ABSTRACT
Introduction
Acute promyelocytic leukemia (APL) is a distinct subtype of acute myeloid leukemia (AML) with a unique clinical presentation and prognosis. This study aimed to investigate the epidemiology, clinical characteristics, treatments, and clinical outcomes of Thai APL patients dominantly treated with all-trans-retinoic acid (ATRA) combined with a chemotherapy-based therapy.
Methods
This was an eight-year prospective, observational study from nine academic hospitals in the Thai Acute Leukemia Working Group (TALWG) of the Thai Society of Hematology, which included newly diagnosed Thai APL patients, aged 18 years or older. The web-based registration collected baseline charateristic, and clinical outcomes.
Results
From 992 newly diagnosed AML patients, 79 APL patients were enrolled in this study. Almost all subjects were de novo APL (94.9%), while the others were therapy-related APL. The commonest clinical presentation was disseminated intravascular coagulation (38%). One-third of the patients were categorized as high risk according to the initial WBC. Almost all patients received ATRA combined with idarubicin regimen. The complete response rate was as high as 95.7%, which translated into excellent four-year overall survival (OS) (75.6%) and four-year leukemia-free survival (LFS) (75.4%). The multivariate analysis demonstrated that the older age and WBC count >20 × 109/L conferred a significantly unfavorable OS with the hazard ratios of 3.03 (95% confidence interval [CI]: 1.14–8.05) and 4.18 (95%CI: 1.69–10.35), respectively. Similarly, these two parameters remained independent of the poor prognosis factors for LFS.
Conclusion
This report confirmed that APL had a favorable prognosis. However, advanced age and high WBC count >20 × 109/L contributed to a worse outcome.
Abbreviations
APL; acute promyelocytic leukemia; ATRA; all-transretinoic acid; CR; complete remission; DS; differentiation syndrome; ECOG; Eastern Cooperative Oncology Group; ED; early death; HR; hazard ratio; IQR; interquartile range; LFS; leukemia-free survival; OS; overall survival; WBC; white blood cell.
KEYWORDS:
Introduction
Acute promyelocytic leukemia (APL) is a distinct subtype of acute myeloid leukemia (AML) with a unique clinical presentation and prognosis. APL accounted for 18% of all AML patients in a previous study from Thailand [Citation1]. The diagnosis of APL requires the presence of APL-specific chromosomal translocation t(15; 17)(q22; q21) and other variant translocations by a conventional cytogenetic method, and/or the PML-RARA fusion product by molecular analyses. Other molecular variants of the RARA gene are rare, but also diagnostic of APL. However, APL is well-known for its favorable prognosis with the all-trans retinoic acid (ATRA)-based treatment. According to the European APL group, a 10-year survival was reported to be as high as 77% [Citation2]. ATRA and chemotherapy combination had been the standard of care for a long period of time until the advent of arsenic trioxide (ATO). The combination of ATO and ATRA treatment had at least equally efficacy and less toxicity profiles compared to a chemotherapy-based regimen in low and intermediate risk APL [Citation3]. The superior anti-leukemia effect of ATRA-ATO was demonstrated in all risk groups, including high-risk patients in a randomized trial conducted by the National Cancer Research Institute in the United Kingdom (AML17 trial) [Citation4]. Due to various evidence supporting the clinical benefits of a chemotherapy-free strategy, some institutions in our group had adopted the ATRA-ATO regimen as the first-line induction in patients with APL. However, the incorporation of ATO was inconvenient due to its route of administration and schedule. Moreover, ATO is not widely available in Thailand. Taken together, the chemotherapy-based regimens were still utilized in most of the institutions. Therefore, this study aimed to investigate the epidemiology, clinical characteristics, treatments, and clinical outcomes of Thai APL patients in the era of ATRA with limited availability of ATO.
