ABSTRACT
Objectives:
To describe the incorporation of monoclonal antibodies (mAb) in real-world (RW) practice for the treatment of patients with relapsed refractory multiple myeloma (RRMM) in a setting with other treatment alternatives.
Methods:
This was an observational, multicenter, ambispective study of RRMM treated with or without a mAb.
Results:
A total of 171 patients were included. For the group treated without mAb, the median (95% CI) progression-free survival (PFS) to relapse was 22.4 (17.8-27.0) months; partial response or better (≥PR) and complete response or better (≥CR) was observed in 74.1% and 24.1% of patients, respectively; and median time to first response in first relapse was 2.0 months and in second relapse was 2.5 months. For the group of patients treated with mAb in first or second relapse, the median PFS was 20.9 (95% CI, could not be evaluated) months; the ≥ PR and ≥ CR rates were 76,2% and 28.6%, respectively; and the median time to first response in first relapse was 1.2 month and in second relapse was 1.0 months. The safety profiles for the combinations were consistent with those expected.
Conclusions:
The incorporation of mAb in RW practice for the treatment of RRMM has shown good quality and speed of response with a similar safety profile shown in randomized clinical trials.
Introduction
Multiple myeloma (MM) accounts for approximately 1% of all cancers and 10% of all hematologic malignancies, representing the second most common blood cancer [Citation1]. The incidence of MM in Spain evaluated over a 23-year study period (1994-2016) has been rather stable, ranging between 4.09 and 4.44 cases per 100,000 inhabitants [Citation2]. The disease has a typical course characterized by a chronic phase lasting several years and an aggressive terminal phase.
The therapeutic landscape in MM has changed markedly in the last decade with the introduction of the immunomodulatory (IMiDs) agents thalidomide, lenalidomide, and pomalidomide as well as proteasome inhibitors (PI) bortezomib and carfilzomib [Citation3,Citation4]. Significant increases in the overall response rate (ORR), duration of progression-free survival (PFS), and overall survival (OS) were obtained with these new drugs [Citation4–6].
The possible combinations of these drugs make the treatment of relapsed refractory multiple myeloma (RRMM) very heterogeneous. Some of the combinations that have demonstrated safety and efficacy according to phase 3 randomized clinical trials (RCTs) include lenalidomide with dexamethasone (Rd) [Citation7,Citation8], bortezomib either alone or in combination with dexamethasone [Citation9,Citation10], KRd (carfilzomib/lenalidomide/dexamethasone), Kd (carfilzomib/lenalidomide/dexamethasone), and Pd (pomalidomide/dexamethasone) [Citation11–13].
However, despite significant therapeutic advances in its treatment over the past years [Citation14], MM remains an incurable disease, and the majority of patients relapse after achieving remission due to inherent drug resistance characterized by highly complex and heterogeneous molecular abnormalities [Citation15,Citation16]. In the search for new treatment options with alternative mechanisms of action addressing different molecular targets that might overcome the above mentioned therapy limitations, monoclonal antibodies (mAbs) have emerged as remarkably effective drugs in the treatment of several hematologic malignancies [Citation17].
Daratumumab is a human monoclonal IgG1κ antibody that targets high affinity CD38, a cell surface protein overexpressed on MM cells. Daratumumab induces rapid, deep, and durable clinical responses in patients with MM through a multifaceted mechanism of action. Direct on-tumor actions include complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and induction of apoptosis by crosslinking [Citation18,Citation19]. Daratumumab should also be considered an active immunotherapy due to its immunomodulatory effects as it exerts immunomodulatory effects via T-cell induction/expansion, T-cell activity enhancement, and reduction of immune-suppressive cell populations [Citation20]. This activity may contribute to prolonged and deep clinical responses [Citation21].
Due to the positive results in several clinical trials, daratumumab is approved in Europe in different settings both as a first-line treatment and in relapse [Citation22]. Currently, more than 274,000 patients have been treated with daratumumab worldwide [Citation23].
This incorporation of daratumumab for the treatment of myeloma in Spain began mainly in the relapsed setting through daratumumab, bortezomib and dexamethasone (DVd) as well as daratumumab, lenalidomide, dexamethasone (DRd) combinations, after these combinations have demonstrated safety and efficacy for the treatment of RRMM in the context of RCTs [Citation24,Citation25].
In POLLUX (NCT02076009) and CASTOR (NCT02136134) phase III clinical trials, DRd versus Rd and DVd versus Vd in RRMM showed superior PFS, ORR, and minimal residual disease (MRD)-negative rates for both standard- and high-risk patients. After 4 years of follow-up, the greatest clinical benefit of DRd was observed in patients who had received one prior line of therapy [Citation24,Citation26,Citation27]. The benefit of triple therapy with DVd occurred regardless of whether the previous regimen included bortezomib or lenalidomide but was more pronounced in patients who had received one prior line of treatment [Citation25,Citation28–30].
