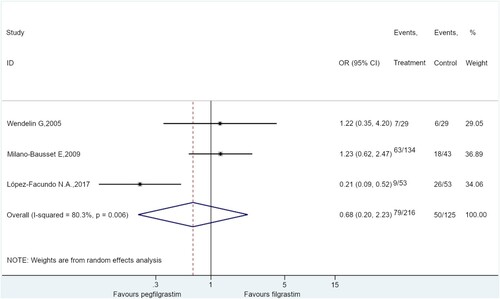
Three studies compared pegfilgrastim vs. filgrastim for the rate of FN [Citation35,Citation39,Citation41]. The results showed no difference between pegfilgrastim and filgrastim for the rate of FN in children receiving chemotherapy (OR = 0.68, 95%CI: 0.20–2.23, P = 0.520; I2 = 80.3%) ().
Rate of grade 4 FN
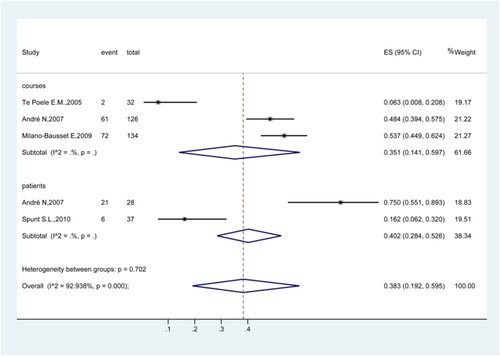
Four studies analyzed the rate of grade 4 FN [Citation34,Citation37–39]. The results showed that the rate of grade 4 FN was 38.3% (95%CI: 19.2%−59.5%; I2 = 92.9%) (). The rates were 35.1% (95%CI: 14.1%−59.7%) in the courses of treatment [Citation34,Citation38,Citation39] and 40.2% (95%CI: 28.4%−52.6%) in patients.
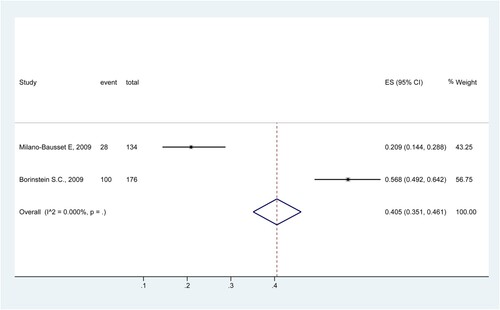
Rate of SN
Two studies analyzed the rate of SN [Citation39,Citation40]. The results showed that the rate of SN was 40.5% (95%CI: 35.1%−46.1%) ().
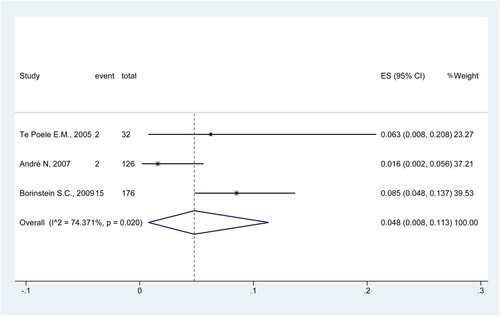
Treatment delays
Three studies examined the treatment delays due to FN [Citation34,Citation38,Citation40]. The results showed that the rate of treatment delays due to FN was 4.8% (95%CI: 0.8%−11.3%; I2 = 74.4%) ().
Sensitivity analysis
When considering the occurrence of FN for pegfilgrastim vs. filgrastim, no single study influenced the results (Supplementary Figure S1).
Discussion
The results suggest that compared with filgrastim, pegfilgrastim had a similar rate of FN. When using pegfilgrastim, the rate of FN was 25.6%, the rate of grade 4 FN was 38.3%, the rate of SN was 40.5%, and the rate of treatment delays due to FN was 4.8%.
