ABSTRACT
Background
In patients with tumors, inflammation, and blood disorders, hyperferritinemia has been associated with the severity of the underlying disease and is frequently accompanied by a co-occurring low platelet count or thrombocytopenia. Despite this, no established correlation has been identified between hyperferritinemia and platelet count. In this retrospective, double-center study, we sought to describe the prevalence and severity of thrombocytopenia in patients with hyperferritinemia.
Study and design
A total of 901 samples were enrolled in this study, all of which had significantly high ferritin levels (>2000 μg/L) between January 2019 and June 2021. We analyzed the general distribution, incidence of thrombocytopenia in patients with hyperferritinemia, and the relationship between ferritin level and platelet count. p-values < 0.05 were considered statistically significant.
Results
The total incidence of thrombocytopenia in patients with hyperferritinemia was 64.7%. Hematological diseases were the most frequent cause of hyperferritinemia (43.1%), followed by solid tumors (29.5%) and infectious diseases (11.7%). Patients with thrombocytopenia (<150 × 109/L) had significantly higher ferritin levels than those with platelet counts exceeding 150 × 109/L, with median ferritin levels of 4011 and 3221 μg/L, respectively (P < 0.001). Additionally, the results showed that the incidence of thrombocytopenia was higher in hematological patients with chronic transfusion than in those without chronic blood transfusions (93% vs 69%).
Conclusions
In conclusion, our results suggest that hematological diseases are the most common cause of hyperferritinemia and that patients with chronic blood transfusions are more susceptible to thrombocytopenia. Elevated ferritin levels may act as a trigger for thrombocytopenia.
Introduction
Ferritin, a molecule that binds to iron, is critical in the storage and release of iron in the body. Severe hyperferritinemia indicates an overload of iron and reflects the severity and poor prognosis of various diseases, including tumors, inflammation, and blood disorders [Citation1–3], which are often accompanied by low platelet counts. Lodha et al. [Citation4] conducted a study on 200 dengue patients, measuring serum ferritin levels at initial presentation and monitoring platelet counts serially. The results showed that a high serum ferritin level upon initial contact could indicate potentially severe disease, with levels ≥593 ng/mL helping to identify patients at risk for developing severe thrombocytopenia and aiding doctors in prognosis. Thrombocytopenia and elevated ferritin levels have also been observed in critically ill COVID-19 patients, indicating poor prognosis [Citation5,Citation6]. Cheong et al. [Citation7] conducted a study of 96 patients with myelodysplastic syndromes (MDS) or aplastic anemia (AA) and reported that elevated platelet counts were associated with a significant decrease in serum ferritin and liver iron concentrations. Thrombocytopenia has also been observed in patients receiving gastrointestinal iron supplementation [Citation8,Citation9]. In patients with acquired iron overload, such as those who receive blood transfusions and/or have abnormal erythropoiesis, oral iron chelators can increase platelet counts [Citation10]. To sum up, iron reserves may be related to platelet counts in certain patients.
However, the extent of the association between elevated serum ferritin levels and platelet counts has been inadequately documented in the scientific literature, particularly in studies involving large sample sizes. Therefore, in this retrospective study, we sought to investigate the prevalence and severity of thrombocytopenia in patients with elevated serum ferritin levels and examine the relationship between hyperferritinemia and platelet counts.
Materials and methods
The patients
We retrospectively reviewed patients with significantly elevated ferritin levels admitted between January 2019 and June 2021 at Zhejiang Provincial Hospital of Traditional Chinese Medicine and Second Affiliated Hospital, Zhejiang University School of Medicine. Inclusion criteria: Patients with elevated ferritin (>2000 μg/L) were reviewed, for patients having multiple ferritin levels greater than 2000 μg/L, the highest value was used. Exclusion criteria: Possible platelet transfusion history ≤1 month, lack of platelet count data within three days, usage of iron supplementation or iron chelators agent within 6 months. Finally, 901 samples were included in the current study.
Study design
We collected medical records (diagnosis, sex, age, platelet count, ferritin content, and blood transfusion history) to analyze the distribution profile of patients with hyperferritinemia, the incidence of thrombocytopenia in patients with hyperferritinemia and the relationship between ferritin level and platelet count. The serum ferritin levels were determined to use Roche E601 automatic electrochemical immunoluminescence apparatus (Basel, Switzerland).