Recently, after more than 6 years of follow-up, a statistically significant and clinically meaningful OS benefit was also confirmed with DRd (OS in the DRd arm was 67.6 (95% CI, 53.1-80.5) months versus 51.8 (95% CI, 44.0-60.0) months in the Rd arm) and DVd (in 1 prior line, OS was NR (95% CI, 59.7 months-not estimable) in the DVd arm versus 47.0 (95% CI, 32.6-58.7) months in the Vd arm) [Citation31,Citation32].
Although some studies have evaluated the effectiveness of daratumumab-containing regimens in RRMM in a real-world (RW) setting, most of the studies were retrospective and enrolled a limited number of patients [Citation33–38]. The present study was performed to describe the incorporation of mAb in RW practice for the treatment of patients with RRMM in a setting with other standard treatment alternatives that do not include mAb.
Materials and methods
Study design
This was an RW, observational, multicenter, ambispective (with retrospective and prospective phases) descriptive study conducted in 52 hospitals throughout Spain (Supplementary Table 1). Patients diagnosed with RRMM (first and second relapse) who started antineoplastic treatment within RW practice were included in the study. Two patient groups were evaluated: RRMM patients treated with standard regimens without mAb (Group A) and treated with a mAb-containing regimen (Group B) (Supplementary Figure 1). Group A started treatment with a combination of ≥ 2 drugs between October 2017 and March 2018, and Group B started treatment between April 2018 and September 2018. Patient recruitment occurred from 15 November 2018 to 15 June 2019 (for Group A) and lasted until 31 October 2019 for Group B in an attempt to counteract the significant imbalance in the number of patients included in the latter group. The study included a retrospective phase from MM diagnosis to study recruitment, during which baseline clinical and demographic data were collected. Subsequently, the study subjects were prospectively followed up for a 12-month observation period and were evaluated at 6-month intervals.
The study was reviewed and approved by all the ethics committees of the participating sites, and it was performed in accordance with all the applicable local regulations on noninterventional studies and/or observational studies. Written informed consent was obtained from all living study participants (or from their legal representative) prior to enrollment in the study.
Study population
Patients were eligible for the study if they were 18 years of age or older, had a confirmed diagnosis of RRMM (with first or second relapse), had started antineoplastic treatment before study enrollment, were not eligible for autologous stem cell transplant at relapse, and, in the opinion of the investigator, had an estimated life expectancy longer than 6 months. Additionally, patients should have started antineoplastic treatment either with any regimen that included at least two drugs or with a mAb-containing regimen in the time period indicated previously.
Patients were excluded if they had a primary refractory MM (nonresponsive while on primary or salvage therapy or progressed within 60 days of last therapy), had a ≥ 3rd relapse of MM, were receiving monotherapy for the 1st or 2nd relapses, and/or were participating or included in an interventional clinical trial.
Patients were recruited by convenience sampling. Specifically, each investigator recruited the first 5–6 patients who met the criteria for selection and who came consecutively to consultation since the opening of the participating center.
Study objectives and assessments
The primary objective of the study was to describe the incorporation of the mAb daratumumab into RW practice for the treatment of RRMM in a period of time that covered the 6 months before and after the establishment of pricing and reimbursement within the Spanish Public Health System. This objective was assessed by means of the PFS for the following combinations: daratumumab-containing and alternative standard of care treatment regimens.
The secondary objectives of this study included the following: (1) to assess the clinical responses by treatment group (evaluated by the IMWG criteria [Citation39]); (2) to estimate the OS by treatment group; (3) to assess the safety and tolerability profile evaluated by the reported adverse events (AEs) and serious adverse events (SAEs) (whether related or not to the study drug) by treatment group; and (4) to describe the standard of care regimens used for RRMM in Spain before the availability of the combinations with daratumumab.
The main data source of the study was the patients’ medical records at each site. All the data entered in the study patients’ clinical record files (CRFs) were originally recorded in their respective medical charts.
The following medical information was collected during the study: baseline demographic data; previous diagnosis and relevant medical history; former and current features of the MM, lab tests and radiologic assessments; cytogenetic evaluation by fluorescence in situ hybridization (FISH) analysis, considering a high risk (HR) profile the presence of one of the following cytogenetic abnormalities: t(4;14), t(14;16) and/or Del17p13(P53) and extended HR cytogenetic profile any of the above plus add(1q); disease staging according to the International Staging System (ISS) and the revised version (R-ISS) [Citation40]; all previously received MM treatments; some concomitant medications; and measures of clinical efficacy/response (tumor response to treatment and disease progression were evaluated according to the IMWG criteria [Citation39] and applied in RW practice). To measure the response, the following assessments were reviewed: quantification of the monoclonal protein (serum and urine protein electrophoresis and immunofixation, serum free light chain), bone marrow examination, radiological imaging assessment, evaluation of extramedullary plasmacytomas, and measurements of serum calcium corrected for albumin.
