ABSTRACT
Anemia is common in older adults, but often unexplained. Previously, we conducted a randomized, controlled trial of intravenous (IV) iron sucrose to study its impact on the 6-minute walk test and hemoglobin in older adults with unexplained anemia and ferritin levels of 20–200 ng/mL. In this report, we present for the first time the response of hemoglobin, as well as the dynamic response of biomarkers of erythropoiesis and iron indices, in a pooled analysis of the initially IV iron-treated group of 9 subjects and the subsequently IV iron treated 10 subjects from the delayed treatment group. We hypothesized that there would be a reproducible hemoglobin response from IV iron, and that iron indices and erythropoietic markers would reflect appropriate iron loading and reduced erythropoietic stress. To investigate the biochemical response of anemia to IV iron, we studied the dynamics of soluble transferrin receptor (STfR), hepcidin, erythropoietin (EPO), and iron indices over 12 weeks after treatment. In total, all 19 treated subjects were evaluable: 9 from initial treatment and 10 after cross-over. Hemoglobin rose from 11.0 to 11.7 g/dL, 12 weeks after initiating IV iron treatment of 1000 mg divided weekly over 5 weeks. We found early changes of iron loading after 1–2 IV iron dose: serum iron increased by 184 mcg/dL from a baseline of 66 mcg/dL, ferritin by 184 ng/mL from 68 ng/mL, and hepcidin by 7.49 ng/mL from 19.2 ng/mL, while STfR and serum EPO declined by 0.55 mg/L and 3.5 mU/mL from 19.2 ng/mL and 14 mU/mL, respectively. The erythroid response and evidence of enhanced iron trafficking are consistent with the hypothesis that IV iron overcomes iron deficient or iron-restricted erythropoiesis. These data provide new insight that iron-restricted erythropoiesis is a potential and targetable mechanism for patients diagnosed with unexplained anemia of the elderly and offers support for larger prospective trials of IV iron among anemic older adults of low to normal ferritin.
KEYWORDS:
Introduction
Anemia affects 10% to 49% of older adults, with etiologies ranging from nutritional deficiencies, chronic kidney disease and anemia of chronic disease [Citation1–4]. However, in population studies approximately one-third of anemia in the older population are classified as unexplained anemia of the elderly (UAE) [Citation1,Citation5]. Even after an intensive hematologic evaluation, a similar proportion of anemic older adults in a referral clinic have been categorized as UAE [Citation6]. Irrespective of the cause, anemia has been independently associated with adverse consequences such as frailty, hospitalization, and mortality [Citation7–10].
Further characterization of the mechanisms of UAE may aid in categorizing subsets to test anemia corrective therapies [Citation11]. Although overt inflammation is uncommon in UAE, subtle features of inflammation may be present such as iron restriction and elevation in certain inflammatory markers [Citation12–14]. Other potential contributors include age-related changes in hematopoietic stem cells, emerging myelodysplastic syndromes, and androgen decline [Citation15,Citation16]. The blunted EPO response of anemia in UAE is a consistent finding relative to iron deficiency anemia [Citation6,Citation17,Citation18]. Patients with mild iron deficiency and/or iron restricted erythropoiesis may be a targetable subset of UAE in older adults. Research definitions of UAE attempt to fully exclude iron deficiency by low serum ferritin. However, as ferritin represents an acute phase reactant and older adults often have inflammation from disease or ageing, the diagnosis of iron deficiency in older adults often employ a higher ferritin cutoff, such as 30–50 ng/mL [Citation5,Citation6,Citation19]. Ferritin thresholds are imprecise; in one study, 6% of older patients with a ferritin above 50 was shown to have iron deficiency by bone marrow examination for iron stores [Citation20]. Likewise, intravenous iron treatment in heart failure patients with ferritin values up to 300 ng/mL may improve hemoglobin and physical activity [Citation21].
