ABSTRACT
Objective
Accumulating evidence suggests the role of immune-inflammatory markers in early risk stratification and prognostication of COVID-19 patients. We aimed to evaluate their association with severity and the development of diagnostic scores with optimal thresholds in critical patients.
Setting and participants
This retrospective case study includes hospitalized COVID-19 patients from March 2019 to March, 2022, in the developing area teaching hospital in Pakistan. Polymerase chain reaction (PCR) positive patients, n = 467 were investigated for clinical outcomes, comorbidities and disease prognosis. The plasma levels of Interleukin-6 (IL-6), Lactate dehydrogenase (LDH), C-reactive protein (CRP), Procalcitonin (PCT), ferritin and Complete blood count markers were measured.
Results
Majority were males (58.8%) and patients with comorbidities had more severe disease. Hypertension and diabetes mellitus were the commonest comorbidities. Shortness of breath, myalgia and cough were the main symptoms. The hematological markers NLR, as well as the plasma levels of immune-inflammatory variables, IL-6, LDH, Procalcitonin, Erythrocyte sedimentation rate, Ferritin were markedly raised in severe and critical patients (p < 0.0001 for these markers). ROC analysis supports IL-6 as the most accurate marker with high prognostic relevance with proposed cut-off threshold (43 pg/ml), determining >90% of patients in terms of COVID-19 severity (AUC = 0.93, 91.7%, se; 90.3%sp). Furthermore, positive correlation with all other markers including NLR with cut-off = 2.99 (AUC = 0.87, se = 89.8%, sp = 88.4%), CRP with cut-offs at 42.9 mg/l, (AUC = 0.883, se = 89.3% and sp = 78.6%), LDH cut-off at 267μg/L, evidenced in >80% patients (AUC = 0.834 se = 84% and sp = 80%). Additionally, ESR and ferritin have the corresponding AUC 0.81 and 0.813 with cut-off at 55 mm/hr and 370, respectively.
Conclusion
Investigating the immune-inflammatory markers can assist physicians in providing prompt treatment and ICU admission in terms of COVID-19 severity. As a result, which may reduce the overall mortality of COVID-19 patients.
Key messages
Immune-inflammatory markers along with hematological parameters can serve as diagnostic and prognostic markers of disease severity as reported in different studies.
Data from the patients of an underdeveloped division in Pakistan reported a significant elevation of these markers in critical and severe patients.
In low resource areas, routine markers can serve as a key indicator to provide information for the diagnosis and cut-off for differentiating severe from non-severe category along with providing treatment basis for health professionals.
1. Introduction
The horrible COVID-19 pandemic caused by the SARS CoV-2 virus, since 2019, has disproportionately affected the global population [Citation1]. Most of the patients who suffered from this disease had mild symptoms ranging from common flu or cold-like illness to severe symptoms that increased the risk of mortality for these patients. Approximately 50% of the individuals were asymptomatic. However, evidence show that older and immune-comprised population with underlying comorbidities are comparatively more vulnerable to severe disease and mortality [Citation2]. Similarly, a review of literature shows that cytokine storm and immune-inflammatory response are more likely the cause of multiple-organ failure and mortality [Citation3]. Hence, there is a need to find adequate indicators that can appropriately asses the prognosis and severity of the COVID-19 disease in order to reduce the mortality rate in these patients. Several observational studies revealed that critical COVID-19 patients have elevated plasma levels of several inflammatory markers, mainly C-reactive protein, interleukin-6 (IL-6), LDH, ESR including the hematological parameters [Citation4–7]. Additionally, the autopsy analyses reports have shown that elevated levels of these inflammatory markers are most likely due to interstitial macrophage infiltrations in the cardiac, pulmonary and GIT systems along with extensive tissue necrosis in severely affected individuals [Citation8,Citation9]. Disruption of the angiotensin-converting enzyme (ACE2) and Cytokine storms are responsible for pathogenesis of this disease [Citation10,Citation11]. As the virus activates the macrophages, natural killer (NK) cells, and other immune-inflammatory cells release chemokines by invading the lung endothelial cells after attaching with the ACE2 receptors on the surface epithelial cells [Citation12]. This dysregulated inflammatory response causes cytokine storm leading to multiple-organ damage [Citation13]. Similarly, serum PCT levels are also associated with poor outcomes comprising mortality, ARDS and critical individuals requiring ICU care or severe COVID-19 infection [Citation14]. Procalcitonin is a precursor protein for calcitonin hormone. PCT has been studied extensively as a potential biomarker for early detection of bacterial infection [Citation15]. It is recommended to test PCT values in critical patients as elevated levels may suggest a superimposed bacterial co-infection. Hence, increasing the severity of disease and more chances of developing systemic sepsis. Consequently, promoting antibiotic stewardship by allowing the targeted use of antimicrobials in COVID-19 [Citation16]. Moreover, due to research conducted on potential risk factors for COVID-19, it is becoming clear that comorbidities like HTN, diabetes, Heart diseases, liver and kidney diseases, COPD, Asthma and HIV/AIDS, etc. also poses a great risk for COVID-19 mortality [Citation2].
