ABSTRACT
Background: Inhibitors of programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) have been used in the treatment of relapsed and refractory Hodgkin's lymphoma (R/R HL) recently. To further understand the safety and efficacy of PD-1/PD-L1 inhibitors in R/R HL, we conducted this meta-analysis.
Methods: Databases and the Clinical Registration Platforms have been systematically searched for related studies by March 2022. For safety analysis, the incidence and exhibition of any grade and grade 3 or higher adverse effects (AEs) were evaluated. Besides, severe AEs (SAEs), treatment-related deaths, and AEs leading to treatment discontinuation were summarized. The overall response rate (ORR), complete response (CR) rate, partial response (PR) rate, progression-free survival (PFS), overall survival (OS), and duration of response (DOR) were calculated for efficacy analysis. All processes were implemented mainly through the package Meta and MetaSurv of software R 4.1.2.
Results: Overall 20 studies and 1440 patients were enrolled. The pooled incidence of any grade and grade 3 or higher AEs were 92% and 26%, respectively. The pooled ORR, CR rate and PR rate were 79%, 44% and 34%, respectively. The most common AEs were neuropathy (29%), nausea (27%), pyrexia (26%), and leukopenia (25%), and the most common grade 3 or higher AEs included leukopenia (10%), infusion reaction (8%), weight gain (3%), and neutropenia (2.7%). In survival analysis, pembrolizumab monotherapy appeared to perform better compared to nivolumab monotherapy.
Conclusions: PD-1/PD-L1 inhibitors show promising efficacy and tolerable AEs in the treatment of R/R HL.
Introduction
Hodgkin's lymphoma (HL) is a rare malignancy that accounts for about 15% of all lymphomas and has a bimodal distribution, mainly affecting the young and the elderly [Citation1]. Currently, after conventional chemotherapy, 80% of young patients can achieve remission. However, 5–10% of patients do not respond to first-line treatment, and up to half of these patients can be cured by high dose therapy and autologous stem cell transplant (HDT/ASCT) [Citation2]. However, prior to the advent of targeted agents, the median overall survival (OS) of patients who relapsed after ASCT was only 12–24 months [Citation3]. Brentuximab vedotin (BV) is a novel CD30-specific antibody–drug conjugate that was approved by the U.S. Food and Drug Administration (FDA) in 2011 for HL relapsed or refractory after ASCT failure or 2 prior therapies. In the pivotal phase 2 trial of BV, the overall response rate (ORR) and complete response (CR) rate were 75% and 33%, respectively, and the estimated 5-year OS rate and progression-free survival (PFS) rate were 41% and 22%, respectively [Citation4]. However, BV-resistant HL patients have a poor prognosis and the duration of disease control is short. None of the patients remained in remission for 18 months after initiation of treatment, regardless of sequential therapy after BV [Citation5].
For relapsed and refractory HL (RR HL) patients who have failed both ASCT and BV, the invention of immune checkpoint inhibitors (ICIs), especially programmed cell death protein 1(PD-1) and programmed cell death ligand 1(PD-L1) inhibitors, provides them with new therapeutic choice. Pembrolizumab and nivolumab are two of the most dominate PD-1 inhibitors. Nivolumab was approved to treat classic HL (cHL) patients who received BV treatment after ASCT failure and still relapsed or progressed. Based on the trial KEYNOTE-087, pembrolizumab has been approved in the United States (refractory cHL in adult and pediatric patients, or those who have relapsed after three or more lines of therapy) and Europe (adults with ASCT and BV failure, or those who were not eligible for ASCT and have failed BV) [Citation6]. In China, the PD-1 inhibitor sintilimab was approved by the China Food and Drug Administration in 2018 for the treatment of relapsed and refractory cHL (RR cHL) after at least two lines of chemotherapy, and the PD-1 inhibitor camrelizumab was approved in 2019 as a third-line therapy for R/R cHL patients.
However, currently there is a lack of meta-analysis of PD-1 and PD-L1 inhibitors in R/R HL, and PD-1/PD-L1 inhibitors show unique adverse events (AEs) during treatment. So it is important and urgent to summarize these results. Therefore, the purpose of this meta-analysis was to evaluate the safety and efficacy of PD-1 and PD-L1 inhibitors in the treatment of R/R HL, and to provide evidence-based reference for clinicians. The study was registered in PROSPERO (CRD42021262408).
Methods
This systematic review was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [Citation7].
Search strategy
We searched PubMed, Embase, the Cochrane Library and Web of Science databases using free-language terms and medical subject headings (MESH) for studies until March 2022. The search terms mainly include: ‘Programmed Cell Death 1 Receptor’, ‘PD-1’, 'PD-L1’, 'Pembrolizumab/lambrolizumab/Keytruda/MK-3475’, 'Nivolumab/MDX-1106 / ONO-4538 / BMS-936558’, ‘Camrelizumab/SHR-1210’, 'tislelizumab’, ‘sintilimab’, ‘Disease, Hodgkin’, 'Hodgkin lymphoma’. In addition, clinicaltrials.gov and the International Clinical Trials Registry Platform (ICTRP) were scanned, as well as references of reviews, so as not to miss any eligible studies.
