ABSTRACT
Objectives
Multiple myeloma (MM) remains an incurable disease despite advances in treatment options. Recently, selinexor has shown promising efficacy for relapsed/refractory multiple myeloma (RRMM), whereas its optimal timing and drug combination remain unclear. In order to assess the various regimens that incorporate selinexor, a systematic review and meta-analysis was conducted.
Methods
Clinical trials and real-world studies involving MM patients treated with selinexor were included. Pooled risk ratio (RR) was calculated to compare the rates, along with a 95% confidence interval (CI) and concurrent p-value assessment. A random-effects model was employed to provide a more conservative evaluation.
Results
A total of 16 studies enrolling 817 patients were reviewed. The usage of selinexor as the fifth-line or prior therapy achieved a higher objective response rate (ORR) (65.9% versus 23.4%, p < 0.01) and longer pooled progression-free survival (PFS) (median: 12.5 months versus 2.9 months, p < 0.01) than those after the fifth-line usage. In addition, early usage also resulted in a consistent trend of pooled overall survival (median: 22.7 months versus 8.9 months, p = 0.26), compared with post-fifth-line usage. Selinexor and dexamethasone (Xd) plus either protease inhibitors (PIs) or immunomodulatory drugs (IMiDs) achieved better ORRs than the Xd-only regimen for RRMM, with ORRs of 56.1%, 52.5% and 24.6%, respectively (p < 0.01).
Conclusion
In conclusion, using selinexor as the fifth-line or prior therapy had a beneficial impact on RRMM. The regimen of Xd plus PIs or IMiDs was recommended.
Introduction
Despite the introduction of innovative therapeutic interventions, multiple myeloma (MM) continues to be an uncurable hematological cancer [Citation1]. MM cells usually develop by-pass cell signaling after anti-MM treatment and escape the drug-induced cancer containment with the impact of the pro-MM tumor microenvironment and the emergence of pivotal genetic alterations in MM cells [Citation2]. Thus, relapsed/refractory multiple myeloma (RRMM) is commonly seen in almost all patients. To cure the disease, one concept for MM management is to target multiple key-cell signaling in MM cells, as well as to target the tumor microenvironment, using the combination or integration of treatment with diverse mechanisms of action [Citation3].
In July 2019, selinexor was initially approved by the United States Food & Drug Administration (FDA) to be used in combination with dexamethasone (Xd) as a fifth-line therapy in RRMM patients who were resistant to standard regimens of proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs) or anti-CD38 monoclonal antibodies. In December 2020, selinexor in combination with bortezomib and dexamethasone (XVd) as the second-line therapy or beyond for the treatment of adult MM patients was approved by FDA based on the BOSTON trial [Citation4]. Selinexor is an oral, first-in-class selective inhibitor of nuclear export (SINE) targeting exportin 1 (XPO1) which regulates the transportation of specific proteins and RNA-protein complexes from the nucleus to the cytoplasm [Citation5]. Cancer cells including MM highly depend on XPO1 to maintain cellular homeostasis for hyper-proliferation. Mechanistically, selinexor exposure resulted in nuclear retention and activation of tumor suppressor proteins, such as p53, and glucocorticoid receptor, the molecular target of dexamethasone. In addition, selinexor caused nuclear retention of eIF4E, known as a rate-limit mRNA translation initiation factor required for many oncoproteins’ mRNA translation in the cytoplasm, such as c-myc [Citation6]. What’s more, preclinical studies supported that the inhibition of XPO1 is related to higher levels of nuclear retention with glucocorticoids, PIs and IMiDs, indicating a strong synergistic effect with other anti-MM drugs [Citation7–9]. Overall, XPO1 is a novel druggable target in addition to current stand-of-care therapies in MM. Selinexor is receiving great expectations as the agent has the unique mechanism of action amongst all approved anti-MM medications, therefore may rescue heavily pre-treated MM patients who are refractory to the other none-selinexor regimens [Citation10]. To demonstrate the optimal timing and drug combination of selinexor usage in RRMM, this systematic review and meta-analysis was performed.