Materials and methods
The study was an eight-year prospective, observational study from nine teaching and referral hospitals in the Thai Acute Leukemia Working Group (TALWG) of the Thai Society of Hematology. The inclusion criteria consisted of (1) Thai patients with a diagnosis of APL based on the 2008 and 2016 World Health Organization classification of myeloid neoplasms and acute leukemia [Citation5,Citation6]; (2) aged 18 years or older; and (3) patients diagnosed with APL during January 1, 2014, and December 31, 2021. The Ethics Committee for Research in Human Subject approved the protocol, and all participants signed the informed consent before enrollment into the study. The web-based registration collected clinical manifestations, laboratory results, treatment regimens, and clinical outcomes, including the complete remission (CR) rate, overall survival (OS), and leukemia-free survival (LFS). Each institution’s policy determined the treatment regimen, including induction, consolidation, and maintenance phases. The ATRA-idarubicin combination regimen was mainly based on AIDA 0493 protocol [Citation7,Citation8]. The primary outcomes were OS and LFS. The study was conducted in compliance with good clinical practice and the Declaration of Helsinki. The Thai Society of Hematology provided funding for this study.
Terminology
CR was defined as: (1) a bone marrow blast count of <5%, (2) the absence of circulating blasts and blasts with Auer rods, (3) the absence of extramedullary disease, (4) an absolute neutrophil count of ≥1.0 × 109/L, and (5) a platelet count of ≥100 × 109/L [Citation9]. OS was defined as the time from diagnosis to death from any cause or last follow-up. LFS was defined as the time from CR to the date of the molecular relapse, hematologic relapse, or death from any cause, whichever came first [Citation10]. The APL risk classification was defined according to the National Comprehensive Cancer Network (NCCN)’s AML guidelines [Citation11]. Patients with a white blood cell (WBC) count ≤ 10 × 109/L were categorized as having low-risk disease, while those with a higher WBC count had a high-risk disease. The differentiation syndrome (DS) was composed of the presence signs and symptoms of fever, weight increase caused by fluid retention, lung infiltrates, pleural effusions, respiratory failure, pericardial effusion, and acute renal failure. The International Society of Thrombosis and Haemostasis (ISTH)- disseminated intravascular coagulation (DIC) scoring system was determined in all eligible patients at diagnosis [Citation12]. The DIC score of ≥5 was defined as overt DIC [Citation12]. Early death (ED) was determined as death within 30 days after APL diagnosis [Citation13].
Statistical analysis
Categorical variables were presented as frequency and percentage and compared between the groups with Fisher’s exact test or Chi-square where appropriate. Continuous variables were presented as median and interquartile range (IQR) or mean ± standard deviation (SD) as appropriate. The comparison between the groups was performed using the Mann–Whitney U test or student’s t-test as appropriate. Survival analyses were done using the Kaplan-Meier’s method, and the comparison between the survival curves was done using the log-rank test. Univariate analyses were performed to determine the effect of the variables on the survival outcomes using the Cox-proportional hazard model, and variables with a p-value < 0.2 were included into the multivariate analysis. All statistical analyses were considered two-sided, and a p-value < 0.05 was defined as being statistically significant. PASW Statistics for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA) was used for all data analyses.
Results
Baseline characteristics
From 992 newly diagnosed AML patients during the eight-year study period, 79 APL patients were enrolled with the prevalence of APL of 7.9%. Most of them were de novo APL (94.9%), while the others were therapy-related APL (5.1%). The median age was 45 years (range 15–74 years) with a slight female predominance (55.7%). The commonest initial clinical manifestations were overt DIC, which occurred in 38% of the patients, followed by hepatomegaly (6.3%), splenomegaly (5.1%), and lymphadenopathy (2.5%).
Regarding the initial complete blood count (CBC), the mean hemoglobin was 8.4 ± 2.2 g/dL, median WBC count was 3.6 × 109/L (IQR: 1.4–15.6 × 109/L), and the median platelet count was 31 × 109/L (IQR: 18–54 × 109/L). The median bone marrow blasts with promyelocytes were 80% (IQR: 70–90%). Among 75 cases with available cytogenetic results, 57 patients (76%) had the t(15; 17) abnormality. Approximately one-fifth (21.3%) of the cases had normal cytogenetic, and 2.6% had t(11; 17). The PML-RARA gene detected by the polymerase chain reaction method was found in all cases, including those with normal cytogenetic or lack of cytogenetic results to confirm the APL diagnosis. Based on the WBC count, patients were categorized into the high-risk group (31.6%) and low-risk group (68.4%). demonstrates the detailed baseline characteristics of the patients.