In adults, studies showed that long-acting G-CSF achieves better outcomes than short-acting G-CSF [Citation17–21], and meta-analyses in adults also indicated the superiority of long-acting G-CSF [Citation22–25]. Nevertheless, only one guideline [Citation4] and no meta-analysis is currently available specifically for the management of FN in pediatric patients, and the guidelines highlight the need for additional evidence [Citation4]. The results in children and adolescents in the present meta-analysis are supported by the results in adults [Citation22–25]. Nevertheless, analyses in adults showed that pegfilgrastim prevented treatment delays and dose reductions [Citation22–25] since the neutrophil values have a higher likelihood of being within the adequate ranges, but this could not be observed in the present meta-analysis because of the available data. The FN rate with pegfilgrastim could be compared with filgrastim, and there was no statistically significant difference. In addition, the rates of FN, grade 4 FN, SN, and treatment delays were high (25.6%, 38.3%, 40.5%, and 4.8%, respectively), and it is surprising since chemotherapy-induced SN and FN are considered the first reason for dose-limiting toxicity in pediatric cancer [Citation34], but it has been reported that only small numbers of cycles were started later in pediatric patients treated with G-CSF, as the incidence of grade 4 SN and FN which may result in intolerance to the treatment was relatively low, reportedly about 6%[Citation34,Citation38]. A previous meta-analysis showed that filgrastim reduced the rate of FN by 20% and shortened hospitalization time, but with no impact on infection-related mortality [Citation42]. The lack of difference in the present study between pegfilgrastim and filgrastim could be because G-CSF stimulates the hematopoietic cells to produce more neutrophils. The hematopoietic system in children is more active than the adult counterpart, and the composition of hematopoietic stem cells and their location in the body are also different [Citation43,Citation44]. Therefore, future study designs should consider the stratification of children and adults to explore the effects of G-CSF. Furthermore, different chemotherapy drugs have different myelosuppression potentials [Citation45,Citation46], but they could not be taken into account in the present meta-analysis because of the small number of included studies.
The conclusions of this meta-analysis must be considered within its limitations. The number of studies that could be included was small, leading to a small number of patients and courses of treatment. Because of this, no selection was based on the type of cancer or chemotherapy, introducing bias due to the different aggressiveness of the diseases and treatments. If a control group was included, the control group was filgrastim or a placebo. The studies were conducted in various countries with different guidelines for using G-CSF. These reasons led to high heterogeneity in all analyses. Furthermore, the safety issues could not be analyzed because of the too important differences in safety outcomes among the included studies. In addition, future studies should examine the effect of pegfilgrastim biosimilars in pediatric patients with cancer.
In conclusion, the results suggest no difference between pegfilgrastim and filgrastim for the rate of FN. The rates of FN, grade 4 FN, SN, and treatment delays were 25.6%, 38.3%, 40.5%, and 4.8%, respectively, in pediatric patients receiving chemotherapy. However, high-level evidence is needed to define the role of pegfilgrastim in pediatric/adolescent cancer patients.
Availability of data and material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
This is a systematic review and single-arm meta-analysis. The Ethics Committee of Beijing Tongren Hospital, Capital Medical University, has confirmed that no ethical approval is required.
Acknowledgments
The authors acknowledge the help of all co-workers.
Authors’ contributions: Dongsheng Huang contributed to the conception and design of the study. Huimin Hu organized the database. Yi Zhang and Yizhuo Wang performed the statistical analysis. Xia Zhu wrote the first draft of the manuscript. Xia Zhu and Weiling Zhang wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American society of clinical oncology and infectious diseases society of America clinical practice guideline update. J Clin Oncol. 2018;36(14):1443–1453.
- Wang XJ, Chan A. Optimizing symptoms and management of febrile neutropenia among cancer patients: current status and future directions. Curr Oncol Rep. 2017;19(3):20.
- Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2011;52(4):e56–e93.
- Lehrnbecher T, Robinson P, Fisher B, et al. Guideline for the management of fever and neutropenia in children With cancer and hematopoietic stem-cell transplantation recipients: 2017 update. J Clin Oncol. 2017;35(18):2082–2094.