Our diagnostic criteria include respiratory rate, fever, oxygen saturation, parenchymal infiltrates to classify patients based on severity. The National Health Commission of the People’s Republic of China issued the ‘novel coronavirus infected pneumonia treatment scheme-Sixth edition’ [Citation11], the asymptomatic and mild or moderate group having symptoms related to fever, respiratory tract infection and radiographic evidence of pneumonia. The severe group include patients with oxygen saturation below 93% or radiographic evidence of pulmonary infiltrates along with shortness of breath and PaO2/FiO2 is 300 mmHg or below this. Similarly, critical group has multi-organ failure, shock or respiratory failure occurs, and need for mechanical ventilation [Citation11].
Our study aims to investigate whether comorbidities, NLR, IL-6, LDH, PCT, CRP, ESR levels are associated with risk stratification and coarse of diagnosis for COVID-19. Additionally, we aimed to determine the diagnostic score along with optimal threshold in critical and severe patients along with the outcome of disease.
2. Materials and methods
Study population and Sample collection
In this single-centered retrospective case study, patients were admitted in the various isolation wards of Sahiwal Teaching Hospital in Sahiwal division, Pakistan. Patients’ information was extracted from the hospital information system. The clinical records of recruited patients were particularly scrutinized by searching for the presence of a bacterial co-infection. The microbiological analyses, i.e. positive blood cultures or lower respiratory tract including sputum, broncho-alveolar lavage fluid and bronchial aspirate was also done.
A total of n = 467 COVID-19 positive patients were included with written consent taken from all of them. Demographical information including age, gender, address, occupation, date of admission and comorbidities was also recorded. Complete case analysis was ensured by following patients until death in case of hospitalized patients or discharge from hospital, whichever occurred first. Data were independently recruited by two investigators from the patient’s files and hospitals record. Corrected and validated data were entered into the databases. Considering it as an observational study, there was no limitation in terms of sample collection and predefined sample size, adding as many patient’s data as was available, those meeting the inclusion criteria. In order to examine the ability of medical tests to clearly categorize the patients having that target medical condition, ‘Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)’ [Citation17] and ‘Standards for Reporting Diagnostic accuracy Studies STARDS’ guidelines for reporting of diagnostic studies were followed [Citation18].
Sample processing
A venous blood sample (∼3 mL) was taken in an EDTA vacutainer tube on the 2nd day of admission to the hospital from each patient. For leukocytes measurement, we used Automatic Hematology Analyzer Swelab Alfa Standard (Boule Medical AB, Sweden). Similarly, Atellica Solution Immunoassay & Clinical Chemistry Analyzers (Siemens Healthcare Diagnostics, Erlangen, Germany) were used to measure immune-inflammatory markers. The collected samples for PCT testing were centrifuged at 3000–6000 rpm for 10 min to obtain a clear supernatant serum. The samples were tested with Chemiluminescence immunoassay (CLIA) using an automated analyzer Architect system of the Abbott diagnostic services. The CLIA was used to measure the concentration of procalcitonin (PCT).
Inclusion Criteria
The hospitalized patients with COVID-19 positive real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) in ICU as well as isolation wards, were recruited.
Exclusion Criteria
Individuals with hematological and oncological abnormalities, under 18 years and over age 70 years, and those symptomatic individuals with PCR status unknown were excluded.
Statistical analysis
The statistical analysis of the data was performed using R statistical software (Version 4.2.0) and Medcalc (Version 19.3). The demographical characteristics of the patients were expressed as number and percentage n (%). To assess the normality of the data distribution, the Kolmogorov–Smirnov test, Q-Q plots and histogram were used. For continuous variables, mean and standard deviation were used to mention them and categorical variables were described as frequency and percentage. The qualitative analysis of the data was performed by the Chi-square test. Continuous data with a skewed distribution were compared with the Wilcoxon rank-sum test. One samples t-test was used for analysis of parametric continued inflammatory markers compared with their reference values. Severity was taken as dependent variable and univariate logistic regression analysis was evaluated to determine the prognostic value of immune-inflammatory markers. Spearman’s rank correlation analysis was used to examine the correlation between IL-6 and all other variables. A receiver-operating characteristic (ROC) curve was also generated to determine the efficacy of these inflammatory markers to distinguish critical cases from the non-severe ones. Optimal discriminating cut-off values were determined by Youden index. Fisher’s exact test was used to calculate the odds ratio (95% confidence interval) from the cut-off values. The p-value <0.05 was considered significant. The correlation between different variables was examined.