Finally, for the safety assessment, AEs related to myeloma treatment in the retrospective phase and all AEs reported in the prospective phase regardless of whether these AEs were related to the study drugs and regardless of their severity were recorded in the CRFs.
Statistical analysis
This study is descriptive/exploratory; therefore, no formal sample size calculation was performed.
The efficacy analysis comprised all study patients who met all the selection criteria, had provided written informed consent, and had at least one response assessment. The safety population consisted of all the study subjects who met all the selection criteria, had provided written informed consent, and had received at least one dose of the study drugs.
For the descriptive analysis, quantitative continuous variables were summarized as the mean, standard deviation (SD), median, and interquartile range (IQR), whereas categorical variables were described by means of absolute and relative frequencies. Missing values were not considered for the calculation of the respective percentages.
PFS was defined as the time elapsed between treatment initiation for the first relapse and the second disease progression (no significant or significant biochemical progression, symptomatic progression, whichever would have been identified first) or death from any cause, whichever occurs first. For PFS, data were censored either if patients started a subsequent treatment and no confirmation of disease progression was available or at the last available follow-up date. OS was defined as the time elapsed between the date of initiation of first or second relapse treatment (depending on patient study registry) to the date of death due to any cause. For OS, patients who were alive at the time of the analysis data cutoff were censored at the last date they were known to be alive. PFS and OS were estimated using the Kaplan–Meier method.
Time to first response (TTFR) was defined as the period of time between treatment initiation for the first/second relapse and the first response in first/second relapse [strict complete response (sCR), complete response (CR), very good partial response (VGPR), partial response (PR)], and time to best response (TTBR) was defined as the time from the beginning of the treatment initiation for the first/second relapse to the date of best response (sCR, CR, VGPR, PR). TTFR and TTBR were analyzed as continuous variables, and their respective median and IQR were estimated.
For the safety analysis, AEs were coded using the Medical Dictionary of Regulatory Activities (MedDRA) [Citation41], and all documented AEs were included in the analysis. In addition, all the safety parameters with a predefined toxicity level were summarized according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE v5.0) [Citation42].
Results
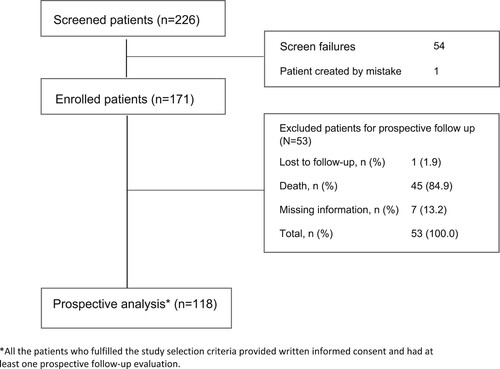
From November 2018 to October 2019, 226 patients were screened, and 171 patients were finally recruited at 52 sites in Spain (Supplementary Table 1). Sixty-three patients received a mAb combination (Group B), and 108 were treated with other standard of care regimens (Group A). The 171 enrolled patients were included in the efficacy and safety analyses; however, 55 patients were excluded from the prospective analysis of the study for not meeting all the eligibility criteria (). The two main reasons for exclusion from the prospective analysis were inclusion of autologous hematopoietic stem cell transplantation as part of the relapsed treatment (40.7%) and not having started treatment with a mAb regimen within the first 6 months after its reimbursement in Spain (35.2%).
Seventy-two patients discontinued the study treatment, including 50 in Group A (46.3% of patients treated in Group A) and 22 in Group B (34.9% of patients treated in Group B). Reasons for study discontinuation are displayed in Supplementary Table 2.
First and second MM relapse: Patient population
A total of 171 patients were included: 121 after first-line therapy and 50 after second-line therapy. Overall, there were 96 (56.1%) men, and the median (IQR) age was 73.0 (67.0-78.0) years. In total, 111 patients (64.9%) were ≤ 75 years old. Comorbidities were common, especially heart (30.3%) and renal failure (15.8%).
One hundred twenty-one patients with first relapse were included in the study. Eighty patients were included in Group A, and 41 were included in Group B. A total of 50 patients were included at the time of second relapse. Specifically, twenty-eight and twenty-two patients were included in Groups A and B, respectively.
Clinical characteristics are presented in . Both groups’ clinical characteristics were comparable, except for ISS and R-ISS stage II, which were the most prevalent in Group B.
Table 1. Patient characteristics and type of therapy at relapse.
A greater number of patients started treatment in symptomatic relapse in both groups (59%). The most frequent treatments resulting in relapse in Group A included Rd and KRd regimens with 36% and 27%, respectively. In Group B, therapy options for relapse included DVd in 60% of patients and DRd in 40%. A low percentage of patients were considered refractory to front-line lenalidomide in both groups: 10.2% (9 patients) and 17.1% (7 patients) in Groups A and B, respectively.