Exclusion and inclusion criteria
Inclusion criteria were as follows: (1) prospective clinical studies (including randomized controlled trials and single-arm trials); (2) a confirmed diagnosis of HL with all subtypes and stages of HL, undergoing first-line treatment, had relapsed or refractory, without restrictions; (3) studies involving patients treated with PD-1 or PD-L1inhibitors, both monotherapy and in combination with other drugs; (4) studies reporting efficacy and/or safety endpoints, including ORR, CR rate, partial response (PR) rate, PFS, OS, duration of response (DOR), and adverse effects (AEs).
The exclusion criteria were as follows: (1) sample size (enrolled R/R HL patients) less than 10; (2) retrospective or observational studies; (3) conference abstracts, reviews, commentaries, case reports, experiments with incomplete information, cellular or animal studies.
Data extraction and quality of studies
Data was collected and screened by two independent reviewers, and any disagreements between them would be resolved through discussion with a third reviewer.
Study name, study design, locations, clinical trial government number, treatment strategy, number of patients, median age, gender, follow-up time, ORR, CR, PR, PFS, OS, DOR were obtained from each included study. Any grade and grade 3 or higher AEs were evaluated if the AE was reported in at least two trials. Besides, severe AEs (SAEs), treatment-related deaths, and AEs leading to treatment discontinuation were summarized. Although original survival data were hardly accessed, we extracted data from Kaplan-Meier curves (KM curves) using software Engauge Digitizer version 10.8. The number at risk, published under the x-axis of K-M curves, together with the estimated number of censored and number of events, was recorded [Citation8].
The included single-arm studies were assessed for the risk of bias by Methodological Index for Non-Randomized Studies (MINORS). There were 12 evaluation indicators, each of which was scored from 0 to 2. The first 8 items were for studies without a control group, with a maximum score of 16, including a clearly stated aim, inclusion of consecutive patients, prospective collection of data, endpoints appropriate to the aim of the study, unbiased assessment of the study endpoint, follow-up period appropriate to the aim of the study, loss of follow up less than 5% and prospective calculation of the study size. A score of 0 indicated that it was not reported; a score of 1 indicated that it was reported but with insufficient information; and a score of 2 indicated that it was reported and sufficient information was provided. Modified Jadad scale was used to assess the risk of bias of the randomized controlled studies.
Statistical analysis
The Cochrane Q chi-square test and I2 statistic were used to evaluate the heterogeneity among studies. When I2 value > 50% and p value < 0.05, heterogeneity existed among studies and the random-effects model was used. Otherwise, the fixed-effects model was applied. Subgroup analysis and sensitivity analysis were used to analyze the sources of heterogeneity. Z test was used to compare the differences between two subgroups [Citation9]. The number of deaths divided by the total number of patients was used to access the profile of treatment-related deaths, which is simple division and not meta-analysis. The studies were sequentially removed for sensitivity analysis to assess the relative impact of each study comprehensively. Qualitative analysis of publication bias was examined by funnel plot. If no obvious asymmetry was found in classical and modified funnel plot, it indicated that there was no significant publication bias, which was further confirmed by Egger test. ORR, CR, PR, and AEs were summarized using the package Meta of software R 4.1.2. The pooled K-M curves were estimated and analyzed using the package MetaSurv of software R 4.1.2 [Citation10].
Results
Study selection and characteristics
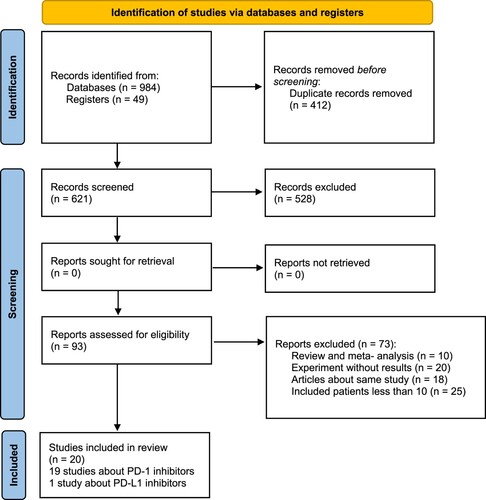
The initial search yielded 1033 relevant records in PubMed (n = 524), Embase (n = 352), the Cochrane Library (n = 4), Web of Science (n = 104), and the Clinical Registration Platforms (n = 49). Ultimately, after removing duplicates, screening titles and abstracts, and scanning the full text of potential studies, 20 prospective studies were enrolled, including 4 phase I studies [Citation11–14], 2 phase I/II studies [Citation15, Citation16], 13 phase II studies [Citation17–29] and a phase III study [Citation30]. These studies covered 2 randomized controlled trials (RCTs) [Citation25, Citation30] and 18 single-arm trials [Citation11–17, Citation19–23, Citation26, Citation28, Citation29, Citation31], and the specific screening process is shown in .