Materials and methods
Registration
This study has been registered at the International Prospective Register of Systematic Reviews (PROSPERO, registration ID: CRD 42021283980).
Literature search
PubMed, Embase, Web of Science, ClinicalTrials.gov, and conference abstracts from the American Society of Hematology (ASH), the European Hematology Association (EHA), and the American Society of Clinical Oncology (ASCO) were searched for prospective trials and real-world studies. The search was restricted to all the published literature in English before 2022.2.1. Clinical trials or real-world studies involving RRMM patients treated with selinexor were included. The detailed search terms were described in Supplemental Materials.
Study selection
Studies were considered as eligible if all the following criteria were met: (1) clinical trials or real-world studies regardless of publication type (article, conference abstract, case series); (2) studies including patients confirmed with RRMM according to The International Myeloma Working Group; (3) studies involving RRMM patients treated with selinexor; (4) studies reporting either efficacy or safety endpoints. If a clinical trial contains multiple arms using the selinexor-based regimens, each arm will be included in this study separately. Two independent investigators screened all the included studies, and any disagreements were resolved by negotiation.
Data extraction
Two independent reviewers extracted information from all the eligible studies using Microsoft Excel (Microsoft Corporation, Redmond, WA). Basic information about the study was extracted, including the name of the first author, year of publication, study design, registration ID, study name, study phase, treatment regimen, number of patients included, the age of included patients, and prior lines of treatments. Indicators evaluating efficacy and safety were extracted, including objective response rates (ORRs), progression-free survival (PFS), overall survival (OS), and adverse events (AEs).
Quality assessment
The quality of the studies included in this review was evaluated through the use of a combination of assessment tools, including Jadad Scale for randomized controlled trials (RCTs), Methodological Index for Non-randomized Studies (MINORS) for non-randomized clinical trials and Joanna Briggs Institute (JBI) Critical Appraisal Checklist for case series. Two authors independently assessed the included studies.
Statistical analysis
Pooled risk ratio (RR) was calculated for rate comparison with a 95% confidence interval (CI) and p-value accessed concurrently. Engauge Digitizer (version 11.1) was utilized to extract data from the Kaplan-Meier curves when original survival data were not available. The heterogeneity of the studies and subgroups was evaluated by conducting a Cochrane χ2 test and calculating the I2 statistic. Subgroup analysis was used to explore the possible causes of heterogeneity and refine the results. Funnel plots were used to examine the potential publication bias, and the sensitivity analysis was performed by the trim method with high heterogeneity (Figure S1). Meta-analyses for rates and survival data were conducted with package Meta and MetaSurv separately, with R software (version 4.1.2).
Results
Study selection
The process of identifying and selecting studies for the analysis was displayed in a flowchart (). After searching multiple databases, 764 publications were initially identified. Then, 578 publications were removed due to repetition and irrelevance. Among the 186 publications accessed for eligibility, article types including reviews, basic reports, case reports, correspondences and comments were excluded. Out of the 36 publications screened for quantitative synthesis, 16 publications were eventually included in the systematic review and meta-analysis.
Study characteristics and quality assessment
After screening, 16 published or ongoing studies with published results were identified that evaluated 817 patients with RRMM receiving selinexor-containing combinations (). All the studies were published between 2015 and 2021. Among all the included studies, 1 study used selinexor as a single agent [Citation11], 3 studies used Xd doublet therapy [Citation12–14], 11 studies used Xd-based triplet therapies [Citation4,Citation15–24], and 1 study used an Xd-based quartet therapy [Citation25]. According to the quality assessment, 1 RCT (Jadad score = 5), 14 non-randomized clinical trials (MINORS score ≥ 10), and 1 case series (JBI overall appraisal = include) were considered to have acceptable quality (Table S1).
Table 1. Characteristics of included studies.