First and second MM relapse: Efficacy
The median (IQR) patient follow-up time was 48.9 (34.9-65.4) months for Group A and 43.5 (35.5-58.7) months for Group B.
Progression-free survival (PFS)
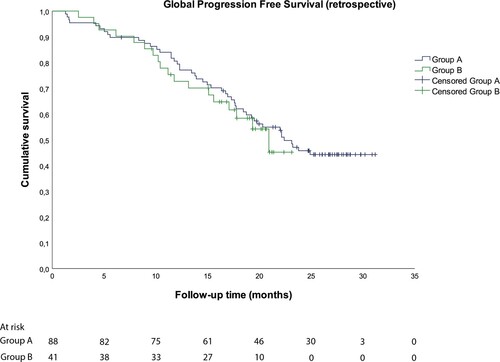
The median (95% CI) PFS to relapse was 22.4 (17.8-27.0) months for Group A and 20.9 (95% CI, could not be evaluated) months for Group B (). Of note, 46.6% and 56.1% of patients in Groups A and B, respectively, were censored; most patients were censored after 15–18 months of follow-up mainly because a subsequent treatment was initiated, and no confirmation of disease progression was available.
Overall survival
Median OS was not achieved in any of the study groups (Supplementary Figure 2). The high proportion of censored patients in both groups (greater than 87%) may have been attributed to the relatively short follow-up time to assess OS.
Clinical responses to the respective therapy regimens by treatment group
Regarding the response rates to relapse therapy, PR or better (≥PR) was observed in 74.1% of the patients in Group A and 76,2% of those in Group B; CR or better (≥CR) was obtained in 24.1% and 28.6% of the patients in Groups A and B, respectively (). Median TTFR in first relapse was 2.0 months in group A and 1.2 months in group B; in second relapse the corresponding figures were 2.5 and 1.0 months, respectively. Median TTBR in first relapse was 4.9 months in group A and 3.6 months in group B; and for the second relapse 7.4 months in group A and 5.4 in Group B ().
Table 2. Best response to relapse therapy per treatment group.
Table 3. Time to response (months).
Only 12 out of 68 patients (17.6%) at first relapse (9 in Group A and 3 in Group B) and 2 of 28 patients (7.1%) at second relapse (1 in Group A and 1 in Group B) who met the criteria for MRD evaluation (IMWG 2016 [Citation39]) underwent MRD evaluation. At first relapse, MRD negativity was achieved in 4 of the 9 patients in Group A and 2 of the 3 patients in Group B. At second relapse, the evaluable single patient in Group A achieved MRD negativity, but MRD negativity was not achieved by the evaluable patient in Group B.
First and second MM relapse: Safety and tolerability
During the retrospective phase of the study, a total of 17 and 35 related adverse events in Groups A and B, respectively, were recorded (Supplementary Table 3). The most frequent adverse event in either group was peripheral neuropathy, as noted in 6 cases among patients in Group A and another 6 cases in Group B. Similarly, the most frequent grade 3–4 adverse event was peripheral neuropathy, as noted in 3 cases in patients in Group A and 2 cases among patients in Group B (Supplementary Table 4).
During the prospective phase, 205 adverse events occurred among the 74 patients in Group A and 131 adverse events among the 39 patients in Group B (Supplementary Table 5). The most commonly reported adverse events in Group A were asthenia (17.6%), thrombocytopenia (14.9%), and respiratory tract infection (14.9%). In Group B, the most frequent adverse events were diarrhea (20.5%) and asthenia (17.9%). Grade 3–4 AEs were reported in 28.4% and 40.9% of patients from Groups A and B, respectively, during the prospective phase of the study (). Hematological toxicity, including anemia (1.4% and 10.3% for Groups A and B, respectively), neutropenia (9.5% and 7.7%) and thrombocytopenia (5.4% and 2.6%), was the most frequently reported grade 3–4 adverse for both groups.
Table 4. Severe adverse events reported during the prospective phase of the study.
Forty-three patients who received antibacterial prophylaxis were identified (27 in Group A and 16 in Group B), most of whom had primary disease treated with oral trimethoprim/sulfamethoxazole.
The treatment discontinuation rate for poor tolerance/adverse reactions for treated patients was 28% in Group A and 18% in Group B (Supplementary Table 2).
In daratumumab-treated patients (63), only 10 infusion-related reactions were recorded, including 9 grade 1 reactions and 1 grade 2 reaction. Forty-one patients had received treatment with montelukast as premedication to prevent the development of IRRs. Eleven patients required red blood cells transfusion support, and none of them presented transfusion reactions.