A total of 20 studies and 1440 RR HL patients were enrolled, and 19 studies were about PD-1 inhibitors with 1 study about PD-L1 inhibitors. Among the included patients, 322 of them received nivolumab monotherapy and 419 of them received pembrolizumab monotherapy. 94 patients were treated with camrelizumab monotherapy, with 70, 30, 96, and 85 patients receiving tislelizumab, avelumab, sintilimab and zimberelimab monotherapy, respectively. A total of 161 patients received nivolumab combination therapy (30 patients received nivolumab plus bendamustine, 110 were treated with nivolumab plus brentuximab vedotin and 22 were treated with nivolumab, ipilimumab and brentuximab vedotin). 69 patients were treated with pembrolizumab combination therapy (39 patients received pembrolizumab plus GVD and 30 patients were treated with pembrolizumab plus AFM13). 93 patients received camrelizumab plus decitabine therapy. The characteristics of each study were summarized in Table S1.
Safety
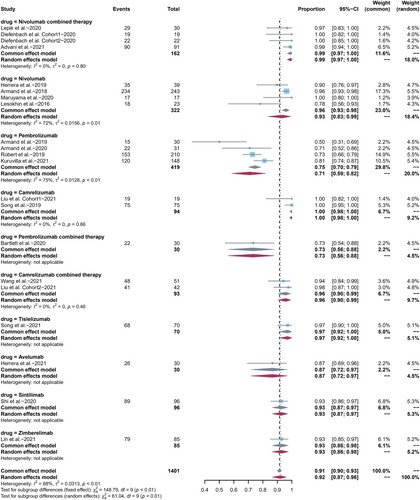
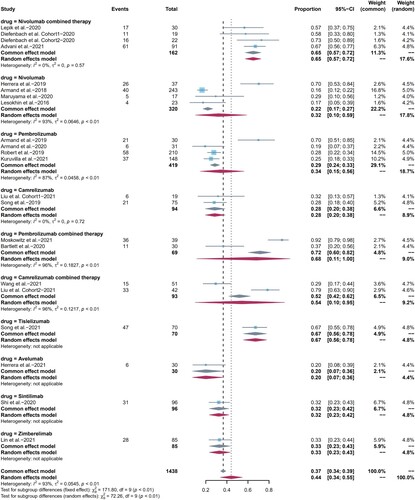
19 studies were included in the analysis of any grade AEs and all studies were included in the analysis of grade 3 or higher AEs. The pooled risk for any grade, grade 3 or higher AEs were 92% (95%CI: 87-96, I2 = 88%) () and 26% (95% CI: 21–31, I2 = 73%) ((b)), respectively. AEs can occur in various body systems, and neuropathy (29%), nausea (27%), pyrexia (26%), and leukopenia (25%) were the most common, as shown in Table S2, The most common grade 3 or higher AEs included leukopenia (10%), infusion reactions (8%), weight gain (3%), and neutropenia (2.7%), as shown in Table S2. When we performed subgroup analysis by drug type, the incidence of any grade AEs appeared to be higher in pembrolizumab combination therapy than that in pembrolizumab monotherapy (p = 0.84), but it was not statistically significant. There was no statistically significant difference between camrelizumab, nivolumab combination therapy with their corresponding monotherapy. Then, we compared camrelizumab, pembrolizumab, and nivolumab one to one, and the results showed that camrelizumab was related to higher incidence of any grade AEs compared with nivolumab (p = 0.003). On the contrary, there were no statistically significant differences among subgroups regarding grade 3 or higher AEs. In addition, treatment-related severe adverse effects (SAEs), deaths and discontinuations due to AEs were summarized in Table S3. The incidence of SAEs was 22% (95%CI 8.0-35, I2 = 91.0%), and a total of 7 treatment-related deaths (3 pneumonitis, 1 pneumonia, 1 hypovolemic shock, 1 psychomotor impairment, and 1 case with unknown cause) were reported, with an overall incidence of 0.53% (7 of 1321), while the incidence of discontinuations was 7% (95% CI 5.0–9.0, I2 = 50.0%).
Figure 2. Forest plots of pooled AEs in each subgroup. (a) Any grade AEs, (b) grade 3 or higher AEs.
We also compared the distinctions between nivolumab, pembrolizumab, and camrelizumab monotherapy one to one in most common AEs, and summarized statistically significant data. Nivolumab had a higher incidence of fatigue than pembrolizumab (p = 0). Camrelizumab had a higher incidence of pyrexia than both nivolumab (p = 0) and pembrolizumab (p = 0). Nivolumab had a lower incidence of leukopenia than pembrolizumab (p = 0.0017), while camrelizumab had a higher incidence compared to pembrolizumab (p = 0.02). Nivolumab had a higher incidence of pneumonitis than pembrolizumab (p = 0.75), but it was not statistically significant, also with only one nivolumab study involved. Besides, comparisons in the incidence of pruritus, rash, nausea, neuropathy and hypothyroidism were not statistically significant. The differences between subgroups of grade 3 or higher AEs could not be compared because of the small number of events.