Beneficial outcome of selinexor as the fifth-line or prior therapy
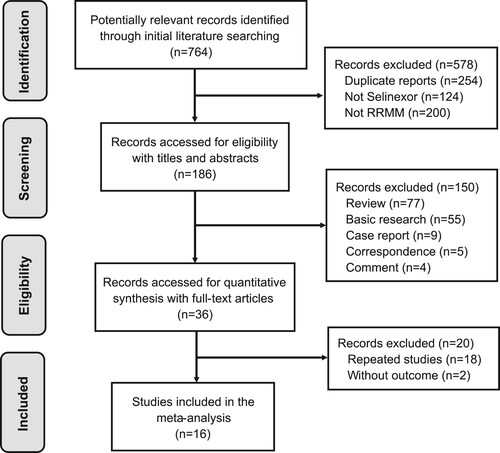
Among all the included patients, 399 of 817 response-evaluable RRMM patients (48.8%) who had received selinexor-based therapy achieved an OR or better. Using selinexor as the fifth-line or previous therapy showed an encouraging shift to an improved pooled ORR of 65.9% (95% CI: 58.3%−72.8%, I2 = 62%, p < 0.01), suggesting a possible benefit with over 50% patients in the group; which compared favorably with 23.4% (95% CI: 19.3%−28.1%, I2 = 0, p = 0.88) beyond the fifth-line treatment ((A)). Using selinexor after fifth-line therapy could bring about a decline in ORR with over 40% of RRMM patients. The estimated ORR of all selinexor-treated patients was 45.4% (95% CI: 33.4%−58.0%, I2 = 91%, p < 0.01).
Figure 2. Meta-analysis of the ORR by subgroups according to different treatment lines and selinexor-based drug combinations in RRMM patients. (A) Objective response rate (ORR) of selinexor-based regimens used in the fifth-line or prior treatment (treatment line = 2-5) vs selinexor-based regimens used after fifth-line treatment (treatment line > 5) in relapsed/refractory multiple myeloma (RRMM); (B) ORR of Xd + PIs, Xd + IMiDs and Xd.
Combination therapy in selinexor-based regimens was also explored. The subgroup analysis indicated that the addition of PIs or IMiDs to the Xd regimen was associated with improved ORRs. When used in combination with PIs, selinexor demonstrated optimal efficacy, with approximately 56.1% (95% CI: 37.9%−73.5%, I2 = 84%, p < 0.01) of RRMM patients achieving OR ((B)). IMiDS also showed clinical benefits, establishing an ORR in RRMM patients of 52.5% (95% CI: 41.6%−63.3%, I2 = 0%, p = 0.44), about twice the ORR of the Xd-only regimen (24.6%, 95% CI: 19.5%−30.0%, I2 = 0, p = 0.59). At present, the treatment efficacy of Xd plus other potential anti-MM drugs, such as anti-CD38 antibodies, could not be evaluated due to the inadequacy of trial data. Nonetheless, results from the STOMP trial suggested a prominent efficacy of Xd in combination with daratumumab (XDd) [Citation17]. Of the 32 RRMM patients treated with XDd therapy, 22 patients (68.9%) achieved an OR.
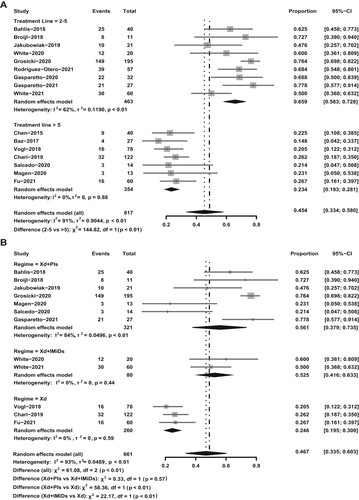
The pooled analysis on survival indicated that the use of selinexor in combination with other treatments prior to the fifth-line therapy was advantageous for RRMM patients, and the median progression-free survival (mPFS) was found to be 12.5 months, compared to 2.9 months for those receiving the fifth-line therapy or beyond (p < 0.01, (A)). Receiving selinexor-based regimens before the fifth line engendered a distinctively superior PFS in RRMM patients to using selinexor afterward, with a hazard ratio (HR) of 0.28 (95% CI: 0.22-0.36). In the pooled analysis including three studies reporting OS curves, an improved OS when using selinexor before the fifth-line therapy was observed with HR of 0.46 (95% CI: 0.28-0.76) and median of 22.7 months, compared with 8.9 months when selinexor was used after the fifth-line (p = 0.26, (B)).
