ABSTRACT
Objective:
Antinuclear antibody (ANA)-positive immune thrombocytopenia (ITP) patients have an unsatisfactory prognosis due to the more severe conditions of these patients and poor response to first-line glucocorticoids (GCs). The current study intended to compare the efficacy and safety of AZA plus prednisone and prednisone alone as first-line treatment in ANA-positive ITP patients.
Methods:
Fifteen ANA-positive ITP patients receiving AZA plus prednisone (AZA + GC group) and eighteen ANA-positive ITP patients receiving prednisone alone (GC group) as first-line treatment were retrospectively enrolled.
Results:
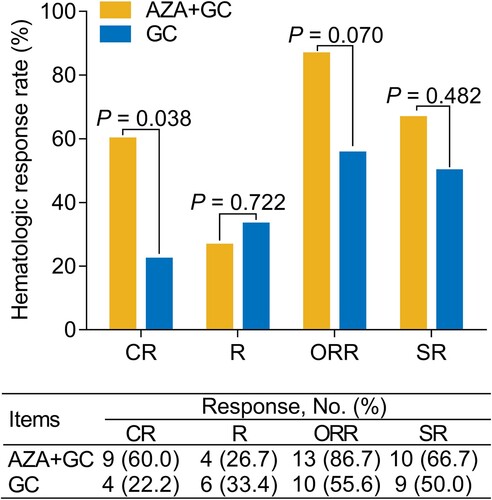
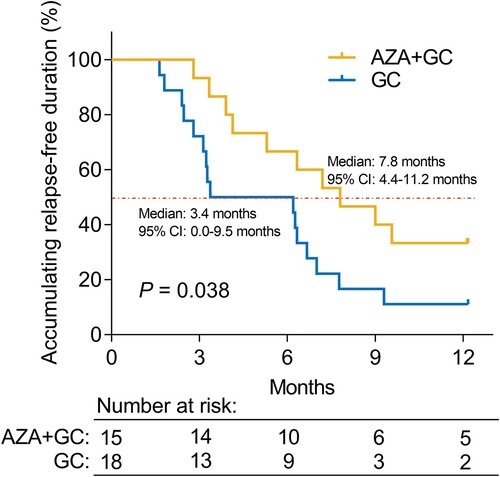
The complete response (CR) rate (60.0% versus 22.2%) (P = 0.038) was increased in the AZA + GC group versus the GC group, while the overall response rate (86.7% versus 55.6%) (P = 0.070) only showed an increasing trend that did not achieve statistical significance. In addition, multivariate analysis revealed that AZA + GC (versus GC) (odds ratio = 31.331, P = 0.018) was independently associated with a higher possibility of achieving CR. Additionally, accumulating relapse-free duration was prolonged in the AZA + GC group versus the GC group (median: 7.8 months versus 3.4 months) (P = 0.038). Additionally, the multivariate analysis suggested that AZA + GC (versus GC) (hazard ratio = 0.306, P = 0.007) was independently correlated with longer accumulating relapse-free duration. The incidence of adverse events did not differ between the two groups (all P > 0.05), and the common adverse events in the AZA + GC group were pneumonia (13.3%), anemia (13.3%), cough (13.3%), nausea (6.7%), and granulocytopenia (6.7%), which were all tolerable and manageable.
Conclusion:
First-line AZA plus prednisone realizes a better hematological response and relapse-free duration with acceptable adverse events compared to prednisone alone in ANA-positive ITP patients.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune hematologic disorder characterized by isolated blood platelet count reduction, which is common in both pediatric and adult patients [Citation1]. Notably, nearly 20%−30% of ITP patients who test positive for antinuclear antibody (ANA) are more likely to suffer from life-threatening bleeding and carry a higher risk of developing connective tissue diseases (CTDs), such as systemic lupus erythematosus (SLE) [Citation2–5]. Generally, the current first-line treatments for ITP patients are glucocorticoids (GCs) (such as high-dose dexamethasone (HD-DXM) and prednisone); however, ANA-positive ITP patients have a poor initial treatment response to GCs compared to ANA-negative ITP patients [Citation6–9]. Therefore, exploring potential first-line treatment options for ANA-positive ITP patients is imperative.