Thrombocytopenia criteria [Citation11]: mild 101–150 × 109/L, moderate 51–100 × 109/L, and severe 21–50 × 109/L, extreme severe <20 × 109/L. Our clinical laboratory considers a normal serum ferritin to be 11.0–306.8 μg/L (female) and 23.9–336.2 μg/L (male). Ferritin levels >306.8 μg/L in females and >336.2 μg/L in males are considered as elevated ferritin. Having a higher ferritin cutoff value is typically considered more valuable in predicting the presence of inflammatory diseases. According to previous reports [Citation1,Citation2], ferritin levels ‘cutoff’ (2000 μg/L) was used in statistical analysis as screening criteria for severe hyperferritinemia [Citation2]. Based on serum ferritin levels, data were divided into five groups: 2001–4000 μg/L, 4001–6000 μg/L, 6001–8000 μg/L, 8001–10,000 μg/L and >10,000 μg/L.
Statistical analysis
The distribution of continuous data was assessed using the Kolmogorov–Smirnov test. Descriptive statistics for continuous and categorical variables were summarized as medians (range: min–max), and numbers (percentages), respectively. One-way analysis of variance (ANOVA) model was used to reveal the significant differences between the compared groups. The Pearson's correlation coefficient was used to measure the strength of a linear association between two variables, where the value r = 1 means a perfect positive correlation and r = −1 means a perfect negative correlation. Statistical calculations were carried out using SPSS software (v.18.0) (Chicago, IL, USA). p-values < 0.05 were considered statistically significant.
Results
Characteristics of patients with hyperferritinemia
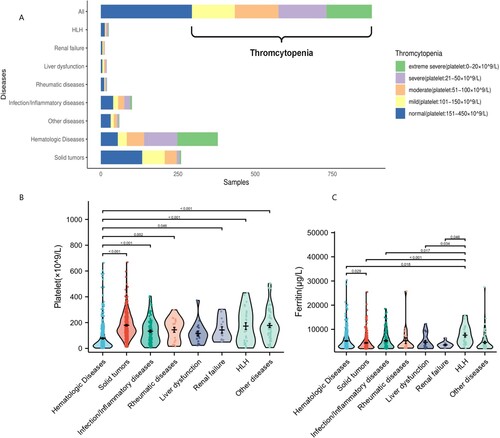
A total of 901 samples with ferritin levels >2000 μg/L were included in this study, including 532 (59.1%) males and 369 (41.0%) females. The proportion of males was slightly higher, the majority of patients were middle-aged and elderly, the median age was 58.0 (range: 43–68) years. Diseases associated with hyperferritinemia were divided into the following groups: solid tumors, hemophagocytic syndrome (HLH), hematologic diseases, liver diseases, kidney diseases, infectious or inflammatory diseases, rheumatic immune diseases, and other diseases (hypertension, diabetes, cerebral infarction, chronic obstructive pulmonary disease, cerebral hemorrhage, and myocardial infarction). Statistical analysis showed that hematologic diseases (n = 388, 43.1%, 95% CI: 39.8–46.4%) were the most common cause of hyperferritinemia, followed by solid tumors (n = 266, 29.5%, 95% CI: 26.6–32.6%), infectious diseases(n = 105, 11.7%, 95% CI: 9.7–13.9%), hemophagocytic lymphohistiocytosis (HLH) (n = 26, 2.9%, 95% CI:1.9–4.2%), rheumatic immune diseases (n = 20, 2.2%, 95% CI: 1.4–3.4%), liver disease (n = 19, 2.1%, 95% CI: 1.4–3.3%) and kidney disease (n = 13, 1.4%, 95% CI: 0.8–2.4%) ().
Table 1. Characteristics of patients with hyperferritinemia.
