ABSTRACT
Objective:
Polycythemia Vera (PV) is a myeloproliferative neoplasm characterized by the overproduction of red blood cells. First-line therapies are directed at lowering hematocrit levels. After the discovery of a mutation in the Janus kinase 2 (JAK2V617F), JAK2 inhibitors have been tested as second-line therapies. Despite these approaches, there is still the need for a major comprehension of the mechanisms involved in PV erythrocytosis and of more effective therapies. Angiotensin-converting enzyme (ACE) stimulates hematopoietic precursors proliferation and erythroid differentiation. We thus hypothesized that ACE inhibition could help in controlling erythrocytosis in PV.
Methods:
We assessed the clonogenic potential by colony-forming unit (CFU) assay of mononuclear cells isolated from PV JAK2 positive or JAK2 negative patients with erythrocytosis treated with enalaprilat or losartan.
Results:
Treatment with drugs led to a decrease of erythroid precursor frequency both in the presence and absence of JAK2 mutation, with a high extent in JAK2 positive cells and without affecting other types of precursors. No dose-dependent effect was observed.
Conclusions:
Our results demonstrate that ACE inhibition reduces erythroid precursor frequency, confirming the involvement of ACE in erythrocytosis despite the presence of JAK2 mutation and encouraging the hypothesis that ACE inhibitors and AT1R antagonists could help in directly managing erythrocytosis in PV.
Introduction
Polycythemia vera (PV) is a myeloproliferative neoplasm characterized by the overproduction of red blood cells, often in association with leukocytosis and thrombocytosis [Citation1]. Current first-line therapies are primarily directed at lowering hematocrit levels in order to decrease the risk of thrombotic events or cardiovascular complications, and comprise treatments with cytoreductive drugs, interferon-α and phlebotomy. After the discovery of a mutation in the Janus kinase 2 (JAK2V617F) that constitutively activates the JAK-STAT pathway and consequently cell proliferation in hematopoietic cells from PV patients, JAK2 inhibitors have been developed and tested as second-line therapies [Citation1,Citation2]. Despite these approaches, there is still the need for a major comprehension of the mechanisms involved in PV erythrocytosis in order to develop more effective therapies targeting this process.
The renin–angiotensin system (RAS) is a hormone system that primarily controls blood pressure, fluid and electrolytes, and is involved in other processes including hematopoiesis. Angiotensin-converting enzyme (ACE) is a key component of RAS and primarily converts angiotensin-I in the vasoconstrictor angiotensin-II (Ang-II) which, through its type 1 receptor (AT1R), stimulates hematopoietic precursors proliferation and erythroid differentiation [Citation3,Citation4]. Moreover, ACE is expressed on the membrane of hematopoietic stem cells and throughout all stages of hematopoiesis [Citation4,Citation5]. Beyond Ang-II, ACE has several other substrates, some of which in turn are involved in erythropoiesis, such as the peptide AcDKP and Substance P [Citation3,Citation4]. The JAK-STAT signaling pathway is activated by Ang-II/AT1R interaction as well as by many cytokines involved in hematopoiesis, therefore representing the possible link between RAS and hematopoiesis [Citation6] and suggesting that RAS itself could be an important player in pathological erythropoiesis in PV. Interestingly, besides the circulating RAS, a local RAS has been identified in the bone marrow (BM), associated with both physiological and pathological hematopoiesis [Citation7]. Since RAS components respond to drug treatment, they represent an interesting therapeutic target. ACE inhibitors (ACEi) and ATR1 antagonists (ARBs) are among the most used drugs as antihypertensive agents. Thus, considering the direct involvement of RAS and ACE in hematopoietic mechanisms and their link with the JAK/STAT pathway, we hypothesized that ACE inhibition through ACEi or ARBs could help in directly controlling erythrocytosis in patients with PV. The use of these drugs in post-renal transplant erythrocytosis (PTE) is known to lower haematocrit levels and ACEi administration is in some cases associated with anemia development [Citation8]. A retrospective study reported that, in a cohort of PV patients, the group receiving ACEi for treating hypertension underwent chemotherapy less frequently than patients treated with other antihypertensive drugs or without treatment [Citation9]. Hence, in the present study, we tested the effects in vitro of ACE inhibition or AT1R blockage by enalaprilat, the active metabolite of ACEi enalapril, and losartan respectively, on hematopoietic progenitors from JAK2V617F positive PV patients.