Azathioprine (AZA) is an immunosuppressant that inhibits the synthesis of purines, thereby reducing the production of white blood cells and ultimately leading to immunosuppression [Citation10]. Clinically, AZA is commonly administered to patients with autoimmune diseases, such as inflammatory bowel disease (IBD), lupus nephritis, and SLE [Citation11–14]. In terms of ITP, the guidelines suggest that AZA could serve as a subsequent treatment for these patients based on the consideration of its benefit and toxicity [Citation15–17]; meanwhile, some clinical trials have also reported the efficacy of AZA as a second-line treatment for ITP [Citation18–20]. For example, a previous study reveals that second-line AZA is effective for ITP patients, which realizes 71.4% of the response rate [Citation19]; notably, the response rate of AZA reported by this previous study is higher than that reported in other studies, which might be due to the difference in patient enrollment and the definition of response; therefore, the response rate might be overestimated [Citation17]. However, ANA-positive ITP patients usually bear a more severe condition; additionally, the potency of AZA as first-line treatment for this type of patient deserves to be investigated.
Accordingly, the current study intended to retrospectively compare the hematological response, relapse-free duration, and safety profile between AZA plus prednisone and prednisone alone as the first-line treatment in ANA-positive ITP patients.
Methods
Patients
From January 2018 to December 2021, a total of 33 ANA-positive ITP patients from our hospital were screened in this retrospective study. Patients who met the following criteria were included: a) diagnosed with primary ITP according to the International Working Group (IWG) definition [Citation21]; b) with ANA-positive, which was defined as a titer of 1:80 or more; c) age ≥18 years old; d) without human immunodeficiency virus infection; and e) negative hepatitis B and hepatitis C serology. The exclusion criteria were a) with aplastic anemia; b) with pregnant or lactating females; c) with incomplete clinical data, including laboratory test data, follow-up data, and efficacy- and safety-related information. This study was approved by the Ethics Committee. Written informed consent was obtained from each patient.
Data
The sample size was determined by convenience sampling. Demographics and laboratory test-related information of ITP patients were reviewed, in which ANA was determined by the indirect immunofluorescent technique on the HEp-2 cell substrates [Citation22]. All laboratory tests before treatment were performed by standard methods.
Treatment
Patients were divided into the GC group and the AZA + GC group according to the treatment they received. Notably, only prednisone was applied, and no patients used dexamethasone. For the GC group, patients were given first-line prednisone 1 mg/kg per day as the initial dose; the median duration of prednisone was 2.1 months with a range from 1.7–2.4 months. In addition, 10 patients were given the combination of IVIg plus platelet transfusion in this group. For the AZA + GC group, patients were given first-line AZA 50 mg twice daily in addition to the standard dose of prednisone therapy; the median duration of AZA was 6.0 months with a range from 3.2–6.6 months, and the median duration of prednisone was 2.1 months with a range from 1.9–2.3 months. Furthermore, 11 patients were given the combination of IVIg plus platelet transfusion in this group. Meanwhile, the use of AZA was based on the following considerations: (1) according to physicians’ clinical experiences and previous studies, ANA-positive ITP patients had a poor treatment response to GCs and second-line drugs, as well as a shorter remission period [Citation23–25]; thus, combining prednisone and AZA might be an option for these patients; and (2) patients’ willingness and doctors’ recommendation. Notably, two patients adjusted the dose to 50 mg once a day, but they experienced a decrease in platelets after 5 days of adjustment; therefore, the dose of AZA was elevated back to 50 mg twice a day in these two patients. Apart from these two patients, no other patients underwent AZA adjustment. For the patients who achieved CR (defined as platelet count of ≥100 × 109/L), prednisone was tapered within several weeks [Citation26].