Table 1. Baseline characteristics of the APL patients in each therapeutic group.
Subgroup analysis depending on the risk groups
The baseline characteristics demonstrated no significant difference between these two groups, including age, gender, initial platelet count, cytogenetic results, and the incidence of DIC. However, the high-risk group showed a significantly higher proportion of bone marrow blasts (90% vs. 80%; p = 0.009). The overall differentiation syndrome (DS) was experienced accounting for 15.2% with almost all cases occurring in the high-risk group. No patients developed DS succumbing to death from this complication.
Treatment outcomes
The majority of the patients (72 patients; 91.1%) received treatment with the ATRA-idarubicin combination, while only one case was treated with ATRA plus ATO. A minority of patients received palliative treatment due to comorbidities and poor performance status (six patients; 7.6%). The median follow-up time for all patients was 50.1 months (IQR; 12.1-63.8 months). The CR rate for patients who underwent induction therapy was 95.7%, and all of these patients had completed consolidation therapy. The rate of CR was not different between patients with low-risk and high-risk diseases (95.9% vs. 95.0%; p = 0.865). Twelve patients (15.2%) had ED, with a median ED duration of 10 days (2-25 days). Maintenance therapy was given in 87.5% of all patients. A few cases did not receive maintenance therapy because they were denied continuing chemotherapy.
Survival outcomes and predictive factors associated with the survival outcomes
The two- and four-year OSs for all patients were 77.1%, and 75.6%, respectively ((A)). The OS of patients aged more than 60 years was inferior when compared to those aged below 60 years with four-year OSs of 49.5% vs. 81.2%; p = 0.012 ((A)). Patients with poor performance status (Eastern Cooperative Oncology Group [ECOG] 2-4) had a significantly lower OS with four-year OSs of 68.2% vs. 84.3%; p = 0.032; (B). Nevertheless, the OS in the high-risk patients was comparable to those with low-risk disease with four-year OSs of 63.8% vs. 81.3%, respectively (p = 0.057; (C)). Therefore, the higher WBC cut-off (20 × 109/L) was additionally analyzed. Patients with WBC ≤20 × 109/L illustrated a survival benefit when compared to those with a higher WBC (four-year OSs of 82.7% vs. 46.7%; p < 0.001; (D)). The OS in patients with disseminated intravascular coagulation was no different from those without the complication (p = 0.537). On the multivariate analysis, the older age and higher WBC (>20 × 109/L) were the factors associated with a poorer OS with an adjusted hazard ratio (aHR) 3.03; 95% confidence interval (CI): 1.14–8.05; p = 0.026, and aHR 4.18; 95%CI: 1.69-10.35; p = 0.002, respectively ().
Figure 1. Survival outcomes of Thai patients with an acute promyelocytic leukemia (A) overall survival and (B) leukemia-free survival.
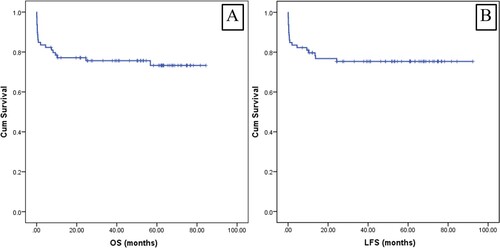
Figure 2. Kaplan Meier curves of the overall survival outcome categorized by (A) age ≤60 years vs. >60 years, (B) ECOG 0–1 vs. ECOG 2-4, (C) WBC ≤ 10 × 109/L vs. > 10 × 109/L, and (D) WBC ≤ 20 × 109/L vs. >20 × 109/L.
Table 2. Univariate and multivariate analyses for the overall survival of the APL patients.
During the follow-up, one patient (1.8%) in the low-risk group and three patients (12%) in the high-risk group had relapsed APL. The two- and four-year LFSs for the patients were 76.9% and 75.4%, respectively ((B)). The patients in the age ≤60 years group, ECOG 0–1 group, and WBC ≤ 20 × 109/L demonstrated significantly better LFS (). Similar to OS, the older age and WBC > 20 × 109/L was related with inferior LFS from the multivariate analysis with aHR 2.96; 95%CI: 1.12–7.83; p = 0.029 and aHR 3.47; 95%CI: 1.37–8.78; p = 0.009, respectively ().