Materials and methods
Ethical approval
Samples were collected after patient informed consent (Ethics committee act 60/18 of the 10.07.2018).
Isolation of PBMCs from peripheral blood and bone marrow samples
Phlebotomy bags were collected from JAK2 positive PV and JAK2 negative primary erythrocytosis patients and bone marrow (BM) samples from JAK2 positive PV patients.
Mononuclear cells (MNCs) were isolated from peripheral blood (PB) or bone marrow samples after density gradient centrifugation with Lymphoprep® (STEMCELL Technologies Inc., Vancouver, Canada, ρ = 1.077 g/ml). Cells were resuspended in IMDM (SIGMA ALDRICH, St. Louis, MO, USA) + 2% FBS (Invitrogen, Waltham, MA, USA) for CFU assay or maintained in X-vivo 20 medium (Lonza, Basel, Switzerland) supplemented with human AB serum (Euroclone, Pero, Italy), thrombopoietin (TPO, 50 ng/ml; R&D Systems, Minneapolis, MN, USA), Fms-like tyrosine kinase 3 ligand (FLT3-L, 50 ng/ml; R&D Systems), stem cell factor (SCF, 0.5U; R&D Systems) and interleukin-3 (IL-3, 100U; R&D Systems) for 24 hours, with or without drugs, before CFU plating (Figure S1).
Colony-forming unit assay
Cells were seeded at 2 × 105/ml density in Methocult™ semisolid medium supplemented with cytokines (H4435, STEMCELL Technologies Inc., Vancouver, Canada) (rhEPO, rh SCF, rh GM-CSF, rh IL-3, rh IL-6, rh G-CSF) or with EPO only (H4330, STEMCELL Technologies Inc., Vancouver, Canada) for the CFU assay. On day14, burst-forming unit erythroid (BFU-E), granulocyte–erythroid–macrophage–megakaryocyte (GEMM) and granulocyte–macrophage (GM) colonies were counted based on their morphology (Figure S1).
Enalaprilat or losartan (both from Abcam, Cambridge, UK) was added at a final concentration of 0.01, 0.1, 1 or 10 µg/ml at the time of CFU plating or 24 hours before (Figure S1). Cells without treatment were used as control.
Results are presented in histograms as mean ± SEM and are a ratio to control values, corresponding to value 1 and represented by a threshold line.
Statistical analyses
Statistical analyses were performed by one sample t-test with Prism (GraphPad Software, Boston, MA, US). Statistical significance is reported as * when p ≤ 0.05, ** when p ≤ 0.01, and ***p ≤ 0.001.