Evaluation
The primary outcome was hematologic responses, and the secondary outcome was accumulating relapse-free duration. Hematologic responses were used to estimate the overall efficacy of current treatments, including complete response (CR) and response (R) [Citation21]. The overall response rate (ORR) was defined as the CR plus R rate. Sustained response (SR) was defined as a platelet count remaining ≥30 × 109/L for 6 consecutive months after the initial response was achieved. In addition, all patients underwent routine follow-ups, and the relapse-free duration was calculated. Relapse was defined as platelet count <30 × 109/L or bleeding. Adverse events were recorded to analyze the safety of current treatments. Notably, according to Chinese guidelines, the bleeding score for ITP patients = age score + bleeding symptom score (highest score of all bleeding symptoms) [Citation17].
Statistics
SPSS v.24.0 and GraphPad Prism v.8.0 were applied to the data processing and figure construction. The comparison analyzes used a t test, Wilcoxon rank sum test, χ2 test, or Fisher's exact test. Kaplan‒Meier curves were plotted to show the difference in accumulating relapse-free duration between the AZA + GC and GC groups, and the log-rank test was used. Univariate and forward-multivariate logistic or Cox regression analyzes were used to determine the factors related to CR or accumulating relapse-free duration, respectively. P < 0.05 indicated significance.
Results
Clinical characteristics
The median (interquartile range (IQR)) age of ANA-positive ITP patients was 52.0 (34.0-66.0) years in the AZA + GC group and 37.5 (23.8-66.5) years in the GC group (P = 0.164). Meanwhile, there were 8 (53.3%) males and 7 (46.7%) females in the AZA + GC group, as well as 5 (27.8%) males and 13 (72.2%) females in the GC group (P = 0.169). In addition, bleeding occurrence was not different between the AZA + GC group (40.0%) and the GC group (27.8%) (P = 0.458). Apart from that, other clinical features were also not different between the AZA + GC group and the GC group (all P > 0.05), including bleeding score, platelet count, lymphocytes, C-reactive protein, erythrocyte sediment rate, globulin, etc. The specific clinical information is listed in .
Table 1. Clinical characteristics of ANA-positive ITP patients.
Treatment response
CR rate was increased in the AZA + GC group compared to the GC group (60.0% versus 22.2%) (P = 0.038). Meanwhile, the ORR was numerically increased in the AZA + GC group compared with the GC group but did not achieve statistical significance (86.7% versus 55.6%) (P = 0.070). However, the R rate (26.7% versus 33.4%) (P = 0.722) and SR rate (66.7% versus 50.0%) (P = 0.482) were not different between the AZA + GC group and GC group ().
Logistics regression analysis for CR
Univariate regression analysis revealed that treatment (AZA + GC versus GC) (odds ratio (OR) = 5.250, P = 0.032) was associated with a higher possibility of achieving CR. Lymphocyte (LY) count (≥1.6 × 109/L vs. < 1.6 × 109/L) (OR = 0.121, P = 0.018) was correlated with a lower possibility of achieving CR. Further forward-multivariate regression analysis revealed that treatment (AZA + GC versus GC) (OR = 31.331, P = 0.018) was independently associated with an increased possibility of reaching CR, while LY (≥1.6 × 109/L vs. < 1.6 × 109/L) (OR = 0.071, P = 0.033) was independently related to a decreased possibility of reaching CR ().
Table 2. The logistics regression analysis of factors related to CR in ANA-positive ITP patients.
Correlation of albumin and prothrombin time with hematological responses
Albumin was only correlated with SR in the AZA + GC group (P = 0.037); however, albumin was not associated with CR, R, or ORR in this group (all P > 0.05). Regarding the GC group, albumin was not correlated with CR, R, ORR, or SR (all P > 0.05). In addition, the prothrombin time was not related to any of the hematological responses, including CR, R, ORR, and SR, in either the AZA + GC group or the GC group (all P > 0.05) (Supplementary Table 1).
Relapse-free duration
ANA-positive ITP patients underwent routine follow-up, and none of these patients exhibited any clinical suspicion of CTD. Accumulating relapse-free duration was increased in the AZA + GC group compared to the GC group (P = 0.038). The median [95% confidence interval (CI)] relapse-free duration was 7.8 (4.4-11.2) months in the AZA + GC group, while it was 3.4 (0.0-9.5) months in the GC group ().