MM diagnosis and front-line treatment
No differences in relevant clinical characteristic were noted between the study groups, with the exception that, compared to Group B, patients in Group A were slightly older, showed a higher frequency of lytic bone lesions (66.7% vs. 49.2%), and exhibited a longer time from initial diagnosis to signed informed consent (median of 51.1 and 38.1 for Groups A and B, respectively). Cytogenetic profiling was performed in 114 patients (70 in Group A and 44 in Group B) at first diagnosis (data on the high-risk profile for both groups are shown in Supplementary Table 6).
Only 35 patients (20.6%) had plasmacytoma radiologic evaluations at MM diagnosis. Most of these patients presented bone and soft tissue involvement (80% in Group A and 60% in Group B).
With respect to the study on antineoplastic therapy, the most frequent front-line treatment regimen was intensive (biweekly bortezomib) VMP (bortezomib-melphalan-prednisone), which was administered in 24% of the total study population, followed by VCd (bortezomib-cyclophosphamide-dexamethasone) (12.9%) and Vd (12.9%). As shown in Supplementary Table 7, a wide variability in drug combinations was observed. In addition, data on treatment response were available for 169 patients (98.8% of the study population). A total of 67.5% and 25% of the evaluated subjects presented VGPR or better and CR or better as the best response, respectively. Response data are displayed in Supplementary Table 8.
Discussion
The introduction of the mAb daratumumab represented a significant breakthrough within the therapeutic arsenal for the treatment of MM. The hallmark RCTs, CASTOR [Citation28] and POLLUX [Citation24], confirmed that daratumumab combined with Vd resulted in significant improvements in depth of response (including MRD negativity), PFS, time to disease progression and OS in patients with RRMM with a good and manageable safety profile. However, only a limited number of publications on RW treatment with daratumumab are available, and these studies present variable results with short follow-up times in general [Citation33,Citation35,Citation36].
Our study performed in an RW setting is, to our knowledge, the first large study to describe the incorporation of a mAb, daratumumab, into RW practice for the treatment of RRMM in Spain. After a median patient follow-up time of 48.9 months for Group A and 43.5 months for Group B, we observed a median PFS of 20.9 months in Group B and a median of 22.4 months in Group A. These results were similar to the median PFS for DVd reported for the CASTOR trial [Citation28], but inferior to the median PFS for DRd reported for the POLLUX trial [Citation43]. Similar results have also been observed for patients treated without mAb in Group A, where the most commonly used salvage regimens (Rd and KRd) have slightly lower PFS results than those identified in CTs [Citation5,Citation11,Citation44]. Furthermore, we also observed a lower response rate (ORR and ≥ CR) in our study than in the CASTOR [Citation28], POLLUX [Citation24] and ASPIRE [Citation11] trial results. However, CR or better was observed in 28.6% of patients in the combinations with daratumumab in relapse (DRd/DVd) and 24.1% of patients in the standard treatment group. Usually, depth of response translates into greater survival, a finding that we have not been able to observe in this RW practice study probably due to the high percentage of patients censored during follow-up, especially patients treated with mAb regimens. Median TTFR in first relapse was 2.0 months in the group receiving the standard of care regimens and 1.2 month in the daratumumab group; in second relapse the corresponding figures were 2.5 and 1.0 months, respectively. Median TTBR in first relapse was 4.9 months in the standard of care group and 3.6 months in the daratumumab group; and for the second relapse the corresponding figures were 7.4 and 5.4 months, respectively.
Regarding MRD evaluation, regrettably, the low number of patients with this analysis among those who met the criteria to be analyzed prevents us from drawing any general conclusions. The Spanish Myeloma Group (GEM) is one of the cooperative groups that has internationally contributed the most to generating the scientific evidence that supports the relevance of negative MRD in MM, placing it as a desirable treatment goal [Citation45,Citation46], having even already reported preliminary results of a study in RW practice of MRD evaluation in our country [Citation47], which could complement these results that we have not been able to assess in the present study. To facilitate wider access to MRD RW measurements in Spanish hospitals and under GEM endorsement, the Cavex program makes it available through its analysis by high-sensitivity multidimensional flow cytometry through a national network of 3 international validated reference sites. Since its launch in 2015, 133 hospitals have joined, and 3,438 samples have been analyzed to date [Citation48].
These differences in efficacy between reference RCTs and this RW practice study may be partly attributed to the baseline clinical and demographic characteristics of the patients enrolled in our study, such as an older median age (range 6–10 years older [Citation7,Citation11,Citation12,Citation24,Citation28]) and the presence of comorbidities, including cardiac and renal failure. In addition, delaying therapy initiation until symptomatic relapse can result in worse therapy outcomes [Citation49,Citation50]. In the present study, a large proportion of patients started treatment during symptomatic relapse. Moreover, the median time between the first diagnosis of MM and study enrollment was shorter in the daratumumab group (median of 51.1 and 36.1 for Groups A and B, respectively). However, the speed of response observed in patients treated with daratumumab in combination may contribute to faster control of the disease and could particularly benefit patients with symptomatic relapse.