Prevalence and severity of thrombocytopenia in patients with hyperferritinemia
To explore the influence of hyperferritinemia-associated diseases on platelet count, at first, we analyzed the incidence of thrombocytopenia in patients with hyperferritinemia. Statistically, 583/901 (64.7%, 95% CI: 61.5–67.8%) of patients with hyperferritinemia were associated with thrombocytopenia (<150 × 109/L). Then, we analyzed the incidence of thrombocytopenia in different diseases with hyperferritinemia. As shown in (A), different diseases have different incidence. The highest proportion of thrombocytopenia occurred in patients with hematologic diseases (324/388, 83.5%, 95% CI: 79.5–86.9%), followed by liver disease (14/19, 73.7%, 95% CI: 51.2–88.2%), kidney diseases (8/13, 61.5%, 95% CI: 35.5–82.3%), infectious diseases (61/105, 58.1%, 95% CI: 48.5–67.1%), HLH (13/26, 50.0%, 95% CI: 32.1–67.9%), solid tumors (126/266, 47.3%, 95% CI: 41.4–53.4%) and rheumatic immune diseases (9/20, 45.0%, 95% CI: 25.8–65.8%). The levels of platelet in hematologic diseases were significantly lower than those in other groups ((B)), but there was no significant difference in ferritin levels ((C)). Finally, we calculate the ferritin levels and platelet counts in these patients with different diseases, the median platelet count with hematologic diseases was significantly lower than other subgroups (p < 0.01, hematologic diseases vs. solid tumors, rheumatic diseases, HLH, infectious diseases) ((B)). However, the ferritin levels of HLH patients was significantly higher than other subgroups (p < 0.05, HLH vs. solid tumors, hematologic diseases, (C)).
Ferritin levels affect the platelet count of patients
To further determine whether ferritin levels have an effect on platelet count, we compared the ferritin levels in different groups of thrombocytopenia. Data analysis indicated that patients with severe thrombocytopenia (<50 × 109/L) had significantly higher levels of ferritin than those with normal platelets. (Extrme severe group vs normal group, 4097 vs 3874, p < 0.001.). Severe group vs normal group, 4115 vs 3874, p < 0.01 ((A)). Based on serum ferritin levels, data were divided into five groups: 2001–4000 μg/L (n = 488), 4001–6000 μg/L (n = 190), 6001–8000 μg/L (n = 89), 8001–10,000 μg/L (n = 55) and >10,000 μg/L (n = 79). The results demonstrated that patients with higher ferritin levels (>8000 μg/L) had significantly different platelet counts from those with lower ferritin levels (8001-10000 μg/L vs 2001–4000 μg/L, 50 vs 124, p < 0.05; >10000 μg/L vs 2001–4000 μg/L, 57 vs 124, p < 0.05) ((B)).
Figure 2. Ferritin and platelet classification in patients with hyperferritinemia. (A) Ferritin level of hyperferritinemia with different concentration gradients of platelet counts. (B) The platelet count of hyperferritinemia with different concentration gradients of ferritin levels. (C) The incidence of thrombocytopenia with different concentration gradients of ferritin levels.
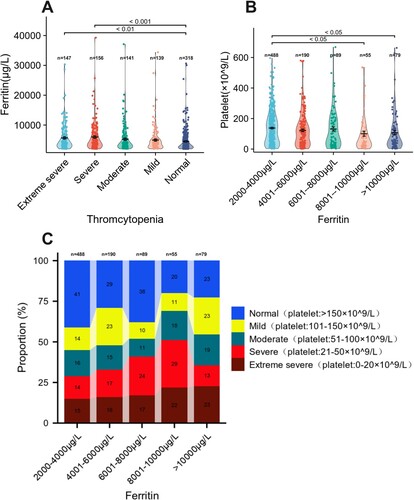
Then, we analyze the incidence of thrombocytopenia with different concentration of ferritin levels when the ferritin levels were 2001–4000 μg/L, 4001–6000 μg/L, 6001–8000 μg/L, the incidence of thrombocytopenia was 59% (288/488, 95% CI: 54.6–63.3%), 71%(135/190, 95% CI: 64.2–77.0%), 62%(55/89, 95% CI: 51.4–71.2%), respectively, see (C). While the ferritin levels were 8001–10000 μg/L and exceeded 10000 μg/L, the incidence of thrombocytopenia was much higher (80% (44/55, 95% CI: 67.6–88.5%) and 77% (61/79, 95% CI: 66.8–85.1%)), (C). With increasing ferritin levels, the incidence of severe thrombocytopenia (platelets <50 × 109/L) elevated significantly, especially when the ferritin levels exceeded 8000 μg/L, suggesting that ferritin >8000 might have a significant effect on platelets counts. And, our data showed that the platelet counts had a weak negative correlation with ferritin levels (Spearman Correlation r = −0.161, p < 0.001, (C)). And the results revealed that the patients with higher levels of serum ferritin were more likely to have thrombocytopenia and results were consistent in both men and women (p < 0.05).