Cox regression analysis for accumulating relapse-free duration
Univariate regression analysis revealed that treatment (AZA + GC versus GC) (hazard ratio (HR) = 0.440, P = 0.044) was related to increased accumulating relapse-free duration. Further forward-multivariate regression analysis revealed that treatment (AZA + GC versus GC) (HR = 0.306, P = 0.007) was independently correlated with prolonged accumulating relapse-free duration, while hemoglobin (Hb) (abnormal versus normal) (HR = 2.816, P = 0.038) and complement C3 (C3) (abnormal versus normal) (HR = 2.478, P = 0.032) were independently associated with shorter accumulating relapse-free duration ().
Table 3. The Cox regression analysis of factors related to accumulating relapse-free duration in ANA-positive ITP patients.
Adverse events
The incidence of each adverse event, including pneumonia, anemia, cough, nausea, and granulocytopenia, was not different between the AZA + GC group and the GC group (all P > 0.05). In detail, the incidence of pneumonia, anemia, cough, nausea, and granulocytopenia was 13.3%, 13.3%, 13.3%, 6.7%, and 6.7%, respectively, in the AZA + GC group, while the incidence of the abovementioned adverse events was 5.6% in the GC group ().
Table 4. Adverse events.
Discussion
AZA is an oral immunosuppressive drug that achieves an acceptable treatment response in ITP patients [Citation19, Citation20]. For instance, one previous study discloses that second-line AZA achieves a response rate of 55.9% in ITP patients [Citation20]. However, the comparison of treatment response between AZA plus prednisone and prednisone alone as the first-line treatment in ANA-positive ITP patients requires further exploration. The current study discovered that first-line AZA plus prednisone achieved a better CR rate (60.0% versus 22.2%) than prednisone alone in ANA-positive ITP patients, and this finding was further confirmed by multivariate logistic regression analysis. The potential reason would be that (1) AZA itself could suppress the immune response by inhibiting the purine pathway, thereby alleviating thrombocytopenia [Citation10, Citation27], and (2) AZA might assist with prednisone to regulate T lymphocytes and natural killer (NK) lymphocytes, thereby contributing to the increase in platelet counts [Citation28, Citation29]. Notably, ORR (86.7% versus 56.6%) was also numerically increased in ANA-positive ITP patients receiving AZA plus prednisone compared to those receiving prednisone alone but did not reach statistical significance. The potential reason might be that the sample size was not large enough; thus, the ORR only slightly increased in patients receiving AZA plus prednisone versus those receiving prednisone alone. Further large-scale studies are required to validate these findings.
In addition, this study also compared the relapse-free duration between ANA-positive ITP patients receiving AZA plus prednisone and those receiving prednisone alone as first-line treatment. The median accumulating relapse-free duration was 7.8 months in ANA-positive ITP patients receiving AZA plus prednisone and 3.4 months in those receiving prednisone alone. Meanwhile, first-line AZA plus prednisone achieved a longer accumulating relapse-free duration than prednisone alone in ANA-positive ITP patients, which was further verified by multivariate Cox regression analysis. A possible reason would be that as discussed above, AZA plus prednisone as the first-line treatment achieved a better treatment response than prednisone alone; therefore, the relapse-free duration was longer in ANA-positive ITP patients receiving AZA + GC than in those receiving prednisone alone. Additionally, this study also found that abnormal Hb and C3 were independent risk factors for achieving shorter accumulating relapse-free duration in ANA-positive ITP patients. The potential reason would be that abnormal Hb and C3 reflected damaged liver function, while liver injury could lead to decreased synthesis of thrombopoietin, resulting in reduced platelet production, which might ultimately contribute to the relapse of ITP [Citation30, Citation31].