Efficacy
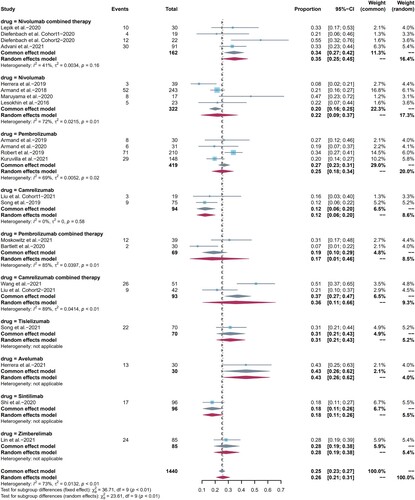
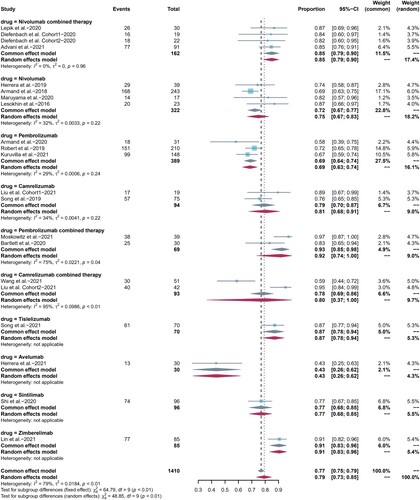
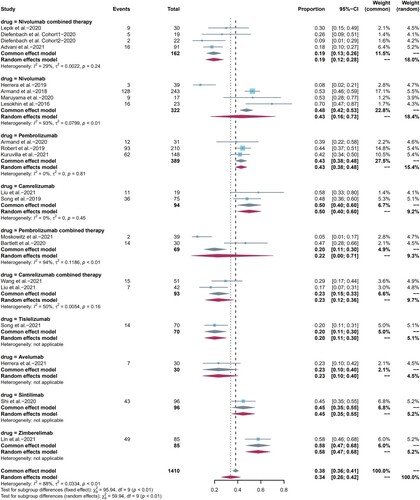
19 included studies reported ORR as clinical efficacy endpoint and the pooled ORR was 79% (95%CI 73-85, I2 = 79%). The ORR of pembrolizumab combination therapy was higher than that of pembrolizumab monotherapy (p = 0.003). We also compared differences between camrelizumab, pembrolizumab, and nivolumab monotherapy one to one, as well as differences between nivolumab and camrelizumab monotherapy and their combination regimen. All the results were not statistically significant with p > 0.05. CR was reported in all included studies, with pooled CR rate of 44% (95%CI 34-55, I2 = 93%). PR was reported in 19 included studies, and the pooled PR rate was 34% (95%CI 26-42, I2 = 88%). Summarized forest plots were shown in (a–c).
Figure 3. Forest plots of pooled efficacy indicators in each subgroup. (a) ORR, (b) CR, (c) PR.
We also analyzed the survival curves showed in the studies. 3 and 2 studies had complete PFS [Citation11, Citation19, Citation20], DOR [Citation19, Citation20] KM curves. The cumulative K-M PFS, DOR curves showed that the median PFS, DOR for patients treated with nivolumab monotherapy were 13.7 (95%CI 11.13–18.92) months and 17.3 (95%CI 11.13–18.92) months, respectively. The 2-year PFS and DOR rates were 33.5% (95%CI 22.5–49.8) and 26.6% (95%CI 12.1–58.2), respectively. They were shown in Figure S1 (a, b). Complete PFS [Citation12, Citation21, Citation22, Citation30], OS [Citation12, Citation21, Citation22], DOR [Citation12, Citation22, Citation30] KM curves of pembrolizumab monotherapy were reported in 4, 3, and 3 studies separately. The cumulative K-M PFS, DOR curves showed that the median PFS, DOR were 17.2 (95%CI 13.16–24.0) months and 18.4 (95%CI 10.17–27.35) months, respectively. The 2-year DOR and PFS rates were 43.2% (95%CI 36.0 − 51.8) and 38.4% (95%CI 25.9–56.9), respectively. The cumulative K-M OS curves showed that the median OS was not reached, and the 2-year OS was 89.8% (95%CI 86.1–93.6). They were shown in Figure S1 (c–e).
Quality assessment
18 single-arm studies were assessed with score between 12 and 14 points by the MINORS index, which is acceptable for the current meta-analysis, and two included RCTs were scored 2 and 5 points through modified Jadad scale, as shown in Table S4. Funnel plots and Egger tests showed that there was no significant publication bias, as shown in Figure S2 (a–e). Sensitivity analysis tests showed that our results were reliable, as shown in Figure S3 (a–e).
Discussion
In recent years, since the approval of nivolumab in 2014 for the treatment of advanced melanoma, at least 500 clinical trials of PD-1/PD-L1 inhibitors have been conducted to treat at least 20 types of solid and hematologic malignancies [Citation32]. We pooled 1440 patients from 20 studies to evaluate the safety and efficacy of PD-1/ PD-L1 inhibitors in RR HL. The incidence of any grade AEs was 92%, and the risk of grade 3 or higher AEs was 26%. About 79% of patients achieved overall response. We also summarized the characteristics of efficacy and AEs in subgroups divided by drug type.