Thrombocytopenia in hematologic diseases with chronic transfusion and non-chronic blood transfusion
Chronic transfusion therapy is generally reserved for patients with hematologic disease and chronic blood transfusions are known to cause iron overload. To analyze the influence of chronic blood transfusion on the platelet counts of hematologic diseases patients. The patients with hematologic diseases were divided into two groups (chronic transfusion and non-chronic blood transfusion) (). Then we found the chronic transfusion patients had significantly higher ferritin levels than no-chronic transfusion patients (ferritin: 4937 μg/L vs. 3565 μg/L, p < 0.001, (A)). Further analysis showed there was no difference in ferritin levels among patients with normal platelet counts (4137 μg/L vs. 2974 μg/L, p > 0.05, (B)), whereas in patients with thrombocytopenia, ferritin levels were significantly higher in the chronic transfusion group than in the non-chronic blood transfusion.(ferritin: 5076 μg/L vs. 3490 μg/L, p < 0.001, (B)), and the patients with chronic transfusion had significantly lower platelet count. The platelet counts of patients with chronic transfusion and non-chronic transfusion were 28 × 109/L and 55 × 109/L, respectively (p < 0.001, (C)). We further analyzed the incidence of thrombocytopenia within the two groups, and found chronic transfusion patients were more likely to have thrombocytopenia, the incidence of thrombocytopenia of chronic blood transfusion patients were 93%(215/231, 95% CI: 89.0–95.7%) respectively, while in non-chronic blood transfusion patients were 69% (109/157, 95% CI: 61.8–76.1%). And found chronic transfusion patients were more likely to have thrombocytopenia. When the ferritin levels were 2001–4000, 4001–6000, 6001–8000, 8001–10,000, >10,000 μg/L, the incidence of thrombocytopenia of chronic blood transfusion patients was 90% (82/91, 95% CI: 82.3–94.1%), 96% (47/49, 95% CI: 86.3–98.9%), 90% (35/39, 95% CI: 76.4–95.9%), 96% (24/25, 95% CI: 80.5–99.3%), 100% (27/27, 95% CI: 84.5–100%) respectively, while in non-chronic blood transfusion patients were 75.5% (80/106, 95% CI: 66.5–82.7%), 51.5% (17/33, 95% CI: 35.2–67.5%), 33.3% (2/6, 95% CI: 9.7–70%), 66.7% (2/3, 95% CI: 20.8–93.9%), 73% (8/11, 95% CI: 43.4–90.3%) respectively ((D)).
Figure 3. The ferritin levels and platelet counts in hematologic diseases with chronic transfusion or non-chronic transfusion. (A) Ferritin levels in patients with hematologic diseases compared with chronic transfusion or non-chronic transfusion. (B) Ferritin level in hematologic diseases patients with thromcytopenia compared with non-thromcytopenia. (C) Platelet counts in patients with hematologic diseases compared with chronic transfusion or non-chronic transfusion. (D) The incidence of thrombocytopenia in hematologic diseases with chronic transfusion or non-chronic transfusion.
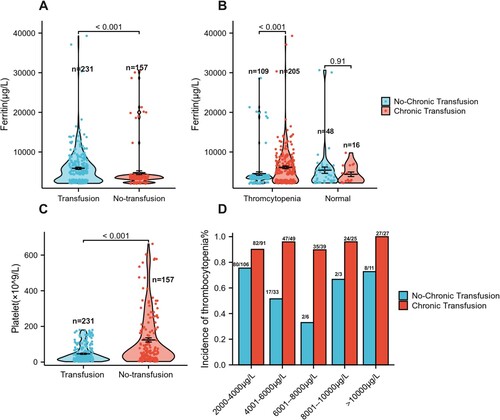
Table 2. Characteristics of hyperferritinemia patients with chronic transfusion.