The toxicity observed in our study seems to be underestimated. This value is lower than that reported in RCTs [Citation11,Citation24,Citation28,Citation44] for the two study groups. This difference may be due to the observational nature of this study as well as the difficulty of conducting this type of study in RW practice. Regarding the standard of care regimens evaluated in the study (Group A), a plausible explanation may also be derived from their greater experience of use and their consequent lower reporting frequency. Regarding the tolerability of the study daratumumab combinations (Group B), the adverse event profile was actually as expected and consistent with previously reported studies [Citation24,Citation28]. Interestingly, although one of the most commonly observed adverse events was infections, the reported use of antimicrobial prophylaxis was low, suggesting that a wider use of antibiotic prophylaxis could have prevented or attenuated the occurrence of those complications.
Not surprisingly, other studies conducted with either mAb or standard of care regimens in an RW setting have also met some methodological issues and, hence, failed to reproduce the same findings from the phase III RCTs [Citation33,Citation35,Citation36,Citation51–53].
Study limitations must, however, be acknowledged. Some are inherent to its observational and nonrandomized design (including the possibility of residual confounding due to unobserved treatment selection biases). Selection bias in patient enrollment cannot be discarded either. In addition, the aggregate analysis in only two treatment groups of different therapeutic options may have underestimated the results with respect to whether they had been obtained independently. Given the reality of complex treatment combinations for RRMM and relatively low patient numbers, assessing individual treatment effectiveness will require substantial cohort sizes and advanced, collaborative analytics even on an international scale.
It is important to highlight is the important recruitment failure identified, largely in Group B, especially given that up to 20.4% of the recruitment failures in this group were because patients began treatment with mAb for more than 6 months after the mAb-regimen Spanish reimbursement. Although this topic is not frequently explored, it may be related to the existing delay in our country in access to new drugs. First, there was a significant delay from drug approval at the European level (European Medicine Agency assessment) until reimbursement in Spain of approximately 22 months for orphan medicines, such as daratumumab [Citation54]. Second, there is additional access delay in those autonomous regions/hospitals that require extra approval processes. Specifically, the time taken from the designation of reimbursement until approval for prescription within the different hospitals shows great variability, ranging between median 5 and 36 months [Citation55]. These administrative barriers could have conditioned the process to incorporate a new drug, such as daratumumab, into RW practice, limiting this therapeutic alternative to more selected patients.
Conclusions
The GeminiS study offers information about the incorporation of mAb in RW practice for the treatment of patients with RRMM in a setting with other standard treatment alternatives that do not include mAbs, showing good quality and speed of the response with a similar safety profile shown in RCTs. Information from the study could help health care providers improve RRMM management in an RW setting.
Supplemental Material
Download MS Word (78.8 KB)Acknowledgements
The authors would like to thank to all the patients and their families who participated in this study, the staff members at the study sites who cared for them and all the researchers of the GeminiS study group. The authors also thank Andrea Barchino and Susana Vara (APICES, Madrid; Spain) for their support with the study setup, coordination and project management, monitoring, and statistical analysis and Fernando Rico-Villademoros (APICES, Madrid, Spain) for writing a draft of this manuscript.
Data availability statement
Data is available from corresponding author upon reasonable request. The data will be provided after its deidentification in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kazandjian D. Multiple myeloma epidemiology and survival: a unique malignancy. Semin Oncol. 2016;43(6):676–681. doi:10.1053/j.seminoncol.2016.11.004.
- Chang-Chan DYL, Ríos-Tamayo R, Rodríguez Barranco M, et al. Trends of incidence, mortality and survival of multiple myeloma in Spain. A twenty-three-year population-based study. Clin Transl Oncol. 2021;23(7):1429–1439. doi:10.1007/s12094-020-02541-1.
- Laubach JP, Mitsiades CS, Mahindra A, et al. Novel therapies in the treatment of multiple myeloma. J Natl Compr Cancer Netw. 2009;7(9):947–960. doi:10.6004/jnccn.2009.0062.
- Orlowski RZ. Novel agents for multiple myeloma to overcome resistance in phase III clinical trials. Semin Oncol. 2013;40(5):634–651. doi:10.1053/j.seminoncol.2013.07.007.
- Siegel DS, Dimopoulos MA, Ludwig H, et al. Improvement in overall survival with carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36(8):728–734. doi:10.1200/JCO.2017.76.5032.
- Dimopoulos MA, Stewart AK, Masszi T, et al. Carfilzomib-lenalidomide-dexamethasone vs lenalidomide-dexamethasone in relapsed multiple myeloma by previous treatment. Blood Cancer J. 2017;7(4):e554. doi:10.1038/bcj.2017.31.
- Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357(21):2133–2142. doi:10.1056/NEJMoa070596.
- Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. doi:10.1056/NEJMoa070594.