3. Results
Demographic and clinical characteristics
Our study participants include (n = 476) patients having COVID-19 positive PCR result. Among them, 196(41.2%) were females and 280(58.5%) were predominantly males. There was no difference in gender based on the severity of disease. Participants were divided into two groups, ‘severe’ and ‘non-severe’ based on the NHS classification [Citation11]. Compared with non-severe group, patients in severe group were older, n = 74(35.1%). The clinical outcomes and comorbidities of patients most frequently include Hypertension (26.8%), and Diabetes Mellitus (24.15%) followed by Ischemic Heart Disease, Liver Diseases (HBV+ve, HCV+ve, Cirrhotic Liver Disease), Asthma, Post-TB, COPD, etc. Compared to non-severe group, nearly all severe patients have associated comorbidities identified .
Prognostic value of inflammatory markers to assess the disease severity
Table 1. Demographic and clinical characteristics of patients with COVID-19.
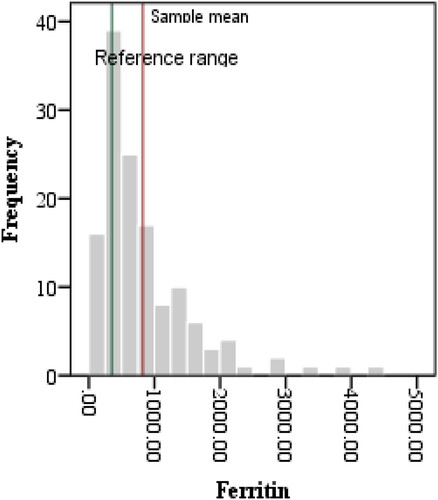
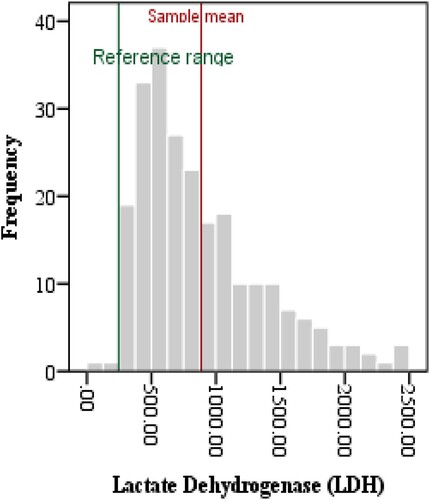
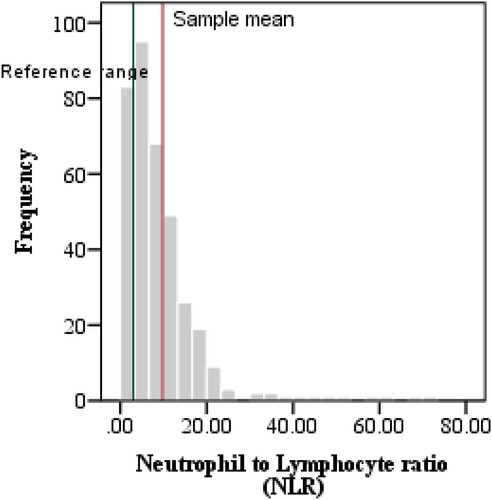
The complete blood count variables indicated Lymphocytopenia and neutrophilia, hence, NLR was elevated in critical patients when compared with reference values (11.16 ± 9.47, p < 0.0001). Similarly, ESR was also raised as compared to its reference value (32.9 ± 21.39, p < 0.0001). Furthermore, the levels of IL6, CRP, LDH, PCT and Ferritin in plasma samples were also compared with international reference ranges as shown in . The above-mentioned parameters were significantly increased as compared to their mean reference values as depicted in .
ROC analysis and diagnostic score
Table 2. Shows immune inflammatory markers among severe cases.
Receiver Operating Characteristics curve analysis was done as there was a statistically significant difference between severe and non-severe cases. The ROC curve analysis performed for the above-mentioned variables depicted a significant area under curve (AUROC) for Interleukin-6 = 0.934, followed by CRP, NLR, PCT, LDH, ESR and ferritin. Their corresponding AUROC were 0.883, 0.871, 0.869, 0.843, 0.835 and 0.811, respectively, reflecting a high prognostic performance of these inflammatory parameters to assess severe forms of COVID-19. As shown in , and .
Figure 1. shows ROC curves of the immune inflammatory markers according to the severity in COVID-19 patients.
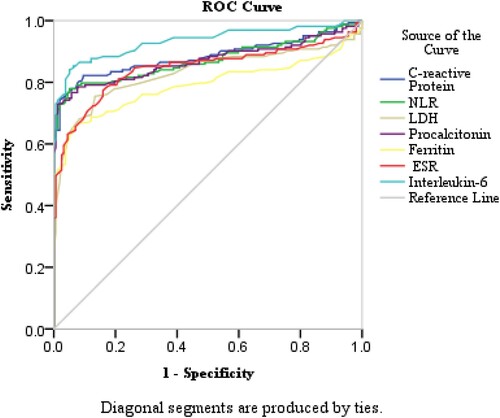
Table 3. Shows optimal cut-off values according to severity in critical COVID-19 patients 95% Confidence Interval.