Discussion
Hyperferritinemia may indicate an increase in iron reserves, but it is more universal in disease with acute phase reactions and when damaged cells release ferritin into peripheral blood. It may also be the result of various stimuli that increase the synthesis and/or secretion of ferritin. French researchers [Citation12] conducted a retrospective study and found that hematological malignancies were the most common cause of hyperferritinemia, followed by serious infections. Studies by Meredith Schaffner showed that liver dysfunction and infection were the most common diagnoses [Citation1]. Another study of 86 patients in the United States with serum ferritin greater than 10,000 μg/L showed that the majority of the cases in the hyperferritinemia population were due to prolonged blood transfusion (35%), followed by liver disease (27%) and hematological malignancies (16%) [Citation2]. Our study of 901 samples found that hematologic diseases (43.1%) were the most common cause of hyperferritinemia, followed by solid tumors (29.5%), infectious diseases (11.7%), this result is basically consistent with related reports.
Hyperferritinemia and thrombocytopenia often co-exist in the patients such as hematological diseases, solid tumor, infectious diseases. Wen-Jing Miao analyzed the clinical data of 35 patients with thrombocytopenia after allo-HSCT(allogeneic hematopoietic stem cell transplantation)and results showed that the serum ferritin level before transplantation was a risk factor for prolonged thrombocytopenia [Citation13]. We studied the prevalence of thrombocytopenia in hyperferritinemia patients and the relationship between the platelet count and the ferritin level and the results show that the overall incidence of thrombocytopenia was 65% in patients with hyperferritinemia and the incidence of thrombocytopenia was as high as 80% when the concentration of serum ferritin was greater than 8000 μg/L, while when the concentration of serum ferritin was <8000 μg/L, the incidence was 59–71%. These results suggest that incidence of thrombocytopenia is associated with elevated serum ferritin levels and the patients with higher levels of serum ferritin were more likely to have thrombocytopenia.
Then we analyzed the correlation between the degree of serum ferritin elevation and platelet count. Based on serum ferritin levels, data were divided into five groups: 2001–4000, 4001–6000, 6001–8000, 8001–10,000 and >10,000 μg/L and the results indicated that patients with extremely high ferritin (ferritin >8000 μg/L) had lower platelet counts compared to mild hyperferritinemia (2001–4000 μg/L).
Our results from disease classification showed that the different diseases had varied effects on the incidence of thrombocytopenia, patients with hematologic diseases had the highest incidence of thrombocytopenia about 84.5%, followed by liver dysfunction and the rheumatic immune diseases had the lowest incidence of thrombocytopenia about 45.0%. Patients with hematologic diseases had the lowest platelet count but the ferritin level was not. Further, the patients with hematologic diseases were divided into two groups as chronic transfusion and non-chronic blood transfusion and we analyze the influence of chronic blood transfusion on the results. The incidence and severity of thrombocytopenia were significantly higher in patients with hematological diseases who received chronic transfusions and but the platelet counts have a week negative correlation with ferritin levels. Meantime, the results showed the two groups had the same ferritin levels but not the same platelet counts levels and platelets counts were lower in the chronic blood transfusion group. It means platelet count is not only dependent on ferritin levels but also related to the type of disease and treatment, and the patients with chronic blood transfusions patients were more prone to severe thrombocytopenia than other hyperferritinemia patients.
The pathogenesis of iron-related thrombocytopenia is unclear [Citation14]. A study in vitro found that excessive iron intake damages normal hematopoietic cells, suggesting that the deleterious effects of iron may be due to its interference with hematopoietic stem cells-progenitor cells and hematopoietic differentiation mediated by reactive oxygen species accumulation [Citation15]. Some researchers suggest the high reactive oxygen species (ROS) state of the microstructure caused by iron overload damages the protein, lipids, and DNA in mitochondria and ATP production, resulting in energy pressure, that ultimately leads to cell apoptosis [Citation16,Citation17].