- Ullmann AJ, Akova M, Herbrecht R, et al. ESCMID* guideline for the diagnosis and management of candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect. 2012;18(Suppl 7):53–67.
- Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199–3212.
- Chu S, McCormick TS, Lazarus HM, et al. Invasive fungal disease and the immunocompromised host including allogeneic hematopoietic cell transplant recipients: improved understanding and new strategic approach with sargramostim. Clin Immunol. 2021;228:108731.
- Scriven JE, Tenforde MW, Levitz SM, et al. Modulating host immune responses to fight invasive fungal infections. Curr Opin Microbiol. 2017;40:95–103.
- Rofail P, Tadros M, Ywakim R, et al. Pegfilgrastim: a review of the pharmacoeconomics for chemotherapy-induced neutropenia. Expert Rev Pharmacoecon Outcomes Res. 2012;12(6):699–709.
- Renwick W, Pettengell R, Green M. Use of filgrastim and pegfilgrastim to support delivery of chemotherapy: twenty years of clinical experience. Bio Drugs. 2009;23(3):175–186.
- Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32.
- Leonard RC, Mansi JL, Keerie C, et al. A randomised trial of secondary prophylaxis using granulocyte colony-stimulating factor (‘SPROG’ trial) for maintaining dose intensity of standard adjuvant chemotherapy for breast cancer by the Anglo-Celtic Cooperative Group and NCRN. Ann Oncol. 2015;26(12):2437–2441.
- Kelly S, Wheatley D. Prevention of febrile neutropenia: use of granulocyte colony-stimulating factors. Br J Cancer. 2009;101(Suppl 1):S6–10.
- Barnes G, Pathak A, Schwartzberg L. G-CSF utilization rate and prescribing patterns in United States: associations between physician and patient factors and GCSF use. Cancer Med. 2014;3(6):1477–1484.
- Link H, Nietsch J, Kerkmann M, et al. Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy–a representative sample survey in Germany. Support Care Cancer. 2016;24(1):367–376.
- Weycker D, Hackett J, Edelsberg JS, et al. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402–407.
- Neulasta AL. Summary of product characteristics 2015 [Available from: https://www.ema.europa.eu/en/documents/product-information/neulasta-epar-product-information_en.pdf.
- Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23(6):1178–1184.
- Mey UJ, Maier A, Schmidt-Wolf IG, et al. Pegfilgrastim as hematopoietic support for dose-dense chemoimmunotherapy with R-CHOP-14 as first-line therapy in elderly patients with diffuse large B cell lymphoma. Support Care Cancer. 2007;15(7):877–884.
- Ozer H, Mirtsching B, Rader M, et al. Neutropenic events in community practices reduced by first and subsequent cycle pegfilgrastim use. Oncologist. 2007;12(4):484–494.
- Pro B, Fayad L, McLaughlin P, et al. Pegfilgrastim administered in a single fixed dose is effective in inducing neutrophil count recovery after paclitaxel and topotecan chemotherapy in patients with relapsed aggressive non-Hodgkin's lymphoma. Leuk Lymphoma. 2006;47(3):481–485.
- Wang Y, Chen L, Liu F, et al. Efficacy and tolerability of granulocyte colony-stimulating factors in cancer patients after chemotherapy: A systematic review and Bayesian network meta-analysis. Sci Rep. 2019;9(1):15374.
- Bond TC, Szabo E, Gabriel S, et al. Meta-analysis and indirect treatment comparison of lipegfilgrastim with pegfilgrastim and filgrastim for the reduction of chemotherapy-induced neutropenia-related events. J Oncol Pharm Pract. 2018;24(6):412–423.
- Cornes P, Gascon P, Chan S, et al. Systematic review and meta-analysis of short- versus long-acting granulocyte colony-stimulating factors for reduction of chemotherapy-induced febrile neutropenia. Adv Ther. 2018;35(11):1816–1829.
- Lyman GH, Reiner M, Morrow PK, et al. The effect of filgrastim or pegfilgrastim on survival outcomes of patients with cancer receiving myelosuppressive chemotherapy. Ann Oncol. 2015;26(7):1452–1458.