Figure 3. Kaplan Meier curves of the leukemia-free survival outcome categorized by (A) age ≤60 years vs. >60 years, (B) ECOG 0–1 vs. ECOG 2–4, (C) WBC ≤ 10 × 109/L vs. >10 × 109/L, and (D) WBC ≤ 20 × 109/L vs. >20 × 109/L.
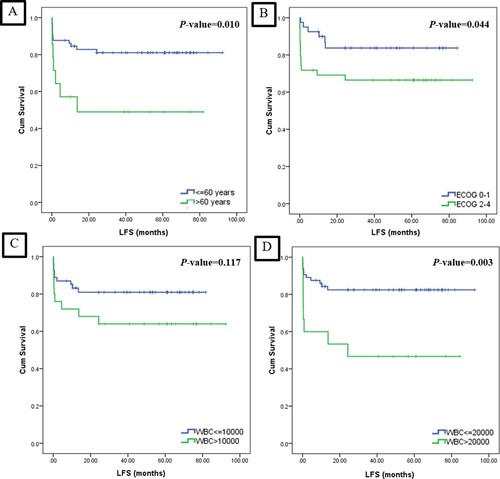
Table 3. Univariate and multivariate analyses for the leukemia-free survival of the APL patients.
Subgroup analysis which excluded palliative cases, was performed. WBC > 20 × 109/L and DS are significant factors impacting the OS and LFS in subgroup analysis remained a significant impact on OS and LFS (Supplementary Tables S1 and S2).
Discussion
This study reported a real-world study of Thai APL patients, which confirmed a favorable outcome with an ATRA-based treatment regimen. The survival outcomes were comparable to several previous reports from Europe and Asia [Citation13–19]. The major difference among these trials was the proportion of patients receiving an ATO containing regimen. One of the most recent population-based studies from China proved to be an appropriate representative of the APL patients who received an ATO-based treatment regimen due to the high prevalence of the ATRA/ATO regimen usage accounting for 78% of patients [Citation13]. Although many randomized clinical trials supported ATO containing regimens to be the preferred option over ATRA-plus-chemotherapy-based regimens, the real-world trials which the majority of patients received ATRA-plus-chemotherapy-based regimens demonstrated excellent survival outcomes that mirrored the ATO-based regimens. Nevertheless, the obvious advantage of utilizing ATO containing regimens was the shorter duration of the treatment course as the maintenance phase was not necessarily incorporated into these regimens [Citation8,Citation20]. Additionally, secondary malignancy was reported to be lower in ATO-based regimens compared to ATRA-plus-chemotherapy-based regimens [Citation21]. The overt DIC incidence in our cohort accounts for 38%, which is lower than previous reports [Citation22,Citation23]. It might be due to an improvement in transfusion support and early ATRA initiation.
The poor prognosis of the elderly and patients with poor performance in this study is mainly due to the high incidence of early mortality, which is comparable to a previous study [Citation24]. It was well recognized that initial WBC >10 × 109/L was the critical factor leading to a poorer clinical outcome in the APL patients [Citation23,Citation25,Citation26]. However, the present work found that WBC >20 × 109/L was the significant cut-off for predicting the APL outcome. The WBC cutoff is also the unfavorable prognostic factor of ED and severe bleeding from a recent Japanese report, in which its patients were predominantly chemotherapy-based regimen users [Citation27]. The reason behind this higher WBC cut-off could be attributed to the improved supportive care over the past decade. Notably, WBC >10 × 109/L was still the significant cut-off for the poorer outcomes in other recent studies [Citation13]. It could be explained by the higher proportion of the usage of the ATO-based regimen contributing to the difficulty in the early control of leukocytosis, which could result in greater DS risk [Citation28]. This was supported by our data, which displayed a lower incidence of DS compared to those with ATO-based regimen (16% vs. 19%) [Citation3]. Taken together, if the chemotherapy-based regimen was employed, it could be reasonable to adjust the WBC cut-off to be more than 20 × 109/L for an unfavorable outcome prediction in the modern era. The platelet count ≤ 40 × 109 /L was once utilized as the cut-off for higher risk APL [Citation25]. However, the current clinical practice guidelines removed the platelet count from the APL risk stratification since multiple studies in recent years had shown no effect of the platelet count on the patients’ survival [Citation11]. Similarly, this analysis demonstrated no significant different survival outcomes in any initial platelet count cut-off. This finding could be driven by the improvement in bleeding control and transfusion management in newly diagnosed APL patients. Older age was also an independent factor for the poor outcome in this cohort, which was similar to the previous study [Citation13]. This highlighted the importance of the supportive measurement for elderly APL patients. Because this study included all APL cases without exclusion criteria [Citation25, Citation29–31], the early mortality rate was as high as real-world data.