Our study showed that the most common AE was neuropathy, and most patients’ manifestations were mild. 7 patients discontinued treatment due to neuropathy (2 peripheral neuropathy, 1 epilepsy, 1seisure, 1 myositis, 1 myelitis and 1 diplopia), with an incidence of approximately 0.52% (7 of 1349). The meta-analysis conducted by Si et al. [Citation33] suggested that in solid tumors, the incidence of peripheral neuropathy caused by PD-1/PD-L1 inhibitors was lower than chemotherapy. However, another study has shown that application of ICIs alone was associated with a higher risk of neurological AE, mostly in patients with solid tumors [Citation34]. In hematological malignancies, further studies are needed. At present, the mechanism of how ICIs cause neurotoxicity remains unclear. But there are some hypotheses. The removal of self-tolerance seems to be the trigger, and the cross reactivity of antigens between tumors and normal tissues contributes to the occurrence. Moreover, the shift towards pro-inflammatory profile of T lymphocytes and release of specific cytokines play important roles in the onset of neuropathy. And there still exist many possible mechanisms which need to be explored further, such as environmental insults, or pre-existing autoantibodies [Citation35–37]. In terms of timing, most neurotoxicity occurs within the first 4–6 months, typically no longer than 12 months. The most common clinical manifestations are myositis and peripheral neuropathy, and it can also invade the central nervous, such as encephalitis, cranial neuropathy [Citation38]. Once neurotoxicity is suspected, ICIs therapy should be suspended. And when it is highly suspected, corticosteroid therapy must be started immediately [Citation18]. Besides, carpal tunnel syndrome and achalasia related to autonomic neuropathy have been reported [Citation39, Citation40].
In addition, the incidence of leukopenia was high and prone to be grade 3 or higher AEs, which was observed to be primarily associated with camrelizumab treatment, consistent with previous performance of the drug in the treatment of other tumors [Citation41–43]. This may be similar to reactive cutaneous capillary hyperplasia, which distinguishes camrelizumab from other ICIs, and additional studies are needed to verify the theory. Moreover, pyrexia is another common AE, and the underlying mechanism is currently thought to be due to cytokine release and non-specific activation of the immune system, which can be treated with antipyretics (e.g. acetaminophen or NSAIDs) [Citation44]. Pneumonitis is an AE requiring high concern, with pooled incidence of 6.3%. And the pooled grade 3 or higher AEs incidence was 0.9%, with incidence of treatment-related death of 0.22% (3 out of 1321), incidence of discontinuations of 3.2% (43 out of 1349) and incidence of SAEs of 2.2% (16 of 742). It has been found that elevated anti-CD74 autoantibodies might help identify patients who tended to develop pneumonitis [Citation45], while the possibility of early diagnosis and treatment can be improved by establishing radiological patterns [Citation46]. Maruyama et al. [Citation20] also observed delayed pulmonary toxicity, which occurred up to 40 months after administration of nivolumab. When pneumonitis is highly suspected, ICIs therapy should be discontinued and immunosuppressive therapy should be started immediately. Meanwhile, the combination with antibiotics is recommended. Grade 2 or higher pneumonitis should be treated with systemic corticosteroid therapy and continuous monitoring is required. In the cases of grade 3 or higher pneumonitis, patients should be hospitalized for parenteral corticosteroid therapy with permanent discontinuation of ICIs therapy. If there is no clinical or radiographic improvement after 48 h, additional drugs such as infliximab, mycophenolate mofetil, or cyclophosphamide should be considered [Citation47]. The immunosuppressive effect of steroids seems to be contradictory to the effect of ICIs. However, early diagnosis and corticosteroid treatment are critical to reduce potentially life-threatening pneumonitis. To date, no data have shown that immunosuppression with corticosteroids decrease the efficacy of ICIs therapy [Citation48].
For RR HL patients, HDT/ASCT is still considered as the standard regimen for eligible patients who are still chemotherapy sensitive to second-line regimen, but 30% of patients are not sensitive to chemotherapy, and some patients are not suitable for ASCT due to age and severe comorbidities. BV can be applied to patients with ASCT failure. However, when the patients have failed both BV and ASCT therapy, the prognosis becomes worse. Prior to the availability of ICIs, treatment was limited to experimental drugs [Citation49]. In the RCT conducted by Kuruvilla et al. [Citation30], all patients were diagnosed as RR cHL who had previously received ASCT or were ineligible to ASCT showed that the ORR and DOR of pembrolizumab were superior to those of BV and the incidence of AEs was lower. Previous studies showed that the ORR of BV in RR cHL was 58–80% and the CR rate was 10–40% [Citation50]. Our meta-analysis suggested that PD-1/PD-L1 inhibitors seemed to perform better in patients with R/R HL, and the pooled ORR, CR and PR rates were 79%, 44% and 34%, respectively. In subgroup analysis, pembrolizumab combination therapy had a higher ORR than pembrolizumab monotherapy. In survival analysis, pembrolizumab monotherapy appeared to perform better in terms of PFS, DOR compared to nivolumab monotherapy. However, in the retrospective studies of advanced non-small cell lung cancer, there was no significant difference in PFS between nivolumab and pembrolizumab [Citation51, Citation52]. Further clinical trials are needed.