Results
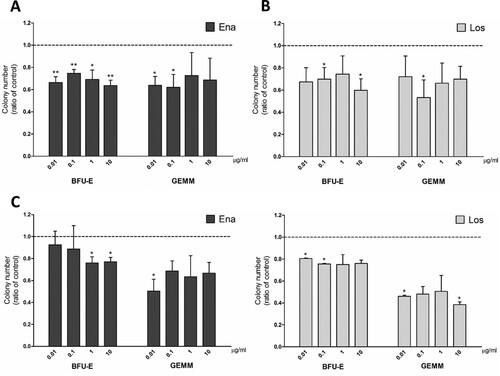
In order to investigate whether ACE inhibition could have an effect on erythrocytosis in PV, we incubated PB-MNCs from JAK2V617F positive PV patients in a medium supplemented with EPO only, thus allowing exclusively erythroid differentiation, testing a range of drug concentrations previously shown to have an effect [Citation5,Citation8]. We observed a significant reduction of erythroid precursor frequency (BFU-E) at all concentrations of enalaprilat ((A)) and 0.1 and 10 µg/ml of losartan ((B)) with respect to controls. GEMM colonies, composed of common myeloid progenitors that give rise to both erythroid and non-erythroid cells, decreased after incubation with 0.01 and 0.1 µg/ml enalaprilat and 0.1 µg/ml losartan. Instead, erythroid differentiation of cells derived from BM samples was impaired at the highest concentrations of enalaprilat (1 and 10 µg/ml) ((C)) and the lowest of losartan (0.01 and 0.1 µg/ml) ((D)).
Figure 1. Clonogenic potential of MNCs from JAK2V617F positive PV patients in medium supplemented with EPO only. CFU obtained from peripheral blood MNCs treated with enalaprilat (A) and losartan (B); n = 3. CFU obtained from bone marrow MNCs treated with enalaprilat (C) and losartan (D); n = 3.
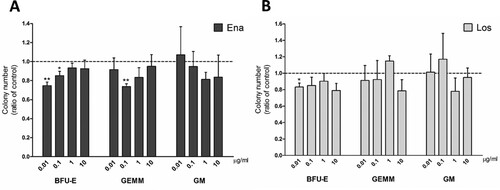
We then performed a CFU assay with a pan-myeloid medium to resemble physiological conditions and to assess whether ACE inhibition affects also non-erythroid precursor differentiation in both the presence and absence of JAK2 mutation. In PB-MNCs from JAK2V617F negative erythrocytosis patients, treatment with drugs caused a decrease of BFU-E at 0.01 and 0.1 µg/ml enalaprilat ((A)) and 0.01 µg/ml losartan ((B)). GEMM colonies significantly decreased only at 0.1 µg/ml enalaprilat concentration ((A)). No statistical variations were observed in monocytic and granulocytic precursor (CFU-GM) frequency with both drugs ((A,B)).
Figure 2. Clonogenic potential of cells isolated from JAK2V617F negative erythrocytosis patients in pan-myeloid differentiation medium. CFU obtained from peripheral blood MNCs treated with enalaprilat (A) and losartan (B), n = 4.
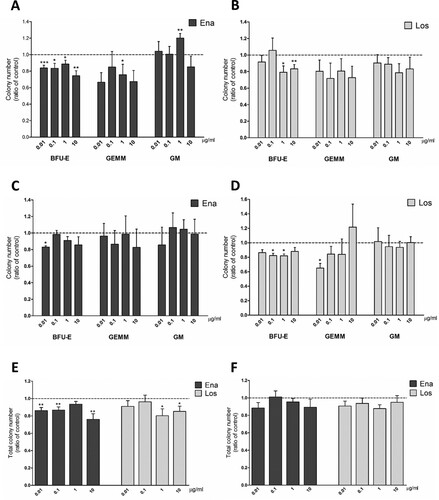
PB-MNCs of JAK2V617F positive PV patients treated with enalaprilat showed a significant reduction in BFU-E number at every dose tested. ((A)). The number of CFU-GEMM decreased significantly with respect to controls only at 1μg/ml of enalaprilat ((A)). Instead, treatment with losartan caused a significant reduction of BFU-E number only at high doses (1 and 10 µg/ml) ((B)). CFU-GM did not show any significant change with both drugs, except for 1 µg/ml enalaprilat concentration in which GM number increased ((A,B)). We tested also samples isolated from BM of PV patients. In these cells, BFU-E frequency decreased only at the lowest dose of enalaprilat ((C)) and 0.1 and 1 µg/ml losartan ((D)). No differences were observed in GEMM and GM colonies with both drugs, except for GEMM reduction with 0.01 µg/ml losartan. The reduction in erythroid colony number observed in PB samples correlates with the reduction in total colony number with both drugs, except at 1 µg/ml enalaprilat ((E)). In BM samples, this correspondence is not present, probably due to higher variability in GEMM and GM counts ((F)).