Although, ATO has proven to be one of the main components in the APL treatment regimen, there were some limitations which precluded its prevalent use, including the limited availability, frequent intravenous administration schedule, and multiple hospital visits. To alleviate these issues, oral arsenic formulations were developed. There was accumulating evidence that oral arsenic had comparable efficacy to an intravenous form [Citation32–34]. Moreover, recent meta-analysis concluded that it had similar efficacy, toxicity, and survival outcome when compared to the intravenous form [Citation35]. All things considered, oral arsenic could be an alternative option for the APL treatment and could play a major role in increasing the utilization of ATO in APL patients in time to come.
Our study is the first prospective multicenter study exploring the treatment outcomes in the ATRA combined with a chemotherapy-based regimen in Thai APL patients, which collected data via a web-based registry. This data evidences that patients with high WBC counts, especially WBC >20 × 109/L, are considered unfavorable outcomes and need to have intensive care. Nevertheless, the survival outcomes of our study might be better than those of the overall Thai APL patients. Because all included institutes are large tertiary hospitals with more facilities for transfusion support and treatment care compared with suburban hospitals.
There are limitations to our study. Firstly, the number of patients was limited, which might lead to some insignificant factors on survival outcomes. Secondly, the ATRA initiation date data was not collected; therefore, the mortality and survival outcomes related to this factor could not be evaluated. Lastly, the measurable residual disease monitoring outcomes after complete consolidation therapy were unavailable.
Conclusions
This report confirmed that APL had a favorable prognosis. In addition, advanced age and high WBC counts >20 × 109/L contributed to a worse outcome.
Supplemental Material
Download MS Word (18.4 KB)Acknowledgements
The authors thank the Thai Society of Hematology for grant support. We thank Ms. Pattaraporn Tunsing for assistance with the data collection and statistical analyses. This study protocol was reviewed and approved by [by the Ethics Committee for Research in Human Subjects at the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand], approval number [Si 575/2013]. All participants signed the informed consent before enrollment into the study. A copy of the consent document is available for review from the Editor-in-Chief of Hematology. All authors collected the data. SK and WO designed the study, performed the statistical analyses, drafted the manuscript, and revised the final manuscript. All authors read and approved the final manuscript.
Data availability statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Auewarakul CU, Promsuwicha O, U-Pratya Y, et al. Immunophenotypic profile of adult acute myeloid leukemia (AML): analysis of 267 cases in Thailand. Asian Pac J Allergy Immunol. 2003;21(3):153.
- Ades L, Guerci A, Raffoux E, et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood. 2010;115(9):1690–1696.
- Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–121.
- Burnett AK, Russell NH, Hills RK, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(13):1295–1305.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–1405.
- Sanz MA, Montesinos P, Rayón C, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all- trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115(25):5137–5146.
- Avvisati G, Lo-Coco F, Paoloni FP, et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood. 2011;117(18):4716–4725.
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21(24):4642–4649.
- National Comprehensive Cancer Network (NCCN). Acute Myeloid Leukemia (version 2.2022). [cited 8 Aug 2022]. https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf.
- Taylor FB, Toh CH, Hoots WK, et al. Scientific subcommittee on disseminated intravascular coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330.
- Zhu H-H, Ma Y-F, Yu K, et al. Early death and survival of patients with acute promyelocytic leukemia in ATRA plus arsenic era: a population-based study. Front Oncol. 2021;11:762653.
- Yedla RP, Bala SC, Pydi VR, et al. Outcomes in adult acute promyelocytic leukemia: a decade experience. Clin Lymph Myeloma Leuk. 2020;20(4):e158–e164.