Regarding the application of ICIs as bridging therapy before ASCT, only several included studies mentioned this part and the detailed data were insufficient. So we could not analyze it statistically and make comparisons. However, some studies demonstrated that using ICIs as bridging therapy before ASCT resulted in promising outcomes [Citation16, Citation29]. For example, Advani et al. [Citation16] reported that a total of 67 patients received Nivolumab combination therapy (Nivolumab plus BV) before ASCT in their study, and the estimated 3-year PFS rate was 91%. In addition, Moskowitz et al. [Citation29] reported that all patients who received ASCT after Pembrolizumab combination therapy (Pembrolizumab plus GVD) were alive and in remission with a median follow-up of 13.5 months. Besides, in a retrospective study, Casadei et al. [Citation53] researched 13 patients who underwent ASCT after pembrolizumab or nivolumab therapy, and 11 patients achieved CR after 2 months, with rapid remission of all AEs that had occurred. For the safety concern, ASCT therapy avoids the challenges of graft-versus-host disease (GVHD) after ICIs treatment [Citation54], but a study suggested that prior anti-PD-1 therapy may be a strong risk factor for life-threatening peri-engraftment respiratory distress syndrome in lymphoma patients receiving ASCT therapy [Citation55].
However, our study had several limitations. Firstly, most of the included studies were single-arm trials with high heterogeneity, and although subgroup analysis was performed according to drug type, heterogeneity still existed in some groups, which may be related to the distinctions of patients’ previous treatment protocols, age, and treatment period. Besides, the number of patients in some subgroups was small. Secondly, the duration of follow-up varied among trials, and some of the survival analysis data included were insufficient because of limited follow-up time. In terms of safety, most studies did not describe the onset time of AEs, so we did not analyze it in the study.
Conclusions
PD-1/PD-L1 inhibitors improve the outcomes of response and survival rates with tolerable AEs in RR HL, and additional attention should be paid to the occurrence of pneumonitis clinically. However, well-designed RCTs are needed to further validate the conclusions of this meta-analysis.
Author contributions
Chenxi Sun: Visualization, Data curation, Software, Writing- Original draft preparation; Huixian Chen: Visualization, Data curation, Writing-Editing and Revision; Yongjing Wang: Conceptualization, Methodology, Writing-Editing and Revision; Chengyun Zheng: Conceptualization, Methodology, Writing-Editing and Revision; All authors participated in the research and gave final approval to the version submitted for publication.
Supplemental Material
Download Zip (10.9 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data was available within the article or its supplementary materials.
Additional information
Funding
References
- Cartwright RA, Watkins G. Epidemiology of Hodgkin's disease: a review. Hematol Oncol. 2004;22(1):11–26. doi:10.1002/hon.723.
- Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359(9323):2065–2071. doi:10.1016/S0140-6736(02)08938-9.
- Mehta-Shah N, Bartlett NL. Management of relapsed/refractory classical Hodgkin lymphoma in transplant-ineligible patients. Blood. 2018;131(15):1698–1703. doi:10.1182/blood-2017-09-772681.
- Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30(18):2183–2189. doi:10.1200/JCO.2011.38.0410.
- Cheah CY, Chihara D, Horowitz S, et al. Patients with classical Hodgkin lymphoma experiencing disease progression after treatment with brentuximab vedotin have poor outcomes. Ann Oncol. 2016;27(7):1317–1323. doi:10.1093/annonc/mdw169.
- Keeping S, Wu E, Chan K, et al. Pembrolizumab versus the standard of care for relapsed and refractory classical Hodgkin's lymphoma progressing after brentuximab vedotin: an indirect treatment comparison. Expert Rev Hematol. 2018;11(6):503–511. doi:10.1080/17474086.2018.1475226.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89–99. doi:10.1186/s13643-021-01626-4.
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9–22. doi:10.1186/1471-2288-12-9.
- Zhang T. How to compare summary estimates of different subgroups in meta-analysis. Chinese J Evid-Based Med. 2017;17(12):1465–1470.
- Combescure C, Foucher Y, Jackson D. Meta-analysis of single-arm survival studies: a distribution-free approach for estimating summary survival curves with random effects. Stat Med. 2014;33(15):2521–2537. doi:10.1002/sim.6111.
- Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients With relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698–2704. doi:10.1200/JCO.2015.65.9789.
- Armand P, Kuruvilla J, Michot JM, et al. KEYNOTE-013 4-year follow-up of pembrolizumab in classical Hodgkin lymphoma after brentuximab vedotin failure. Blood Adv. 2020;4(12):2617–2622. doi:10.1182/bloodadvances.2019001367.
- Herrera AF, Burton C, Radford J, et al. Avelumab in relapsed/refractory classical Hodgkin lymphoma: phase 1b results from the JAVELIN Hodgkins trial. Blood Adv. 2021;5(17):3387–3396. doi:10.1182/bloodadvances.2021004511.