The cut-off point for IL-6 values was found to be 43 pg/mL (AUC = 0.934; p < 0.0001, with 91% sensitivity (se) and 90% specificity (sp). For CRP, cut off point was 11.9 mg/L (AUC = 0.883; p < 0.0001, se = 89%, sp = 87%). Similarly, NLR has cut-off at 2.99 with corresponding AUC = 0.871 (p < 0.0001, se = 90% and sp 88%), LDH has 266.6 U/L cut-off, (AUC = 0.843; p < 0.0001, se = 85% and sp 82%). For Procalcitonin, cut-off point was 0.23 µg/L (AUC = 0.869; p < 0.0001, se = 83%, sp = 82%), ESR has 55 mm/hr threshold (AUC = 0.835; p < 0.0001, se = 82%, sp = 81%), and ferritin has 370 µg/L cut-off value.
4. Discussion
Accumulating evidence suggests the role of inflammatory cytokines in the pathogenesis of severe and critical cases of COVID-19 infection [Citation20,Citation21] A review of literature shows the elevated levels of hematological and biochemical markers including IL6, CRP, ESR, PCT, neutrophil count and NLR in these individuals. Hence, their levels reflect the intensity of cytokine-mediated immune response, and increased mortality [Citation22,Citation23].
In the middle income and developing countries including Pakistan, the COVID-19 crisis has put a huge burden on the health facilities. There is lack of diagnostic facilities and patient management expertise specifically in small cities where health resources are scarce. The focus of our study population, Sahiwal, is also a peripheral city of Punjab where COVID-19 rRT-PCR, CT scan, MRI, are not readily available to lower income population. Hence, there is a need for alternative inexpensive tests as prognostic markers to determine the severity of Covid-19 disease. Laboratory tests including CBC and biochemical tests, widely used in clinical practice, are cost-effective and require comparatively little medical expertise to perform and interpret in many clinical conditions [Citation20,Citation24]. In our study, we observed Complete blood count markers along with immune-inflammatory markers. Additionally, we investigated that people having comorbidities are at increased risk for acquiring COVID-19 and for poor progression. Data related to gender in terms of severity is not statistically significant. However, age categories have shown that older people are more prone to develop disease severity [Citation25]. Comorbidities and associated diseases adversely affected the outcome of illness. Hypertension, Diabetes mellitus were the most common diseases and severe group seems to be more vulnerable to these comorbidities and worse prognosis. Similarly, Lymphopenia is observed in COVID-19 patients by many studies Lymphopenia is associated with variations in NLR ratio, which we want to evaluate in our study as the indicator for making diagnosis, predicting progression and severity of the disease. NLR was significantly elevated in severe and critical patients [Citation26]. Neutrophil and lymphocyte count combines to form NLR. Increased Neutrophil level signifies the severity of systemic inflammation while, lymphopenia refers to their sequestration in the site of inflammation and their apoptosis. Their combined value is a more valuable prognostic marker of COVID-19 severity [Citation27]. Our findings suggest a higher area under curve for NLR, and hence the highest accuracy among all hematological markers in terms of severe and critical cases with the proposed cut-off value of 2.99 (, ). Their values are not symmetrically distributed and the sample mean is greater than the reference mean (). Accumulating evidence in the literature proposes the cut-offs between 3 and 6 (3.13 [Citation28]‘ 3.3 [Citation12] 4 [Citation29]‘ 5 [Citation30]‘ 5.5 [Citation31] 5.87) [Citation32]. Regarding the serum levels of inflammatory markers, IL-6, CRP, PCT, ESR and ferritin were markedly elevated in severe and critical patients. These results correspond to cytokine release syndrome (CRS) hypothesis [Citation33].