Figure 3. Survival curves of selinexor-based regimens in RRMM patients. (A) Pooled progression-free survival (PFS) curves of selinexor-based regimens used prior to fifth-line treatment (median treatment line < 5) vs selinexor-based regimens used as fifth-line or later treatment (median treatment line ≥ 5) in relapsed/refractory multiple myeloma (RRMM); (B) pooled overall survival (OS) curves of selinexor-based regimens used in the fifth-line treatment (median treatment line = 5) vs selinexor-based regimens used after fifth-line treatment (median prior line > 5) in RRMM.
Better safety of selinexor as the fifth-line or prior therapy
In terms of safety, the usage of selinexor combination regimens in the fifth and prior therapy could significantly reduce the incidence of AEs when compared with selinexor usage after the fifth line (Table S2). Among grade 1–4 AEs, significant differences were observed in thrombocytopenia (63.7% vs 71.9%, p = 0.017), anemia (48.9% vs 61.1%, p < 0.01), leukopenia (34.3% vs 48.1%, p = 0.017), nausea (55.3% vs 69.6%, p < 0.01), vomiting (21.3% vs 43.1%, p < 0.001) and weight loss (25.8% vs 50.6%, p < 0.01). Concerning grade 3–4 AEs, the occurrence of anemia (22.7% vs 29.9%, p = 0.032) was significantly reduced when using selinexor in the early stages. However, it is worth noting that selinexor-based regimens used in the fifth-line or prior therapy could lead to an increased probability of grade 3–4 lymphopenia (23.9% vs 11.4%, p = 0.020). In addition, no matter in which time phase selinexor was used, more than half of the RRMM patients would experience grade 1–4 fatigue (56.3% vs 53.6%, p = 0.475). In terms of the overall incidence during selinexor treatment, the most frequently occurred grade 1–4 AE was thrombocytopenia (68.3%), closely followed by nausea (60.8%). Fatigue (55.4%) and anemia (55.0%) were also prevalent. The most prevalent grade 3–4 AE was thrombocytopenia (49.2%).
Discussion
This systematic review and meta-analysis shed illuminating insight into the appropriate timing and drug combinations of selinexor usage in MM treatment. Our findings align with prior studies, which demonstrated that selinexor provided encouraging treatment responses with tolerable AEs [Citation26,Citation27]. Importantly, this study was the first to indicate that the fifth-line therapy was a critical point in the clinical application of selinexor. It is undeniable that no matter which drug is used, the prognosis of patients will deteriorate with the increase in the number of treatment lines. However, it is remarkable that the ORR of RRMM patients using selinexor would witness a huge decline of almost 40% around the fifth-line therapy (treatment line = 2-5 vs treatment line > 5: 65.9% vs 23.4%). The PFS before and after the fifth line also showed significant differences, with mPFS of 2.9 months before the fifth-line treatment and 12.5 months in the fifth-line treatment or beyond. Among our known treatment options for RRMM, no other drug has such a significant waterfall-like decline in efficacy with the increasing number of treatment lines. On the one hand, this study suggests a more appropriate time frame for the use of selinexor, giving a precise range. On the other hand, it challenges the stereotype that the prognosis of MM patients gets worse gradually as treatment lines increase with selinexor usage. In clinical practice, a jumping decline may exist at a critical time point.
Furthermore, this study revealed that triplet therapy, including PIs + Xd or IMiDs + Xd, exhibited more potent treatment outcomes than the Xd-only regimen. This might be explained by the synergistic effect of selinexor in combining with other drugs in MM [Citation9,Citation28,Citation29]. Although a previous study had illustrated the superior efficacy and safety of the Xd + PIs regimen over the Xd-only regimen [Citation26], our study discovered the comparable advantageous performance of Xd + IMiDs regimens. Incorporating more recent publications, this study provides more validated evidence to support the promising selinexor-inclusive triplet regimens. Previously, a network meta-analysis focused on the comparison of XVd with other non-selinexor combinations, establishing a link with other regimens through the mere BOSTON study, and reached the conclusion that the XVd regimen was non-inferior to the other top 5 regimens in both doublet and triplet therapies [Citation30]. The robustness of the argument demands more high-quality RCTs involving different comparisons of other combinations with selinexor-based regimens for strengthening.