- Dayama A, Dass J, Seth T, et al. Clinico-hematological profile and outcome of acute promyelocytic leukemia patients at a tertiary care center in North India. Indian J Cancer. 2015;52(3):309.
- Bajpai J, Sharma A, Kumar L, et al. Acute promyelocytic leukemia: an experience from a tertiary care centre in north India. Indian J Cancer. 2011;48(3):316.
- Sanz MA, Martı́n G, Rayón C, et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia. PETHEMA group. Blood. 1999;94(9):3015–3021.
- Mandelli F, Diverio D, Avvisati G, et al. Molecular remission in PML/RAR alpha-positive acute promyelocytic leukemia by combined all-trans retinoic acid and idarubicin (AIDA) therapy. Gruppo Italiano-Malattie Ematologiche Maligne dell'Adulto and Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood. 1997;90(3):1014–1021.
- Mathews V, George B, Lakshmi KM, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood. 2006;107(7):2627–2632.
- Coutre SE, Othus M, Powell B, et al. Arsenic trioxide during consolidation for patients with previously untreated low/intermediate risk acute promyelocytic leukaemia may eliminate the need for maintenance therapy. Br J Haematol. 2014;165(4):497–503.
- Platzbecker U, Avvisati G, Cicconi L, et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. J Clin Oncol. 2017;35(6):605–612.
- Rahman F, Kabir AL, Khan MR, et al. Disseminated intravascular coagulation in acute promyelocytic leukaemia and its impact on the induction failure: a single centre study. Bangl Med Res Counc Bull. 2013;39(2):57–60.
- Baysal M, Gürsoy V, Hunutlu FC, et al. The evaluation of risk factors leading to early deaths in patients with acute promyelocytic leukemia: a retrospective study. Ann Hematol. 2022;101(5):1049–1057.
- Matsuda K, Jo T, Toyama K, et al. Risk factors for early in-hospital death in patients who developed coagulopathy during induction therapy for acute promyelocytic leukemia: a nationwide analysis in Japan. Ann Hematol. 2021;100(10):2613–2619.
- Sanz MA, Coco FL, Martın G, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96(4):1247–1253.
- Jillella AP, Kota VK. The global problem of early deaths in acute promyelocytic leukemia: a strategy to decrease induction mortality in the most curable leukemia. Blood Rev. 2018;32(2):89–95.
- Minamiguchi H, Fujita H, Atsuta Y, et al. Predictors of early death, serious hemorrhage, and differentiation syndrome in Japanese patients with acute promyelocytic leukemia. Ann Hematol. 2020;99(12):2787–2800.
- Stahl M, Tallman MS. Differentiation syndrome in acute promyelocytic leukaemia. Br J Haematol. 2019;187(2):157–162.
- Lehmann S, Ravn A, Carlsson L, et al. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia. 2011;25(7):1128–1134.
- Park JH, Qiao B, Panageas KS, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118(5):1248–1254.
- Chen Y, Kantarjian H, Wang H, et al. Acute promyelocytic leukemia: a population-based study on incidence and survival in the United States, 1975–2008. Cancer. 2012;118(23):5811–5818.
- Zhu H-H, Wu D-P, Jin J, et al. Oral tetra-arsenic tetra-sulfide formula versus intravenous arsenic trioxide as first-line treatment of acute promyelocytic leukemia: a multicenter randomized controlled trial. J Clin Oncol. 2013;31(33):4215–4221.
- Zhu H-H, Wu D-P, Du X, et al. Oral arsenic plus retinoic acid versus intravenous arsenic plus retinoic acid for non-high-risk acute promyelocytic leukaemia: a non-inferiority, randomised phase 3 trial. Lancet Oncol. 2018;19(7):871–879.
- Zhu H-H, Liu Y-R, Jia J-S, et al. Oral arsenic and all-trans retinoic acid for high-risk acute promyelocytic leukemia. Blood. 2018;131(26):2987–2989.
- Sasijareonrat N, Jahn N, Ungprasert P, et al. Efficacy and the adverse effects of oral versus intravenous arsenic for acute promyelocytic leukemia: a meta-analysis of randomized-controlled studies. Technol Cancer Res Treat. 2020;19:1533033820937008.