Procalcitonin (PCT) is a precursor of hormone calcitonin. In normal individuals, it is produced by the para-follicular C cells of the thyroid. However, in cases of different bacterial infections, the mesodermal cells in various tissues such as the neuroendocrine pulmonary cells secrete it in response to endotoxin bacterial stimuli. Similarly, the release of interleukin-1 (IL-1), Interleukin-6 (IL-6) and Tumor necrosis factor-alpha TNF, and intestine, liver, kidney, fat tissues are also responsible for the production of PCT [Citation15]. Le Moullec et al. in 1984, identified it first time in patients and Assicot et al. found its association with infection and sepsis [Citation34,Citation35]. One of the main biomarkers of bacterial infection is serum PCT, giving valuable information for supporting antibiotic stewardship and prognostic algorithms while treating lower respiratory tract infections as well as multi-organ failure. Recent literature reported its elevated level in critical and deceased COVID-19 patients [Citation7]. Besides, PCT is recommended for better prognosis and improving the diagnosis in severe and non-severe COVID-19 patients [Citation34]. Additionally, the Infectious Diseases Society of America (IDSA) Sepsis Task Force [Citation36], Italian Society of Clinical Biochemistry and Clinical Molecular Biology/Academy of Emergency Medicine and Care, and the Society of Critical Care Medicine/European Society of intensive care medicine have also recommended PCT. As it helps not only in diagnosing sepsis and severe bacterial infections but also for Guiding efficiency of antimicrobial therapy [Citation15]. However, not many studies from Pakistan have specifically focused on the role of PCT determination in COVID-19 patients. Due to above-mentioned limitations, PCT was analyzed for those individuals with super-imposed co-infection as it helped in validating the appropriateness of this diagnostic test. Moreover, viral infection hinders PCT levels due to interferon gamma (IFN-γ) production. This explains why in mild cases serum concentration of PCT remains regular although increased value can indicate a concurrent or superimposed bacterial infection. So PCT could be used as an indicator for diagnosis, progression and for monitoring treatment therapy for COVID-19 [Citation37]. Our results have depicted a cut-off value of 0.23 values ng/mL for severity (, and , ). Similar to the previously conducted studies, our results augment the evidence of the super-imposed bacterial infections in severe and critical COVID-19 patients, justifying the need for rational use of antibiotics in them. While, the prior studies, suggested the variable threshold levels from: 0.07 ng/mL to 0.5 ng/mL [Citation15,Citation30].
The role of CRP, the acute phase reactant in systemic inflammation has been discussed in many prior studies. Our results have defined the cut-offs at 42.9 mg/L, with 89.3% se and 78.6% sp. (p < 0.0001). These values fall in between those of results of previous studies: 20.42 mg/L [Citation35]‘ 41.4 mg/L [Citation38]‘ 60 mg/L [Citation36] and 97 mg/L [Citation39] ( and , ).
IL-6, a pro-inflammatory cytokine, causes production of chemokines and many acute-phase proteins. It also regulates the sequestration of neutrophil via STAT3 signaling pathway during an acute inflammatory reaction [Citation40]. In our study, IL-6 proves to be a comparatively valuable prognostic marker with 91.7% sensitivity and 90.3% specificity. Our proposed cut-off value (43 pg/mL) could determine >90% of patients in terms of COVID-19 severity (AUROC = 0.934). Prior studies demonstrate it variably, ranging between 24 and 32 pg/mL [Citation41–43]. Aziz et al. suggested a cut-off value of 55 pg/mL in the severe group in his meta-analysis of 1246 patients [Citation44]. Literature reports included fewer data regarding the predictive value of IL6 in terms of severity and mortality. Similarly, in patients with mechanical ventilation, Herold et al proposed cutoff = 80 pg/mL and median duration of 1.5 days [Citation45]. While, in deceased patients, Chen et al suggested the median range of IL-6 = 72 pg/mL (IQR – 35.6–146.8) [Citation46]. Additionally, in severe and critical patients of COVID-19 patients, the studies have shown that the IL6 levels are 10–200 fold lower as compared to acute respiratory distress syndrome (ARDS) and septic shock patients [Citation47–50]. Hence, lung tissues are the main site of hyper-inflammatory response with raised IL-6 in those sites as compared with their circulating plasma levels [Citation51]. However, this mechanism still remains unclear in case of COVID-19 pathogenesis [Citation52]. Our results have shown a positive correlation among IL6 and other immune-inflammatory markers NLR, CRP while negative correlation with lymphocyte levels ( and , ). Moreover, in case of COVID-19, studies have shown that IL-6 causes lymphocytopenia [Citation53]. Additionally, Tocilizumab (anti-IL-6 receptor) has been proved effective in severe and critical COVID-19 patients with decreased lymphocyte count.
LDH, an intracellular enzyme in almost all cells of the body, has been used as a cardiac marker since a long time. However, its elevated levels in plasma suggest multiple organ injury with decreased oxygenation and upregulation of glycolytic pathway. The cytokine-mediated cell injury in severe inflammation causes LDH release in the blood. Several studies reported the evidence of raised levels in severe COVID-19 patients [Citation54]. Our results have shown significantly raised median value of 885.54 ± 492.56U/L as compared to the reference values 236U/L ( and , ). The ROC analysis shows AUC = 0.834 with a proposed cut-off value = 267 μg/L, and evidenced in >80% patients with se = 84% and sp = 80%. These results are comparable to a pooled meta-analysis of 2661 patients with AUC = 0.844 and cut-off value = 263.6 μg/L, with 87.5% sensitivity and a specificity of 75% [Citation10]. A review of literature shows LDH as one of the most common markers in patients with ARDS [Citation55] as well as MERS-COV [Citation56], H5N1 [Citation57] infections. Hence, significantly elevated levels of LDH isozyme released from injured lung tissue justifies the pathogenesis in case of COVID-19.