- Bartlett NL, Herrera AF, Domingo-Domenech E, et al. A phase 1b study of AFM13 in combination with pembrolizumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2020;136(21):2401–2409. doi:10.1182/blood.2019004701.
- Diefenbach CS, Hong F, Ambinder RF, et al. Ipilimumab, nivolumab, and brentuximab vedotin combination therapies in patients with relapsed or refractory Hodgkin lymphoma: phase 1 results of an open-label, multicentre, phase 1/2 trial. Lancet Haematol. 2020;7(9):e660–ee70. doi:10.1016/S2352-3026(20)30221-0.
- Advani RH, Moskowitz AJ, Bartlett NL, et al. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood. 2021;138(6):427–438. doi:10.1182/blood.2020009178.
- Lepik KV, Mikhailova NB, Kondakova EV, et al. A study of safety and efficacy of nivolumab and bendamustine (NB) in patients With relapsed/refractory hodgkin lymphoma after nivolumab monotherapy failure. HemaSphere. 2020;4(3):e401–ee01. doi:10.1097/HS9.0000000000000401.
- Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9(6):e002435–e002467. doi:10.1136/jitc-2021-002435.
- Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-Up of the multicohort single-Arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36(14):1428–1439. doi:10.1200/JCO.2017.76.0793.
- Maruyama D, Terui Y, Yamamoto K, et al. Final results of a phase II study of nivolumab in Japanese patients with relapsed or refractory classical Hodgkin lymphoma. Jpn J Clin Oncol. 2020;50(11):1265–1273. doi:10.1093/jjco/hyaa117.
- Armand P, Chen YB, Redd RA, et al. PD-1 blockade with pembrolizumab for classical Hodgkin lymphoma after autologous stem cell transplantation. Blood. 2019;134(1):22–29. doi: 10.1182/blood.2019000215.
- Robert C, Zinzani PL, Lee HJ, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134(14):1144–1153. doi: 10.1182/blood.2019000324.
- Song Y, Gao Q, Zhang H, et al. Tislelizumab for relapsed/refractory classical hodgkin lymphoma: 3-year follow-up and correlative biomarker analysis. Clin Cancer Res. 2022;28(6):1147–1156. doi:10.1158/1078-0432.CCR-21-2023.
- Wang C, Liu Y, Dong L, et al. Efficacy of decitabine plus anti-PD-1 camrelizumab in patients with hodgkin lymphoma Who progressed or relapsed after PD-1 blockade monotherapy. Clin Cancer Res. 2021;27(10):2782–2791. doi:10.1158/1078-0432.CCR-21-0133.
- Liu Y, Wang C, Li X, et al. Improved clinical outcome in a randomized phase II study of anti-PD-1 camrelizumab plus decitabine in relapsed/refractory Hodgkin lymphoma. J Immunother Cancer. 2021;9(4):e002347–e002357. doi:10.1136/jitc-2021-002347.
- Song Y, Wu J, Chen X, et al. A single-Arm, multicenter, phase II study of camrelizumab in relapsed or refractory classical hodgkin lymphoma. Clin Cancer Res. 2019;25(24):7363–7369. doi:10.1158/1078-0432.CCR-19-1680.
- Shi Y, Su H, Song Y, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2019;6(1):e12–e19. doi:10.1016/S2352-3026(18)30192-3.
- Lin N, Zhang M, Bai H, et al. Efficacy and safety of GLS-010 (zimberelimab) in patients with relapsed or refractory classical Hodgkin lymphoma: A multicenter, single-arm, phase II study. Eur J Cancer. 2022;164:117–126. doi:10.1016/j.ejca.2021.07.021.
- Moskowitz AJ, Shah G, Schöder H, et al. Phase II trial of pembrolizumab plus gemcitabine, vinorelbine, and liposomal doxorubicin as second-line therapy for relapsed or refractory classical Hodgkin lymphoma. J Clin Oncol. 2021;39(28):3109–3117.
- Kuruvilla J, Ramchandren R, Santoro A, et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2021;22(4):512–524. doi:10.1016/S1470-2045(21)00005-X.
- Herrera AF, Chen RW, Palmer J, et al. PET-Adapted nivolumab or nivolumab plus ICE As first salvage therapy in relapsed or refractory hodgkin lymphoma. Blood. 2019;134:239–242. doi:10.1182/blood-2019-123162.
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi:10.1126/science.aar4060.
- Si Z, Zhang S, Yang X, et al. The association between the incidence risk of peripheral neuropathy and PD-1/PD-L1 inhibitors in the treatment for solid tumor patients: A systematic review and meta-analysis. Front Oncol. 2019;9:866–875. doi:10.3389/fonc.2019.00866.
- Mikami T, Liaw B, Asada M, et al. Neuroimmunological adverse events associated with immune checkpoint inhibitor: a retrospective, pharmacovigilance study using FAERS database. J Neurooncol. 2021;152(1):135–144. doi:10.1007/s11060-020-03687-2.