Indeed, some limitations should be considered when generalizing the conclusion of our study. The quality of the included studies will strongly influence the quality of the results. Limited by the modest number of published clinical trials of selinexor, only 1 of the 16 included studies was a randomized controlled trial (RCT), which hindered us from conducting a head-to-head meta-analysis or network analysis with valid control groups. Besides, 8 of the included studies were ongoing with reported interim results, and 14 registered clinical trials are being conducted without published data (Table S3). Therefore, further analysis would be required to evaluate these results.
Notably, the role of selinexor-containing therapies in the MM management is also worth a thorough discussion. In this study, we found that early use of the selinexor-based triplet regimens showed better benefits for RRMM patients. For selinexor, the timing of dosing after the first relapse needs to be sought, as the timing of RRMM treatment and drug selection do not affect prognosis in isolation. Delaying the introduction of selinexor into the treatment plan could lead to a reduction in the number of available drug combinations, as patients will have already undergone exposure and refractory to other treatments. Accordingly, the synergistic effects of selinexor with different types of drugs cannot be well exploited.
Currently, several intriguing BCMA-targeted therapeutics have been approved for RRMM after the fourth or fifth-line treatment, including 2 chimeric antigen receptor (CAR)-T cells, 1 antibody–drug conjugate and 1 bispecific T cell engager [Citation31]. However, options are limited for patients refractory to anti-BCMA therapies. A few studies have explored the potential position of selinexor in the context of relapse after anti-BCMA therapies. In the STOMP trial, 11 patients with prior anti-BCMA therapy were treated with selinexor-containing regimens, among which 9 were triple-refractory and 4 were penta-refractory. Seliexor showed impressive potency and durability with an ORR of 54.5% and clinical benefit rate of 81.8% [Citation32]. Another study that included 7 RRMM patients who relapsed after CAR-T therapy demonstrated a strong benefit of selinexor-inclusive regimens with 1 patient stringent complete, 3 very good partial, 2 partial and 1 minimal response [Citation33]. The use of different targets, like GPRC5D, or different forms of immunotherapy, such as trispecific antibodies and CAR-expressing natural killer cells are also potential options for relapse after anti-BCMA treatment in the future.
At present, based on the limited duration of efficacy of selinexor-containing regimens, it is recommended that the appropriate place for selinexor remains in pre-CAR-T tumor reduction therapies. As more clinical evidence is generated, we will have a clearer understanding of the landscape of anti-MM treatment that incorporates more new drugs and immunotherapies, in which selinexor may have a place in both the pre-CAR-T tumor reduction phase and post-CAR-T relapse salvage phase.
Conclusion
In brief, selinexor has reliable efficacy and safety for RRMM. Incorporating selinexor into earlier lines of therapy can significantly improve the short and long-term outcomes of RRMM. Accordingly, a distinct efficacy reduction, along with a substantial increase in adverse reactions, may occur around the fifth-line therapy of RRMM patients, which deserves clinical attention. Furthermore, the addition of PIs or IMiDs to Xd can improve clinical benefits in RRMM patients, warranting further investigation into more selinexor-combined therapies. The validation of the optimal timing and drug combination of selinexor helps to strike a balance between maximum benefits and minimum risks.
Ethics statement
Ethical approval is not required, because this manuscript is a meta-analysis.
Supplemental Material
Download PDF (683.1 KB)Supplemental Material
Download PDF (542.9 KB)Acknowledgement
The authors gratefully acknowledge Dr. Ting Niu, Dr. Wenjiao Tang, Dr. Zhongqing Zou and Changyang Tang for their constructive suggestions for this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Additional information
Funding
References
- Palumbo A, Anderson K. Multiple myeloma[J]. N Engl J Med. 2011;364(11):1046–1060. doi:10.1056/NEJMra1011442.
- Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annu Rev Pathol. 2011;6:249–274. doi:10.1146/annurev-pathol-011110-130249.