Ferritin levels have been recently cited as indicators of severity and mortality in COVID-19 [Citation58]. Our results demonstrate an AUC of 0.81 with 74.6% sensitivity and 84% specificity with cut-off value 370 µg/L ( and , ). However, in recent COVID-19 sepsis guidelines, ferritin or transferrin were not considered comparative to other inflammatory markers. Its production is related to increased concentration in the cell and the remaining being stored and subsequently sequestered out. It is also reported as a direct indicator of cell damage in case of sepsis. However, different studies have reported hyperferritinemic syndromes as possible modifications in severe and critical cases of COVID-19 [Citation59–63].
Literature reports have shown an increased ESR as COVID-19 severity worsens. Pu et al reported an unexplained elevation in COVID-19 patient after recovery [Citation36]. Similarly, Xie et al., have shown that 72.1% patients have raised ESR while observing disease progression in 16,526 patients. However, the data are scarce and very few studies are available. Our results have shown a cut-off value of 55 mm/hr with 82.4% se and 80.7% sp. Hence, ESR could be valuable marker in predicting COVID-19 severity and mortality.
However, we recommend more studies with large sample population to clearly understand the mechanism of these inflammatory markers in determining the fatalities. Moreover, our study cohort consists of hospitalized patients and those seeking medical care only. Hence, a large-scale study is needed to include a wider population.
Conclusion
Severe and critical COVID-19 patients have elevated immune-inflammatory markers. Hence, supporting their diagnostic and prognostic evidence in determining the course as well as the clinical outcome in terms of COVID-19 severity. The proposed cut-off thresholds can help identifying the patients require anti-inflammatory and IL-6 blockade therapies. Similarly, PCT levels can guide antibiotic therapy in those patients. However, NLR and PCT are cost-effective alternative markers as their blood levels are not influenced by steroids therapy. All the investigated markers could be useful in COVID-19 diagnosis, along with other reference tests in high clinical suspicion and PCR limited settings.
Author contributions
AK was involved in conception, design, analysis and final review. JA did the drafting and revising the paper. MS prepared the final draft. AN and MR collected data and revised the final draft for intellectual review.
Consent for publication
Informed consent was granted from all the study participants and sources whose data and figures were used.
Ethical approval
This study was approved by the Sahiwal medical college ethical committee (vide letter SLMC/DME/345) and Government of Pakistan. This study is in accordance to the principles of the ‘World Medical Association Helsinki Declaration’.
Acknowledgements
We are indebted to all the healthcare professionals and patients who by giving consent to use their data, paved the way towards it.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
- Khalid A, Ali S. COVID-19 and its challenges for the healthcare system in Pakistan. Asian Bioeth Rev. 2020;12(4):551–564.
- Estenssoro E, Loudet CI, Ríos FG, et al. Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): a prospective, multicentre cohort study. Lancet Respir Med. 2021 Sep 1;9(9):989–998.
- Rabaan AA, Al-Ahmed SH, Muhammad J, et al. Role of inflammatory cytokines in COVID-19 patients: a review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines (Basel). 2021;9:436. [Internet]. 2021 Apr 29 [cited 2022 May 11];9(5):436. Available from: https://www.mdpi.com/2076-393X/9/5/436/htm.
- Soraya G, clinica ZU-M. 2020. Undefined. Crucial laboratory parameters in COVID-19 diagnosis and prognosis: an updated meta-analysis. Elsevier [Internet]. [cited 2022 May 12]; Available from: https://www.sciencedirect.com/science/article/pii/S0025775320303444.
- Yuan X, Huang W, Ye B, et al. Changes of hematological and immunological parameters in COVID-19 patients. Int J Hematol. 2020 Oct;112(4):553–559.
- Moderbacher CR, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020 Nov;183(4):996–1012.
- Xu JB, Xu C, Zhang RB, et al. Associations of procalcitonin, C-reaction protein and neutrophil-to-lymphocyte ratio with mortality in hospitalized COVID-19 patients in China. Sci Rep. 2020;10(1):15058. doi:10.1038/s41598-020-72164-7.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Apr 1;8(4):420–422.
- Yao X, Li T, He Z, et al. 2020. Undefined. A pathological report of three COVID-19 cases by minimal invasive autopsies. europepmc.org [Internet]. [cited 2022 May 12]; Available from: https://europepmc.org/article/med/32172546.
- Hariyanto TI, Japar KV, Kwenandar F, et al. Inflammatory and hematologic markers as predictors of severe outcomes in COVID-19 infection: a systematic review and meta-analysis. Am J Emerg Med. 2021 Mar 1;41:110–119.