Importantly, the comparison of safety between first-line AZA plus prednisone and prednisone alone in ANA-positive ITP patients is rarely reported. The present study discovered that the incidence of all adverse events was not different between ANA-positive ITP patients receiving AZA plus prednisone and those receiving prednisone alone as first-line treatment. This finding indicated that first-line AZA plus prednisone had a good safety profile for ANA-positive ITP patients. In addition, the common adverse events induced by AZA are leucopenia, anemia, and hepatobiliary laboratory abnormalities in ITP patients according to a previous study [Citation14]. The prevalent adverse events caused by prednisone are a cushingoid appearance, weight gain, hypertension, hyperglycemia, insomnia, and dizziness in ITP patients [Citation32]. The present study discovered that common adverse events caused by first-line AZA plus prednisone were pneumonia (13.3%), anemia (13.3%), cough (13.3%), nausea (6.7%), and granulocytopenia (6.7%) in ANA-positive ITP patients, which were partly in line with previous studies [Citation14, Citation32]; meanwhile, no new adverse events occurred. Taken together, AZA plus prednisone as a first-line treatment might be safe and tolerable for ANA-positive ITP patients.
Notably, although multivariate regression analyzes were applied in this study, limited by the small sample size, the sensitivity (or precision) of the results might be greatly affected. In addition, the overfitting effect of multivariate regression analysis might also occur due to the small sample size. Based on this consideration, forward regression analysis was used to solve the overfitting problem by putting one variable into the analysis at a time instead of putting all variables into the analysis at once by backward regression analysis. However, the findings revealed by multivariate regression analysis still needed large-scale studies to verify.
Several limitations should be noted in this study: (1) the sample size was small, which led to low statistical power and affected the generalization of this study; (2) this was a retrospective study, and although multivariate regression analysis was conducted, some biases and confounding factors might still exist; and (3) this study only explored the potency of first-line AZA plus prednisone; however, the efficacy of AZA plus other GCs (such as HD-DXM) as first-line treatment for ANA-positive ITP should be further investigated.
To sum up, first-line AZA plus prednisone may achieve superior treatment response and relapse-free duration to a certain extent with acceptable tolerance versus prednisone alone for ANA-positive ITP. However, further large-scale studies are required to validate the findings of this study.
Author’s contribution
Junnan Su, Meihong Xu and Xuyan Chen designed the study. Zhigao Dong, Qingqing Wang, Lili Ma and Pingping Xiao collected the data. Junnan Su, Meihong Xu and Xuyan Chen analyzed the data. All authors contributed to drafting and reviewing the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cooper N, Ghanima W. Immune thrombocytopenia. N Engl J Med. 2019;381(10):945–955.
- Liu Y, Chen S, Yang G, et al. ANA-positive primary immune thrombocytopaenia: a different clinical entity with increased risk of connective tissue diseases. Lupus Sci Med. 2021;8(1):e000523.
- Altintas A, Ozel A, Okur N, et al. Prevalence and clinical significance of elevated antinuclear antibody test in children and adult patients with idiopathic thrombocytopenic purpura. J Thromb Thrombolysis. 2007;24(2):163–168.
- Ahn SM, Choi EJ, Oh JS, et al. Prognostic factors for the development of systemic lupus erythematosus in patients with immune thrombocytopenia. Arthritis Res Ther. 2022;24(1):213.
- Liu Q, Xu H, Guan X, et al. Clinical significance of antinuclear and antiextractable nuclear antigen antibody in childhood immune thrombocytopenia. Semin Thromb Hemost. 2017;43(6):629–634.
- Bolton-Maggs PHB, George JN. Immune thrombocytopenia treatment. N Engl J Med. 2021;385(10):948–950.
- Kochhar M, Neunert C. Immune thrombocytopenia: a review of upfront treatment strategies. Blood Rev. 2021;49:100822.
- Huang QS, Liu Y, Wang JB, et al. All-trans retinoic acid plus high-dose dexamethasone as first-line treatment for patients with newly diagnosed immune thrombocytopenia: a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Haematol. 2021;8(10):e688–e699.
- Abbasi SY, Milhem M, Zaru L. A positive antinuclear antibody test predicts for a poor response to initial steroid therapy in adults with idiopathic thrombocytopenic purpura. Ann Hematol. 2008;87(6):459–462.
- Broen JCA, van Laar JM. Mycophenolate mofetil, azathioprine and tacrolimus: mechanisms in rheumatology. Nat Rev Rheumatol. 2020;16(3):167–178.