Several other markers beyond ferritin and serum iron may illuminate iron status in patients. Hepcidin is produced by hepatocytes and is the primary systemic negative regulator of iron absorption. Hepcidin levels are low in individuals with iron deficiency anemia, while hepcidin is induced in response to iron loading [Citation23]. Hepcidin can also be induced in response to inflammation or infection inhibiting mobilization of iron stores [Citation24]; in contrast, hepcidin is inhibited by hypoxia and augmented erythropoiesis through stimulation of the negative regulator; i.e. erythroferrone [Citation25,Citation26]. Plasma soluble transferrin receptor (STfR) is predominantly derived from the erythrons as a complex of transferrin, the primary carrier protein of iron, and transferrin receptor. STfR when elevated is a marker of iron deficiency but also may suggest effective iron accessibility, as IV iron correction of anemia reduces STfR in inflammatory anemias [Citation27,Citation28]. The STfR index, derived from STfR divided by ferritin, may help to identify a component of iron deficiency in the setting of concurrent inflammation although the clinical utility requires validation [Citation11,Citation29,Citation30].
The widespread use of IV iron in chronic kidney disease and congestive heart failure motivated the targeted use of IV iron for unexplained anemia with low to normal iron stores [Citation22]. We leveraged this small prospective randomized clinical trial using IV iron in anemic older adults with borderline low to normal serum ferritin of 20–200 ng/mL to characterize the IV iron-induced erythropoiesis through biomarkers of iron homeostasis. We hypothesized that ineffective iron utilization may be a component of UAE and providing IV iron would improve hemoglobin and relieve markers of erythropoiesis, with increased hepcidin, decreased EPO and STfR levels.
Methods
Clinical trial design
We previously reported the design, methods, and outcomes of our randomized control trial under the auspices of an NIH-funded consortium to study novel treatments for anemia in older adults [Citation22]. Briefly, participants were community-dwelling men and women age ≥65 years with unexplained anemia and borderline low to normal iron stores. Unexplained anemia was defined by a hemoglobin concentration of ≥9 g/dL, and <11.5 g/dL or <12.7 g/dL respectively for women and men, and serum ferritin 20–200 ng/mL (inclusive) without a known cause for anemia after an extensive evaluation. Screening for other etiologies of anemia required standardized laboratory testing for folate, vitamin B12, creatinine/glomerular filtration rate, liver function test, and fecal occult testing in addition to history and physical performed by the screening clinician. Further diagnostic testing such as bone marrow evaluation was left at the discretion of the provider. Subjects meeting inclusion and exclusion criteria were randomized in an open-label fashion to the immediate treatment group or to the control group [Citation22]. After 12 weeks, the control group crossed over to treatment for 12 weeks following the same schedule as the treatment group, and for this study is referred to as the ‘delayed treatment group’ ((a)). All participants received 1000 mg of IV iron sucrose, Venofer®, supplied by Luitpold Pharmaceuticals, dosed as 200 mg per week for 5 weeks. Follow-up visits occurred for all participants at 6 and 12 weeks after initiation of their first IV iron dose.
Figure 1. (a) Flow diagram of randomized wait-list control trial. The immediate treatment group (blue) received IV iron at randomization. The control group (red) were observed for 12 weeks; all 10 opted to receive IV iron at that time becoming the delayed treatment group. (b) Timeline of laboratory measures drawn at each time point. The pooled analysis includes data from the immediate treatment group (n = 9) and the delayed treatment group (n = 10).
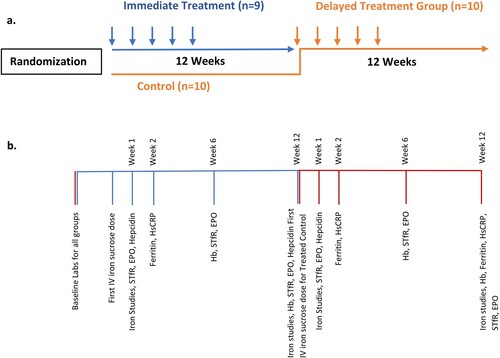
Due to poor accrual, the trial closed after enrolling 19 subjects; 9 randomized to the immediate treatment group and 10 randomized to the control group. All 10 initial control subjects crossed over to IV iron resulting in a total of 19 IV iron-treated patients.