The pathogenesis of thrombocytopenia is closely related to immunity, and abnormal activation of humoral and cellular immunity in diseases such as infectious or inflammatory diseases, rheumatic immune diseases, contributes to the pro-inflammatory features and progression of thrombocytopenia in patients with thrombocytopenia. The balance of M1 and M2 macrophages plays a key role in maintaining normal immune function and has been shown to be involved in the pathogenesis of ITP [Citation18]. Recently, a study showed an increase in the number of M1 macrophages in the spleen and a decrease in the number of M2 macrophages in ITP patients compared to non-ITP control patients through the confocal laser scanning microscopy [Citation19]. Zhao et al. [Citation20] believed that M1 macrophages play a key role in regulating thrombosis and platelet production. M1 polarization is characterized by the production of large amounts of pro-inflammatory cytokines, such as Th1 and interferon INF-γ, which in turn damage megakaryocytes and affect platelet production [Citation21,Citation22]. Another study showed that intracellular iron accumulation induced M1-type macrophage polarization [Citation23]. The exact mechanisms underlying this relationship between hyperferritinemia and thrombocytopenia are not yet fully understood and further research is needed to clarify this relationship.
On the mechanism of serum ferritin in thrombocytopenia, a study in vitro found that excessive iron intake damages normal hematopoietic cells, suggesting that the deleterious effects of iron may be due to its interference with hematopoietic stem cells-progenitor cells and hematopoietic differentiation mediated by reactive oxygen species accumulation [Citation24]. Blood transfusion therapy can improve anemia symptoms, but over a period, chronic blood transfusion is known to be a risk factor for iron overload [Citation25]. Our data also confirmed that ferritin levels in chronic transfusion patients were higher than that in non-chronic transfusion patients. Breccia et al. [Citation26] reported oral iron chelator could obtain hematologic improvements and increases the platelet count in myelodysplastic syndrome (MDS) patients with acquired iron overload such as blood transfusion and/or abnormal erythropoiesis. So we suggested that chronic transfusion patients were need to be monitored for the platelet count and to use iron chelator promptly to prevent the risk of bleeding. It's important to note the limitations of this study, it is a retrospective study with a moderate sample size may not be able to fully capture all aspects of the relationship between hyperferritinemia and thrombocytopenia. A larger prospective study could potentially provide more data and confirm the findings of this study, and further research is needed to determine the prevalence and mechanisms of thrombocytopenia in hyperferritinemia in other patient groups.
In summary, hematologic diseases were the most common cause of hyperferritinemia and patients with hyperferritinemia are more likely to have reduced platelet counts. Increased ferritin levels may be an inducer of thrombocytopenia, especially in chronic transfusion patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Schaffner M, Rosenstein L, Ballas Z, et al. Significance of hyperferritinemia in hospitalized adults. Am J Med Sci. 2017;354(2):152–158.
- Belfeki N, Strazzulla A, Picque M, et al. Extreme hyperferritinemia: etiological spectrum and impact on prognosis. Reumatismo. 2020;71(4):199–202.
- Rosário C, Zandman-Goddard G, Meyron-Holtz EG, et al. The hyperferritinemic syndrome: macrophage activation syndrome, still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11:185.
- Lodha A, Pillai A, Reddy P, et al. Using first-contact serum ferritin to predict severe thrombocytopenia in dengue patients: determination and validation in independent cohorts. Infect Dis. 2022;54(6):425–430.
- Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99(6):1205–1208.
- Gómez-Pastora J, Weigand M, Kim J, et al. Hyperferritinemia in critically ill COVID-19 patients - is ferritin the product of inflammation or a pathogenic mediator? ClinChimActa. 2020;509:249–251.
- Cheong JW, Kim HJ, Lee KH, et al. Korean society of hematology acute myeloid leukemia/myelodysplastic syndrome working party. Deferasirox improves hematologic and hepatic function with effective reduction of serum ferritin and liver iron concentration in transfusional iron overload patients with myelodysplastic syndrome or aplastic anemia. Transfusion. 2014;54(6):1542–1551.