- Keam S, Megawati D, Patel SK, et al. Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection. Rev Med Virol. 2020 Sep;30(5). [cited 2022 May 12] Available from: /pmc/articles/PMC7404843/.
- Fara A, Mitrev Z, Rosalia RA, et al. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020 Sep;10(9). [cited 2022 May 12] Available from: https://pubmed.ncbi.nlm.nih.gov/32961074/.
- Henry BM, Aggarwal G, Wong J, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020 Sep 1;38(9):1722–1726.
- Zeng F, Huang Y, Guo Y, et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020 Jul;96:467–474. [cited 2022 May 11] Available from: http://www.ijidonline.com/article/S1201971220303623/fulltext.
- Lippi G, Cervellin G. Procalcitonin for diagnosing and monitoring bacterial infections: for or against? Clinical chemistry and laboratory medicine. Walter de Gruyter GmbH. 2018;56:1193–1195.
- Waris A, Din M, Iqbal N, et al. Evaluation of serum procalcitonin level as a biomarker for disease severity in COVID-19 patients. New Microbes New Infect. 2021;43:100922.
- Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(1):S31.
- Korevaar DA, Cohen JF, Reitsma JB, et al. Updating standards for reporting diagnostic accuracy: the development of STARD 2015. Res Integr Peer Rev. 2016 Jun;1(1):7. Available from: http://researchintegrityjournal.biomedcentral.com/articles/10.1186s41073-016-0014-7.
- Clinical lab tests by royal college of physicians and surgeons - Google Search. [cited 2023 Feb 12]. Available from: https://www.google.com/search?q = clinical+lab+tests+by+royal+college+of+physicians+and+surgeons&oq = clinical+lab+tests+by+royal+college+&aqs = chrome.2.69i57j33i160l5.18218j0j15&sourceid = chrome&ie = UTF-8#ip = 1.
- Khalid A, Ali Jaffar M, Khan T, et al. Hematological and biochemical parameters as diagnostic and prognostic markers in SARS-COV-2 infected patients of Pakistan: a retrospective comparative analysis. Hematology. 2021;26(1):529–542.
- Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. Wiley-Liss Inc 2020;95:834–847.
- Waris A, Din M, Khalid A, et al. Evaluation of hematological parameters as an indicator of disease severity in Covid-19 patients: Pakistan’s experience. J Clin Lab Anal. 2021;35(6):e23809.
- Lin S, Mao W, Zou Q, et al. Associations between hematological parameters and disease severity in patients with SARS-CoV-2 infection. J Clin Lab Anal. 2021 Jan;35(1).
- Asghar MS, Khan NA, Haider Kazmi SJ, et al. Hematological parameters predicting severity and mortality in COVID-19 patients of Pakistan: a retrospective comparative analysis. J Commun Hosp Intern Med Persp. 2020 Nov;10(6):514–520. doi:10.1080/20009666.2020.1816276.
- Lo Tartaro D, Neroni A, Paolini A, et al. Molecular and cellular immune features of aged patients with severe COVID-19 pneumonia. Commun Biol. 2022 Jun;5(1):590.
- Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020 May;55(5).
- Li X, Liu C, Mao Z, et al. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Crit Care. 2020 Dec;24(1).
- Liu J, Liu Y, Xiang P, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med . 2020 May;18(1). [cited 2022 May 12]; Available from: https://pubmed.ncbi.nlm.nih.gov/32434518/.
- Ciccullo A, Borghetti A, Zileri Dal Verme L, et al. Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19: a report from the Italian front line. Int J Antimicrob Agents. 2020 Aug;56(2):106017. doi:10.1016/j.ijantimicag.2020.106017.
- Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020 Jun;127:104370. doi:10.1016/j.jcv.2020.104370.
- Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. Available from: https://pubmed.ncbi.nlm.nih.gov/32161940.
- Song C-Y, Xu J, He J-Q, et al. COVID-19 early warning score: a multiparameter screening tool to identify highly suspected patients. medRxiv. 2020 Mar. 2020.03.05.20031906
- Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474.
- Cleland DA, Eranki AP. Procalcitonin. StatPearls. 2021 Aug [cited 2022 May 12]; Available from: https://www.ncbi.nlm.nih.gov/books/NBK539794/.
- Dolci A, Robbiano C, Aloisio E, et al. Searching for a role of procalcitonin determination in COVID-19: a study on a selected cohort of hospitalized patients. Clin Chem Lab Med. 2021 Feb 1;59(2):433–440.
- Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629.
- Vanhomwegen C, Veliziotis I, Malinverni S, et al. Procalcitonin accurately predicts mortality but not bacterial infection in COVID-19 patients admitted to intensive care unit. Irish J Med Sci. 1971. [Internet]. 2021 Mar 30; Available from: http://link.springer.com/10.1007s11845-020-02485-z
- Luo X, Zhou W, Yan X, et al. Prognostic value of creactive protein in patients with COVID-19 (internet). Infect Dis (Except HIV/ AIDS). 2020. doi:10.1101/2020.03.21.20040360.
- Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146(1):128–136.e4.
- Fielding CA, McLoughlin RM, McLeod L, et al. IL-6 regulates Neutrophil Trafficking during acute inflammation via STAT3. J Immunol. 2008 Aug;181(3):2189–2195. [cited 2022 May 13]; Available from: https://www.jimmunol.org/content/181/3/2189.
- Grifoni E, Valoriani A, Cei F, et al. Interleukin-6 as prognosticator in patients with COVID-19. J Infect. 2020 Sep;81(3):452–482.
- Chang SH, Minn D, Kim SW, et al. Inflammatory markers and cytokines in moderate and critical cases of COVID-19. Clin Lab. 2021 Sep;67(9):2115–2120.
- Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020.
- Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. 2020 Nov;92(11):2283–2285. [cited 2022 May 13] Available from: https://onlinelibrary.wiley.com/doi/full/10.1002jmv.25948.
- Herold T, Jurinovic Phd V, Arnreich C, et al. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients. medrxiv.org [Internet]. [cited 2022 May 13]; Available from: https://www.medrxiv.org/content/10.11012020.04.01.20047381.abstract.
- Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. bmj.com [Internet]. [cited 2022 May 13]; Available from: https://www.bmj.com/content/368/bmj.m1091.
- Surbatovic M, Popovic N, Vojvodic D, et al. Cytokine profile in severe gram-positive and gram-negative abdominal sepsis. Sci Rep. 2015;5(1):11355.
- Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–620.
- Famous KR, Delucchi K, Ware LB, et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2016;195(3):331–338.
- Sinha P, Delucchi KL, Thompson BT, et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intens Care Med. 2018;44(11):1859–1869.
- Sinha P, Delucchi KL, Thompson BT, et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med. 2018 Nov;44(11):1859–1869. [cited 2022 May 13]; Available from: https://link.springer.com/article/10.1007s00134-018-5378-3.
- Tanaka T. 2014. MN-CSH, undefined. IL-6 in inflammation, immunity, and disease. cshperspectives.cshlp.org [Internet]. [cited 2022 May 13]; Available from: https://cshperspectives.cshlp.org/content/6/10/a016295.short.
- Tavakolpour S, Rakhshandehroo T, Wei EX, et al. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol Lett. 2020 Sep 1;225:31–32.
- Henry BM, Aggarwal G, Wong J, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020 Sep;38(9):1722–1726. [cited 2022 May 12]; Available from: https://pubmed.ncbi.nlm.nih.gov/32738466/.
- Hoeboer SH, Oudemans-van Straaten HM, Groeneveld AB. Albumin rather than C-reactive protein may be valuable in predicting and monitoring the severity and course of acute respiratory distress syndrome in critically ill patients with or at risk for the syndrome after new onset fever. BMC Pulm Med. 2015 Mar 14;15:22. doi:10.1186/s12890-015-0015-1.
- Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013 Sep;13(9):752–761. [Internet] [cited 2022 May 13] Available from: http://www.thelancet.com/article/S1473309913702044/fulltext
- Oner AF, Bay A, Arslan S, et al. Avian Influenza A (H5N1) Infection in Eastern Turkey in 2006. [Internet]. 2009 Oct 8 [cited 2022 May 13];355(21):2179–85. Available from: https://www.nejm.org/doi/full/10.1056NEJMoa060601. https://doi.org/10.1056/NEJMoa060601
- Banchini F, Cattaneo GM, Capelli P. Serum ferritin levels in inflammation: a retrospective comparative analysis between COVID-19 and emergency surgical non-COVID-19 patients. World J Emerg Surg. 2021 Mar 8;16(1):9.
- Ruscitti P, Berardicurti O, Di Benedetto P, et al. Severe COVID-19, another piece in the puzzle of the Hyperferritinemic Syndrome. An immunomodulatory perspective to alleviate the storm. Front Immunol. 2020 May;11:1130.
- Colafrancesco S, Alessandri C, Conti F, et al. COVID-19 gone bad: a new character in the spectrum of the hyperferritinemic syndrome? Autoimmun Rev. 2020 Jul;19(7):102573.
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552.
- Pu SL, Zhang XY, Liu DS, et al. Unexplained elevation of erythrocyte sedimentation rate in a patient recovering from COVID-19: a case report. World J Clin Cases. 2021;9(6):1394–1401. doi:10.12998/wjcc.v9.i6.1394.
- Rendeiro AF, Ravichandran H, Bram Y, et al. The spatial landscape of lung pathology during COVID-19 progression. Nature. 2021 May;593(7860):564–569.