- Psimaras D, Velasco R, Birzu C, et al. Immune checkpoint inhibitors-induced neuromuscular toxicity: From pathogenesis to treatment. J Peripheral Nervous Syst. 2019;24(Suppl 2):S74–S85. doi:10.1111/jns.12339.
- Möhn N, Beutel G, Gutzmer R, et al. Neurological immune related adverse events associated with nivolumab, ipilimumab, and pembrolizumab therapy—Review of the literature and future outlook. J Clin Med. 2019;8(11):1777–1791. doi:10.3390/jcm8111777.
- Johnson DB, Balko JM. Biomarkers for immunotherapy toxicity: Are cytokines the answer? Clin Cancer Res. 2019;25(5):1452–1454. doi:10.1158/1078-0432.CCR-18-3858.
- Vogrig A, Muñiz-Castrillo S, Farina A, et al. How to diagnose and manage neurological toxicities of immune checkpoint inhibitors: an update. J Neurol 2022;269(3):1701–1714. doi:10.1007/s00415-021-10870-6.
- Eisenbud L, Ejadi S, Mar N. Development of carpal tunnel syndrome in association with checkpoint inhibitors. J Oncol Pharm Pract. 2021;27(3):764–765. doi:10.1177/1078155220950430.
- Montes G, Duval F, Eldani C, et al. Esophageal achalasia induced by ipilimumab and nivolumab combination: A rare neurological manifestation of immune-related autonomic neuropathy. J Immunotherapy. 2021;44(9):348–350. doi:10.1097/CJI.0000000000000381.
- Zhang B, Qi L, Wang X, et al. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun (Lond). 2020;40(12):711–720. doi:10.1002/cac2.12119.
- Tian Z, Yang Y, Yang J, et al. Safety and efficacy of PD-1 inhibitors plus chemotherapy in advanced soft tissue sarcomas: A retrospective study. Cancer Manag Res. 2020;12:1339–1346. doi:10.2147/CMAR.S237300.
- Yang W, Xing X, Yeung SJ, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10(1):e003497–e003507. doi:10.1136/jitc-2021-003497.
- Marin-Acevedo JA, Chirila RM, Dronca RS. Immune checkpoint inhibitor toxicities. Mayo Clin Proc. 2019;94(7):1321–1329. doi: 10.1016/j.mayocp.2019.03.012.
- Tahir SA, Gao J, Miura Y, et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc Natl Acad Sci USA. 2019;116(44):22246–22251. doi:10.1073/pnas.1908079116.
- Nishino M, Ramaiya NH, Awad MM, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051–6060. doi:10.1158/1078-0432.CCR-16-1320.
- Palmieri DJ, Carlino MS. Immune checkpoint inhibitor toxicity. Curr Oncol Rep. 2018;20(9):72–83. doi:10.1007/s11912-018-0718-6.
- Horvat TZ, Adel NG, Dang TO, et al. Immune-Related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients With melanoma treated With ipilimumab at memorial sloan kettering cancer center. J Clin Oncol. 2015;33(28):3193–3198. doi: 10.1200/jco.2015.60.8448.
- Vassilakopoulos TP, Asimakopoulos JV, Konstantopoulos K, et al. Optimizing outcomes in relapsed/refractory Hodgkin lymphoma: a review of current and forthcoming therapeutic strategies. Ther Adv Hematol. 2020;11:1–31. doi:10.1177/2040620720902911.
- Zinzani PL, Sasse S, Radford J, et al. Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma: An updated review of published data from the named patient program. Crit Rev Oncol Hematol. 2016;104:65–70. doi:10.1016/j.critrevonc.2016.04.019.
- Cui P, Li R, Huang Z, et al. Comparative effectiveness of pembrolizumab vs. nivolumab in patients with recurrent or advanced NSCLC. Sci Rep. 2020;10(1):13160–13166. doi:10.1038/s41598-020-70207-7.
- Torasawa M, Yoshida T, Yagishita S, et al. Nivolumab versus pembrolizumab in previously-treated advanced non-small cell lung cancer patients: A propensity-matched real-world analysis. Lung Cancer. 2022;167:49–57. doi:10.1016/j.lungcan.2022.03.020.
- Casadei B, Argnani L, Morigi A, et al. Potential survival benefit for patients receiving autologous hematopoietic stem cell transplantation after checkpoint inhibitors for relapsed/refractory Hodgkin lymphoma: A real-life experience. Hematol Oncol. 2020;38(5):737–741. doi:10.1002/hon.2803.
- Pianko MJ, Moskowitz AJ, Lesokhin AM. Immunotherapy of lymphoma and myeloma: facts and hopes. Clin Cancer Res. 2018;24(5):1002–1010. doi:10.1158/1078-0432.CCR-17-0539.
- Bai B, Wang XX, Gao Y, et al. Prior anti-PD-1 therapy as a risk factor for life-threatening peri-engraftment respiratory distress syndrome in patients undergoing autologous stem cell transplantation. Bone Marrow Transplant. 2021;56(5):1151–1158. doi:10.1038/s41409-020-01164-y.