Figure 3. Clonogenic potential of MNCs from JAK2V617F positive PV patients in a medium for pan-myeloid differentiation. CFU obtained from peripheral blood MNCs treated with enalaprilat (A) and losartan (B); n = 13. CFU obtained from bone marrow MNCs treated with enalaprilat (C) and losartan (D); n = 3. Total colony number obtained with both drugs with peripheral blood MNCs (E), n=13; and bone marrow MNCs (F), n = 3.
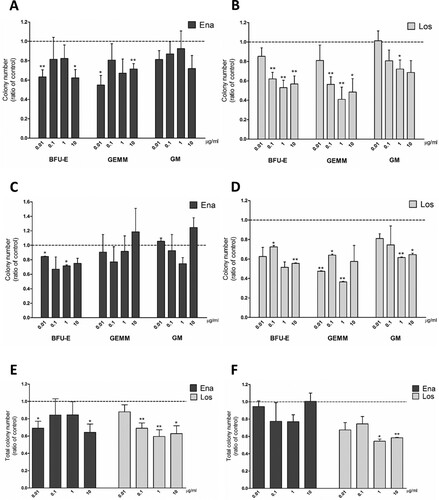
When culturing JAK2 positive PV cells for 24 hours in hematopoietic stem cells medium supplemented with drugs before CFU plating, we observed a decrease in BFU-E and CFU-GEMM frequency in PB samples with 0.01 and 10 µg/ml enalaprilat ((A)) and with every dose of losartan, except for the lowest one ((B)). Losartan at 1 µg/ml led to a decrease in CFU-GM ((B)). In BM-MNCs from PV patients, the treatment with enalaprilat led to fewer significant variations in CFU counts ((C)), whereas cells resulted more susceptible to losartan treatment at lower concentrations ((D)) with respect to PB cells. In fact, BFU-E significantly decreased at 0.01 and 1 µg/ml enalaprilat, while losartan treatment reduced the frequency of all types of precursors at different doses ((D)). In PB-MNCs total colony number decrease correlates with the effects of drug treatment on erythroid precursors ((E)), whereas in BM samples the high variability in GEMM and GM counts probably masked the decrease we observed in BFU-E, except for the highest doses of losartan ((F)).
Figure 4. Clonogenic potential of MNCs from JAK2V617F positive PV patients treated with enalaprilat and losartan for 24-h before plating in a medium for pan-myeloid differentiation. CFU obtained from peripheral blood MNCs treated for 24 hours with enalaprilat (A) and losartan (B); n = 5. CFU obtained from bone marrow MNCs treated for 24 hours with enalaprilat (C) and losartan (D); n = 3. Total colony number obtained with both drugs with peripheral blood MNCs (E), n = 5; and bone marrow MNCs (F), n = 3.
Discussion
ACE is a key player in the RAS system, involved in several physiological processes, including hematopoiesis. ACE can be targeted by drugs, routinely used for treating hypertension, thus opening interesting perspectives for the treatment of other pathological conditions. There are evidences that in post-renal transplant erythrocytosis patients these drugs, used for treating hypertension, led to lowered hematocrit. Another retrospective study reported that PV patients receiving ACEi for hypertension underwent chemotherapy less frequently [Citation9]. Thus, we investigated whether ACE inhibition could help control erythrocytosis in JAK2V617F positive PV patients testing in vitro the effects of enalaprilat and losartan on the clonogenic potential of patients cells.