- Yang Y, Li Y, Gu H, et al. Emerging agents and regimens for multiple myeloma. J Hematol Oncol. 2020;13(1):150. doi:10.1186/s13045-020-00980-5.
- Grosicki S, Simonova M, Spicka I, et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, phase 3 trial. Lancet. 2020;396(10262):1563–1573. doi:10.1016/S0140-6736(20)32292-3.
- Parikh K, Sekhri A, Cang S, et al. Selective inhibitors of nuclear export (SINE)– a novel class of anti-cancer agents. J Hematol Oncol. 2014;7(1):78. doi:10.1186/s13045-014-0078-0.
- Gravina G, Senapedis W, Mccauley D, et al. Nucleo-cytoplasmic transport as a therapeutic target of cancer. J Hematol Oncol. 2014;7(1):85. doi:10.1186/s13045-014-0085-1.
- Tai YT, Landesman Y, Acharya C, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2014;28(1):155–165. doi:10.1038/leu.2013.115.
- Turner JG, Dawson J, Sullivan DM, et al. Inhibition of CRM1-dependent nuclear export sensitizes malignant cells to cytotoxic and targeted agents. Semin Cancer Biol. 2014;27:62–73. doi:10.1016/j.semcancer.2014.03.001.
- Turner JG, Dawson JL, Gomez J, et al. XPO1 inhibitor combination therapy with bortezomib or carfilzomib induces nuclear localization of IκBα and overcomes acquired proteasome inhibitor resistance in human multiple myeloma. Oncotarget. 2016;7(48):78896–78909. doi:10.18632/oncotarget.12969.
- Mo CC, Jagannath S, Chari A, et al. Selinexor for the treatment of patients with previously treated multiple myeloma. Expert Rev Hematol. 2021;14(8):697–706. doi: 10.1080/17474086.2021.1923473.
- Chen C, Garzon R, Gutierrez M, et al. Safety, efficacy, and determination of the recommended phase 2 dose for the oral selective inhibitor of nuclear export (SINE) selinexor (KPT-330). Blood. 2015;126(5):258. doi:10.1182/blood.V126.23.258.258.
- Fu WJ, Xia ZJ, Fu CC, et al. Results of the phase 2 MARCH study: oral ATG-010 (selinexor) plus low dose dexamethasone in Chinese patients with relapsed/refractory multiple myeloma (RRMM) previously treated with an immunomodulatory agent (IMiD) and a proteasome inhibitor (PI). J Clin Oncol. 2021;39(Suppl15):e20002. doi:10.1200/JCO.2021.39.15_suppl.e20002.
- Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor–dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381(8):727–738. doi:10.1056/NEJMoa1903455.
- Vogl DT, Dingli D, Cornell RF, et al. Selective inhibition of nuclear export With oral selinexor for treatment of relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36(9):859–866. doi:10.1200/JCO.2017.75.5207.
- Gasparetto C, Lipe B, Tuchman S, et al. Once weekly selinexor, carfilzomib, and dexamethasone (XKd) in carfilzomib nonrefractory multiple myeloma (MM) patients. J Clin Oncol. 2021;39(Suppl15):8038. doi:10.1200/JCO.2021.39.15_suppl.8038.
- White D, Chen C, Baljevic M, et al. Oral Selinexor, pomalidomide, and dexamethasone (XPd) at recommended phase 2 dose in relapsed refractory multiple myeloma (MM). J Clin Oncol. 2021;39(Suppl15):8018. doi:10.1200/JCO.2021.39.15_suppl.8018.
- Gasparetto C, Lentzsch S, Schiller G, et al. Selinexor, daratumumab, and dexamethasone in patients with relapsed or refractory multiple myeloma. eJHaem. 2021;2:56–65. doi:10.1002/jha2.122.
- White DJ, LeBlanc R, Baljevic M, et al. Selinexor, lenalidomide and dexamethasone (SRD) for patients with relapsed/refractory and newly diagnosed multiple myeloma[J]. Blood. 2020;136(Suppl1):45–46. doi:10.1182/blood-2020-140141.