- Fu Q, Wu C, Dai M, et al. Leflunomide versus azathioprine for maintenance therapy of lupus nephritis: a prospective, multicentre, randomised trial and long-term follow-up. Ann Rheum Dis. 2022;81(11):1549–1555.
- Reynolds JA, Gayed M, Khamashta MA, et al. Outcomes of children born to mothers with systemic lupus erythematosus exposed to hydroxychloroquine or azathioprine. Rheumatology (Oxford). 2022;62(3):1124–1135.
- Wilson L, Tuson S, Yang L, et al. Real-World Use of azathioprine metabolites changes clinical management of inflammatory bowel disease. J Can Assoc Gastroenterol. 2021;4(3):101–109.
- Mishra K, Pramanik S, Sandal R, et al. Safety and efficacy of azathioprine in immune thrombocytopenia. Am J Blood Res. 2021;11(3):217–226.
- Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–4207.
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–3866.
- Thrombosis, Hemostasis Group CSoHCMA. Chinese guideline on the diagnosis and management of adult primary immune thrombocytopenia (version 2020). Zhonghua Xue Ye Xue Za Zhi. 2020;41(8):617–623.
- Poudyal BS, Sapkota B, Shrestha GS, et al. Safety and efficacy of azathioprine as a second line therapy for primary immune thrombocytopenic purpura. JNMA J Nepal Med Assoc. 2016;55(203):16–21.
- Chang H, Tang TC, Hung YS, et al. Immune thrombocytopenia: effectiveness of frontline steroids and comparison of azathioprine, splenectomy, and rituximab as second-line treatment. Eur J Haematol. 2018;101(4):549–555.
- Abdallah GEM, Elbiih EAS, Sayed D, et al. Revisiting the management of chronic ITP; a randomized controlled clinical trial. Platelets. 2021;32(2):243–249.
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393.
- von Muhlen CA, Garcia-De La Torre I, Infantino M, et al. How to report the antinuclear antibodies (anti-cell antibodies) test on HEp-2 cells: guidelines from the ICAP initiative. Immunol Res. 2021;69(6):594–608.
- Li HQ, Zhang L, Zhao H, et al. Chronic idiopathic thrombocytopenic purpura in adult Chinese patients: a retrospective single-centered analysis of 1791 cases. Chin Med J (Engl). 2005;118(1):34–37.
- Baysal M, Bas V, Umit E, et al. Could antinuclear antibody positivity be a factor affecting treatment response in immune thrombocytopenia patients on eltrombopag? Turk J Haematol. 2022;39(1):38–42.
- Wang YM, Yu YF, Liu Y, et al. The association between antinuclear antibody and response to rituximab treatment in adult patients with primary immune thrombocytopenia. Hematology. 2020;25(1):139–144.
- Neunert CE, Cooper N. Evidence-based management of immune thrombocytopenia: ASH guideline update. Hematology Am Soc Hematol Educ Program. 2018;2018(1):568–575.
- Audia S, Mahevas M, Samson M, et al. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 2017;16(6):620–632.
- Xu J, Wang G, Tan S, et al. The clinical effect of Prednisone in combination with mycophenolate mofetil on idiopathic thrombocytopenic purpura (ITP) and its influence on the level of peripheral blood T lymphocytes and NK lymphocytes. Saudi J Biol Sci. 2019;26(8):2108–2112.
- Okoye IS, Xu L, Walker J, et al. The glucocorticoids prednisone and dexamethasone differentially modulate T cell function in response to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade. Cancer Immunol Immunother. 2020;69(8):1423–1436.
- Scharf RE. Thrombocytopenia and hemostatic changes in acute and chronic liver disease: pathophysiology, clinical and laboratory features, and management. J Clin Med. 2021;10(7):1530.
- van Dievoet MA, Eeckhoudt S, Stephenne X. Primary hemostasis in chronic liver disease and cirrhosis: what Did We learn over the past decade? Int J Mol Sci. 2020;21(9):3294.
- Wei Y, Ji XB, Wang YW, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood. 2016;127(3):296–302. quiz 370.