- Huscenot T, Luc D, Ballon OW, et al. Iron deficiency anemia, a rare and potentially underestimated cause of thrombocytopenia and a differential diagnosis of immune thrombocytopenia (ITP): results from a retrospective case-controlled study. Blood. 2018;132(Suppl 1):4984.
- Giordano L, Llanos-Chea A, Monde A, et al. Recurrent severe iron deficiency anemia and thrombocytopenia in an adolescent male. J Pediatr Hematol Oncol. 2019;41(2):e116–e118.
- Breccia M, Voso MT, Aloe Spiriti MA, et al. An increase in hemoglobin, platelets and white blood cells levels by iron chelation as single treatment in multitransfused patients with myelodysplastic syndromes: clinical evidences and possible biological mechanisms. Ann Hematol. 2015;94(5):771–777.
- Getawa S, Aynalem M, Bayleyegn B, et al. The global prevalence of thrombocytopenia among HIV-infected adults: A systematic review and meta-analysis. Int J Infect Dis. 2021;105:495–504.
- Sackett K, Cunderlik M, Sahni N, et al. Extreme hyperferritinemia: causes and impact on diagnostic reasoning. Am J Clin Pathol. 2016;145(5):646–650.
- Miao WJ, Qi JQ, Song BQ, et al. Clinical significance of pretransplant serum ferritin level in patients with prolonged thrombocytopenia after allogeneic hematopoietic stem cell transplantation. Zhongguo Shi Yan Xue Ye XueZaZhi. 2021;29(3):869–875.
- Babikir M, Ahmad R, Soliman A, et al. Iron-Induced thrombocytopenia: a mini-review of the literature and suggested mechanisms. Cureus. 2020;12(9):e10201.
- Tanaka H, Espinoza JL, Fujiwara R, et al. Excessive reactive iron impairs hematopoiesis by affecting both immature hematopoietic cells and stromal cells. Cells. 2019;8(3):226.
- Zheng Q, Zhao Y, Guo J, et al. Iron overload promotes mitochondrial fragmentation in mesenchymal stromal cells frommyelodysplastic syndrome patients through activation of the AMPK/MFF/Drp1 pathway. Cell Death Dis. 2018;9(5):515.
- Sousa L, Oliveira MM, Pessôa MTC, et al. Iron overload: effects on cellular biochemistry. Clin Chim Acta. 2020;504:180–189.
- Chang Y, Chen X, Tian Y, et al. Downregulation of microRNA-155-5p prevents immune thrombocytopenia by promoting macrophage M2 polarization via the SOCS1-dependent PD1/ PDL1 pathway. Life Sci. 2020;257:118057.
- Weigelin B, Bakker G-J, Friedl P. Third harmonic generation microscopy of cells and tissue organization. J. Cell Sci. 2016;129:245–255.
- Zhao HY, Zhang YY, Xing T, et al. M2 macrophages, but not M1 macrophages, support megakaryopoiesis by upregulating PI3K-AKT pathway activity. Sig Transduct Target Ther. 2021;6:234.
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737.
- Zhao Y, Xu P, Guo L, et al. Tumor necrosis factor-α blockade corrects monocyte/macrophage imbalance in primary immune thrombocytopenia. Thromb Haemost. 2021;121(6):767–781.
- Zhou Y, Que KT, Zhang Z, et al. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med. 2018;7(8):4012–4022.
- Zheng Q, Zhao Y, Guo J, et al. Iron overload promotes mitochondrial fragmentation in mesenchymal stromal cells from myelodysplastic syndrome patients through activation of the AMPK/MFF/Drp1 pathway. Cell Death Dis. 2018;9(5):515.
- Bauduer F, Recanzone H. Transfusional iron overload in heavily transfused patients: real-life data from a 10-year retrospective study of 611 cases managed in a French general hospital. Transfus Clin Biol. 2022;29(3):236–242.
- Breccia M, Voso MT, Aloe Spiriti MA, et al. An increase in hemoglobin, platelets and white blood cells levels by iron chelation as single treatment in multitransfused patients with myelodysplastic syndromes: clinical evidences and possible biological mechanisms. Ann Hematol. 2015;94(5):771–777.