- Bahlis NJ, Sutherland H, White D, et al. Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood. 2018;132(24):2546–2554. doi:10.1182/blood-2018-06-858852.
- Salcedo M, Lendvai N, Mastey D, et al. Phase I study of selinexor, Ixazomib, and Low-dose dexamethasone in patients With relapsed or refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2020;20(3):198–200. doi:10.1016/j.clml.2019.12.013.
- Jakubowiak AJ, Jasielec JK, Rosenbaum CA, et al. Phase 1 study of selinexor plus carfilzomib and dexamethasone for the treatment of relapsed/refractory multiple myeloma. Br J Haematol. 2019;186(4):549–560. doi:10.1111/bjh.15969.
- Broijl A, Asselbergs E, Lonergan S, et al. A phase II study of selinexor(KPT-330) combined with bortezomib and dexamethasone (SVD) for induction and consolidation for patients with progressive or refractory multiple myeloma: The selvedex trial. HemaSphere. 2018;2(Suppl2):611–612.
- Baz R, Zonder JA, Shain KH, et al. Phase I/II study of liposomal doxorubicin (DOX) in combination with Selinexor (SEL) and dexamethasone (DEX) for relapsed and refractory multiple myeloma (RRMM)[J]. Blood. 2017;130(Suppl1):3095. doi:10.1182/blood.V130.Suppl_1.3095.3095.
- Magen H, Geva M, Volchik Y, et al. Selinexor, bortezomib, and dexamethasone for heavily pretreated multiple myeloma: A case series. Clin Lymphoma Myeloma Leuk. 2020;20(12):e947–e955. doi: 10.1016/j.clml.2020.07.016.
- Rodríguez-Otero P, González-Calle V, Sureda A, et al. Selinexor in combination with daratumumab-bortezomib and dexamethasone for the treatment of relapse or refractory multiple myeloma: initial results of the phase 2, open-label, multicenter GEM-selibordara study. Blood. 2021;138(Suppl1):1677. doi:10.1182/blood-2021-147725.
- Tao Y, Zhou H, Niu T. Safety and efficacy analysis of selinexor-based treatment in multiple myeloma, a meta-analysis based on prospective clinical trials. Front Pharmacol. 2021;12:758992. doi:10.3389/fphar.2021.758992.
- Abushanab D, Mraiche F, Al-Badriyeh D. Pcn20 efficacy and safety of selinexor for patients with relapsed and refractory multiple myeloma: A meta-analysis. Value Health. 2021;24(Suppl1):S22. doi:10.1016/j.jval.2021.04.112.
- Turner JG, Dawson JL, Meads M, et al. Treatment of acquired drug resistance in multiple myeloma by combination therapy with XPO1 and topoisomerase II inhibitors. J Hematol Oncol. 2016;9(1):73. doi:10.1186/s13045-016-0304-z.
- Kashyap T, Argueta C, Unger T, et al. Selinexor reduces the expression of DNA damage repair proteins and sensitizes cancer cells to DNA damaging agents. Oncotarget. 2018;9(56):30773–30786. doi:10.18632/oncotarget.25637.
- Dolph M, Tremblay G, Gilligan AM, et al. Network meta-analysis of once weekly selinexor-bortezomib-dexamethasone in previously treated multiple myeloma. J Health Econ Outcomes Res. 2021;8(2):26–35. doi:10.36469/jheor.2021.27080.
- Tanenbaum B, Miett T, Patel SA. The emerging therapeutic landscape of relapsed/refractory multiple myeloma. Ann Hematol. 2023;102(1):1–11. doi:10.1007/s00277-022-05058-5.
- Baljevic M, Gasparetto C, Schiller GJ, et al. Selinexor-based regimens in patients with multiple myeloma after prior anti-B-cell maturation antigen treatment. EJHaem. 2022;3(4):1270–1276. doi:10.1002/jha2.572.
- Chari A, Vogl DT, Jagannath S, et al. Selinexor-based regimens for the treatment of myeloma refractory to chimeric antigen receptor T cell therapy. Br J Haematol. 2020;189(4):e126–e130. doi:10.1111/bjh.16550.