- Dimopoulos MA, Orlowski RZ, Facon T, et al. Retrospective matched-pairs analysis of bortezomib plus dexamethasone versus bortezomib monotherapy in relapsed multiple myeloma. Haematologica. 2015;100(1):100–106. doi:10.3324/haematol.2014.112037.
- Kouroukis TC, Baldassarre FG, Haynes AE, et al. Bortezomib in multiple myeloma: systematic review and clinical considerations. Curr Oncol. 2014;21(4):e573–e603. doi:10.3747/co.21.1798.
- Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2014;372(2):142–152. doi:10.1056/NEJMoa1411321.
- Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38. doi:10.1016/S1470-2045(15)00464-7.
- Miguel JS, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055–1066. doi:10.1016/S1470-2045(13)70380-2.
- Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149–157. doi:10.1038/leu.2011.196.
- Weinhold N, Ashby C, Rasche L, et al. Clonal selection and double-hit events involving tumor suppressor genes underlie relapse in myeloma. Blood. 2016;128(13):1735–1744. doi:10.1182/blood-2016-06-723007.
- Keats JJ, Chesi M, Egan JB, et al. Clonal competition with alternating dominance in multiple myeloma. Blood. 2012;120(5):1067–1076. doi:10.1182/blood-2012-01-405985.
- Cuesta-Mateos C, Alcaraz-Serna A, Somovilla-Crespo B, et al. Monoclonal antibody therapies for hematological malignancies: not just lineage-specific targets. Front Immunol. 2018;8:1936. doi:10.3389/fimmu.2017.01936.
- Sanchez L, Wang Y, Siegel DS, et al. Daratumumab: a first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J Hematol Oncol. 2016;9(1):51. doi:10.1186/s13045-016-0283-0.
- Xia C, Ribeiro M, Scott S, et al. Daratumumab: monoclonal antibody therapy to treat multiple myeloma. Drugs Today. 2016;52(10):551–560. doi:10.1358/2016.52.10.2543308.
- Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38 + immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–394. doi:10.1182/blood-2015-12-687749.
- Adams HC, Stevenaert F, Krejcik J, et al. High-parameter mass cytometry evaluation of relapsed/refractory multiple myeloma patients treated with daratumumab demonstrates immune modulation as a novel mechanism of action. Cytom A. 2018;95(3):279–289. doi:10.1002/cyto.a.23693.
- EMA. Summary of product characteristics. DARZALEX 20 ng/mL. 2012. Available from: https://www.ema.europa.eu/en/documents/product-information/darzalex-epar-product-information_en.pdf.
- Janssen [Data on File]. Number of patients treated with DARZALEX worldwide as of June 30th, 2022. RF-228132.
- Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–1331. doi:10.1056/NEJMoa1607751.
- Spencer A, Lentzsch S, Weisel K, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica. 2018;103(12):2079–2087. doi:10.3324/haematol.2018.194118.
- Dimopoulos MA, San-Miguel J, Belch A, et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of POLLUX. Haematologica. 2018;103(12):2088–2096. doi:10.3324/haematol.2018.194282.
- Kaufman JL, Usmani SZ, San-Miguel J, et al. Four-year follow-up of the phase 3 pollux study of daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) alone in relapsed or refractory multiple myeloma (RRMM). Blood. 2019;134(Supplement_1):1866. doi:10.1182/blood-2019-123483.
- Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766. doi:10.1056/NEJMoa1606038.
- Mateos M-V, Sonneveld P, Hungria V, et al. Daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in patients with previously treated multiple myeloma: three-year follow-up of CASTOR. Clin Lymphoma Myeloma Leuk. 2020;20(8):509–518. doi:10.1016/j.clml.2019.09.623.
- Weisel KC, Sonneveld P, Mateos M-V, et al. Efficacy and safety of daratumumab, bortezomib, and dexamethasone (D-Vd) versus bortezomib and dexamethasone (Vd) in first relapse patients (pts) with multiple myeloma (MM): four-year update of castor. Blood. 2019;134(Supplement_1):3192. doi:10.1182/blood-2019-123527.
- Dimopoulos MA, Oriol A, Nahi H, et al. P05: Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with previously treated multiple myeloma: overall survival results from the phase 3 POLLUX trial. HemaSphere. 2022;6:13. doi:10.1097/01.HS9.0000829592.26407.09.
- Sonneveld P, Chanan-Khan A, Weisel K, et al. P04: Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone alone in patients with previously treated multiple myeloma: overall survival results from the phase 3 CASTOR trial. HemaSphere. 2022;6:12. doi:10.1097/01.HS9.0000829588.31575.a9.
- Davies F, Rifkin R, Costello C, et al. Real-world comparative effectiveness of triplets containing bortezomib (B), carfilzomib (C), daratumumab (D), or ixazomib (I) in relapsed/refractory multiple myeloma (RRMM) in the US. Ann Hematol. 2021;100(9):2325–2337. doi:10.1007/s00277-021-04534-8.