- Link H. Current state and future opportunities in granulocyte colony-stimulating factor (G-CSF). Support Care Cancer. 2022;30(9):7067–7077.
- Selcuk AA. A guide for systematic reviews: PRISMA. Turk Arch Otorhinolaryngol. 2019;57(1):57–58.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). London: Cochrane Collaboration; 2019.
- Higgins JP, Altman DG, Gotzsche PC, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. Br Med J. 2011;343:d5928.
- Coen D, Cortellaro F, Pasini S, et al. Towards a less invasive approach to the early goal-directed treatment of septic shock in the ED. Am J Emerg Med. 2014;32(6):563–568.
- Excellence NIfHaC. Appendix 4 Quality of case series form [Available from: https://www.nice.org.uk/guidance/cg3/documents/appendix-4-quality-of-case-series-form2.
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716.
- Ding H, Hu GL, Zheng XY, et al. The method quality of cross-over studies involved in Cochrane Systematic Reviews. PLoS One. 2015;10(4):e0120519.
- te Poele EM, Kamps WA, Tamminga RY, et al. Pegfilgrastim in pediatric cancer patients. J Pediatr Hematol Oncol. 2005;27(11):627–629.
- Wendelin G, Lackner H, Schwinger W, et al. Once-per-cycle pegfilgrastim versus daily filgrastim in pediatric patients with Ewing sarcoma. J Pediatr Hematol Oncol. 2005;27(8):449–451.
- Yousofian S, Miri-Aliabad G, Kiumarsi A, et al. Effectiveness of filgrastim and polyethylene glycol-filgrastim in the treatment of postchemotherapy neutropenia in children: phase I clinical trial. Indian J Med Paed Oncol. 2019;40(1):101–104.
- Spunt SL, Irving H, Frost J, et al. Phase II, randomized, open-label study of pegfilgrastim-supported VDC/IE chemotherapy in pediatric sarcoma patients. J Clin Oncol. 2010;28(8):1329–1336.
- Andre N, Kababri ME, Bertrand P, et al. Safety and efficacy of pegfilgrastim in children with cancer receiving myelosuppressive chemotherapy. Anticancer Drugs. 2007;18(3):277–281.
- Milano-Bausset E, Gaudart J, Rome A, et al. Retrospective comparison of neutropenia in children with Ewing sarcoma treated with chemotherapy and granulocyte colony-stimulating factor (G-CSF) or pegylated G-CSF. Clin Ther. 2009;31(Pt 2):2388–2395.
- Borinstein SC, Pollard J, Winter L, et al. Pegfilgrastim for prevention of chemotherapy-associated neutropenia in pediatric patients with solid tumors. Pediatr Blood Cancer. 2009;53(3):375–378.
- Lopez-Facundo NA, Valois-Escamilla MG, Tejocote-Romero I, et al. Cost-benefit study of febrile neutropenia prophylactic treatment with pegfilgrastim vs. filgrastim in pediatric patients with solid tumors. Gaceta Mexicana dew Oncologia. 2017;16(1):31–35.
- Sung L, Nathan PC, Lange B, et al. Prophylactic granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor decrease febrile neutropenia after chemotherapy in children with cancer: a meta-analysis of randomized controlled trials. J Clin Oncol. 2004;22(16):3350–3356.
- Copley MR, Eaves CJ. Developmental changes in hematopoietic stem cell properties. Exp Mol Med. 2013;45:e55.
- Fernandez KS, de Alarcon PA. Development of the hematopoietic system and disorders of hematopoiesis that present during infancy and early childhood. Pediatr Clin North Am. 2013;60(6):1273–1289.
- Akashi M, Shibuya Y, Kusumoto J, et al. Myelosuppression grading of chemotherapies for hematologic malignancies to facilitate communication between medical and dental staff: lessons from two cases experienced odontogenic septicemia. BMC Oral Health. 2013;13:41.
- Gordon MS. Myelosuppression. Current cancer therapeutics. London: Current Medicine Group; 2001.