Our results showed that treatment with these two drugs affects erythrocytosis, both in the presence and in absence of JAK2 mutation. In fact, we observed a reduction of erythroid progenitors both in cells of JAK2 negative and PV patients, more consistent in JAK2 positive cells. In these cells, we also observed a reduction of BFU-E and GEMM colonies, both containing erythroid precursors. Moreover, the reduction in erythroid colonies did not correspond to an increase in GM colonies or in total colony number. We thus concluded that drug treatment did not lead to erythroid progenitors differentiation into other cell types nor blocked them in early differentiation, but likely pushed them to cell death.
In vitro, we did not observe a dose-dependent effect of enalaprilat and losartan, reflecting the flat dose–response curve of ACEi and ARBs in lowering blood pressure [Citation10] observed in vivo.
After 24 hours of drug treatment in a hematopoietic stem cell medium before the CFU assay, the effect of losartan on lowering erythroid colony frequency was more evident but it also led to a decrement of CFU-GM at the highest doses. Enalaprilat gave similar results during CFU assay or after 24-hours treatment, suggesting that in liquid and semisolid media cells are equally exposed to drugs but stem cells are more susceptible to losartan than committed precursors.
In conclusion, our results confirm ACE (and RAS) involvement in pathological erythropoiesis, in both the presence and absence of JAK2 mutation, confirming that CFU assay is an effective tool to monitor cell response to drug treatment in PV patients. Furthermore, the in vitro data are in agreement with the observation that PV hypertensive patients receiving ACEi and ARBs therapy need less chemotherapy for hematocrit control, opening the possibility of their use in PV treatment. Further studies are necessary to clarify the molecular mechanism of action.
Supplemental Material
Download TIFF Image (3.5 MB)Acknowledgements
The authors are grateful to Omar Perbellini, Eros Di Bona, Maurizio Frezzato and the nurses of the hematology of Vicenza Hospital for sample collection. AB, GA: study design; AB, MB: sample collection; AB: acquisition and analysis of data; AB, MB, DC, KC, AM, GA: data interpretation; AB: manuscript writing; AB, GA: manuscript revision. All authors approved the final version.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Additional information
Funding
References
- Spivak JL. Polycythaemia vera, ruxolitinib, and hydroxyurea: where do we go now? Lancet Haematol. 2020;7:e184–e185.
- Nazha A, Khoury JD, Verstovsek S, et al. Second line therapies in polycythemia vera: what is the optimal strategy after hydroxyurea failure? Crit Rev Oncol Hematol. 2016;105:112–117.
- Shen XZ, Bernstein KE. The peptide network regulated by angiotensin converting enzyme (ACE) in hematopoiesis. Cell Cycle (Georgetown, Tex.). 2011;10:1363–1369.
- Bernstein KE, et al. A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol Rev. 2013;65:1–46.
- Jokubaitis VJ, et al. Angiotensin-converting enzyme (CD143) marks hematopoietic stem cells in human embryonic, fetal, and adult hematopoietic tissues. Blood. 2008;111:4055–4063.
- Haznedaroğlu IC, Arici M, Büyükaşik Y. A unifying hypothesis for the renin-angiotensin system and hematopoiesis: sticking the pieces together with the JAK-STAT pathway. Med Hypotheses. 2000;54:80–83.
- Hubert C, Savary K, Gasc JM, et al. The hematopoietic system: a new niche for the renin-angiotensin system. Nat Clin Pract Cardiovasc Med. 2006;3:80–85.
- Naito M, Kawashima A, Akiba T, et al. Effects of an angiotensin II receptor antagonist and angiotensin-converting enzyme inhibitors on burst forming units-erythroid in chronic hemodialysis patients. Am J Nephrol. 2003;23:287–293.
- Barbui T, Masciulli A, Ghirardi A, et al. ACE inhibitors and cytoreductive therapy in polycythemia vera. Blood. 2017;129:1226–1227.
- Taddei S. RAS inhibitors’ dose-dependent efficacy: myth or reality? Curr Med Res Opin. 2015;31:1245–1256.