- Duarte PJ, Schutz NP, Ochoa P, et al. Real-world outcomes for the treatment of relapsed/refractory multiple myeloma patients with lenalidomide-dexamethasone combinations in a Latin American country. A retrospective cohort study from grupo argentino de mieloma múltiple. Expert Rev Hematol. 2021;14(3):315–322. doi:10.1080/17474086.2021.1886073.
- Harvanová Ľ, Štulajterová V, Guman T, et al. Real-world effectiveness and safety of daratumumab, bortezomib and dexamethasone in relapsed/refractory multiple myeloma in Slovakia. Neoplasma. 2021;68(3):626–630. doi:10.4149/neo_2021_201113N1223.
- Antonioli E, Staderini M, Pilerci S, et al. Daratumumab, lenalidomide, and dexamethasone combination in relapsed/refractory myeloma patients: a real-life single-center experience. Leuk Lymphoma. 2020;61(13):3255–3258. doi:10.1080/10428194.2020.1802452.
- McMillan A, Basu S, Karunanithi K, et al. Daratumumab, bortezomib and dexamethasone (DVd) at first relapse for patients with relapsed/ refractory multiple myeloma (RRMM): a UK myeloma research alliance (UK-MRA) real-world multicentre analysis. Blood. 2021;138(Supplement 1):4120. doi:10.1182/blood-2021-147043.
- Lovas S, Varga G, Farkas P, et al. Real-world data on the efficacy and safety of daratumumab treatment in Hungarian relapsed/refractory multiple myeloma patients. Int J Hematol. 2019;110(5):559–565. doi:10.1007/s12185-019-02715-w.
- Kumar S, Paiva B, Anderson KC, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346.
- Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol. 2015;33(26):2863–2869. doi:10.1200/JCO.2015.61.2267.
- MedDRA. Medical dictionary for regulatory activities (MedDRA). Available from: https://www.meddra.org/.
- CTEP. Common terminology criteria for adverse events (CTCAE) v6.0. 2012. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_60.
- Bahlis NJ, Dimopoulos MA, White DJ, et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia. 2020;34(7):1875–1884. doi:10.1038/s41375-020-0711-6.
- Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23(11):2147–2152. doi:10.1038/leu.2009.147.
- Lahuerta J-J, Paiva B, Vidriales M-B, et al. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35(25):2900–2910. doi:10.1200/JCO.2016.69.2517.
- SEHH. Guía de mieloma múltiple grupo Español de mieloma. 2021. Available from: https://www.sehh.es/publicaciones/guias-recomendaciones/123889-guias-recomendaciones-2020-2019.
- Cedena M, Puig N, Paiva B, et al. Enfermedad mínima residual en la práctica clínica de pacientes con mieloma múltiple, C0-013. LXI Congreso nacional de la sociedad Española de hematología y hemoterapia Valencia, España, 24-26 de Octubre, 2019. Hematologíca. 2019;104(S3):1–470.
- Janssen [Data on File]. Cavex program Spain_ Affidavit. as of June 30th, 2022. RF-234641.
- Palumbo A, Rajkumar SV, San Miguel JF, et al. International myeloma working group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32(6):587–600. doi:10.1200/JCO.2013.48.7934.
- Sonneveld P, Broijl A. Treatment of relapsed and refractory multiple myeloma. Haematologica. 2016;101(4):396–406. doi:10.3324/haematol.2015.129189.
- Delforge M, Vekemans M-C, Anguille S, et al. Real-world outcomes for standard-of-care treatments in patients with relapsed/refractory multiple myeloma. Blood. 2021;138(Supplement 1):4075. doi:10.1182/blood-2021-146250.
- Katodritou E, Vadikolia C, Lalagianni C, et al. “Real-world” data on the efficacy and safety of lenalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma who were treated according to the standard clinical practice: a study of the Greek Myeloma Study Group. Ann Hematol. 2013;93(1):129–139. doi:10.1007/s00277-013-1841-y.
- Rocchi S, Tacchetti P, Pantani L, et al. A real-world efficacy and safety analysis of combined carfilzomib, lenalidomide, and dexamethasone (KRd) in relapsed/refractory multiple myeloma. Hematol Oncol. 2021;39(1):41–50. doi:10.1002/hon.2820.
- EFPIA. EFPIA patients W.A.I.T. indicator 2020 survey. 2021. Available from: https://www.efpia.eu/media/602652/efpia-patient-wait-indicator-final-250521.pdf.
- Rodríguez-Lescure A, de la Peña FA, Aranda E, et al. Study of the Spanish society of medical oncology (SEOM) on the access to oncology drugs and predictive biomarkers in Spain. Clin Transl Oncol. 2020;22(12):2253–2263. doi:10.1007/s12094-020-02366-y.