Sample procurement
Whole blood was collected at baseline and at week 1 and 2 of treatment, and at follow-up visits at week 6 and 12 from the first dose of IV iron ((b)). Complete blood count, serum ferritin, serum iron, percent transferrin saturation, TIBC, high sensitive C-reactive protein (hsCRP) values were all performed at CLIA-approved local laboratories. Samples were shipped to the University of Utah at Associated Regional and University Pathologists (ARUP) laboratory where measurements of STfR, EPO, and hepcidin were obtained. Hepcidin analysis was performed at Eli Lilly laboratories by ELISA based on the assay previously described [Citation31].
Statistical analysis
Descriptive statistics include median and range for the data presented. Comparisons of baseline lab values between immediate treatment and delayed treatment groups were carried out with two sample t-test if the data are normally distributed or a Wilcoxon two-sample test if the data are not normally distributed. Our primary objective was to investigate the change overtime on measures of iron status and the erythroid response to IV iron. Subjects for this report include the immediate treatment group pooled with the subsequently treated delayed treatment who both received the same schedule of IV iron and were studied over 12 weeks. A Wilcoxon signed rank test was used to test if the change in results over two time points was significantly different from zero or not. A p-value of less than or equal to 0.05 is considered as statistically significant. All analyses are carried out in Statistical Analysis System version 9.4.
Results
Baseline values
Previously we reported the baseline characteristics of ferritin, serum iron, total iron binding capacity (TIBC), and transferrin saturation, and hsCRP [Citation22]. Here we additionally compared the values between the immediate treatment group and delayed treatment group for STfR, EPO, and hepcidin prior to the first treatment dose in each group, and as expected no significant differences were found (). The baseline values for the iron indices (ferritin, serum iron, TIBC, and transferrin saturation) used for the 10 patients in the delayed treatment group were approximately 12 weeks prior to IV iron treatment because these patients were initially part of the control group and did not start IV iron treatment until the control period was completed. The delayed treatment group had generally similar characteristics as the immediate treatment group except having higher serum iron and transferrin saturation (). These minor differences between the two groups are likely related to small sample size. Although the cross-over group exhibited measures of more iron restriction with lower serum iron, the cross-over group also had higher median iron stores by ferritin, suggesting the reasonableness of combining cohorts for subsequent biochemical characterization after IV iron. 53% of the pooled subjects had transferrin saturation >20%.
Table 1. Baseline laboratory values.
Sample availability
Sample availability varied at each time point (). Per protocol, during treatment visits, labs for translational studies were to be drawn 30 min after the IV iron infusion. However, unintentionally, the timing of labs for EPO, hepcidin, STfR, serum iron, TIBC, Tsat varied on the week when patients were due for dose 2 of IV iron. For example, 11 subjects had hepcidin samples drawn before infusion versus 4 subjects who had samples drawn after the second dose of IV iron. Nevertheless, hepcidin values were not affected by sampling time (median 37.7 ng/mL before infusion versus 37.2 ng/mL after infusion). Similarly, there were no significant differences for the remaining values other than serum iron, when drawn before or after IV iron and the results were treated as the one-time point.
Table 2. Pooled Analysis of Iron Markers and Serum EPOs.
Erythroid response
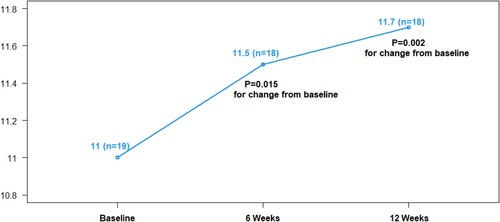
Previously we showed that Hb increased with IV iron and decreased over time in the untreated control group [Citation22]. After pooling the immediate treatment and the delayed treatment groups, the expanded sample of 19 IV iron-treated patients confirmed an overall median increase in Hb from 11.0 to 11.7 g/dL (median change 0.5, p = 0.002) 12 weeks from the first dose of IV iron ().
Figure 2. Median hemoglobin over time with IV iron therapy. Effects of IV iron on hemoglobin levels (g/dL) over 12 weeks as compared to baseline.
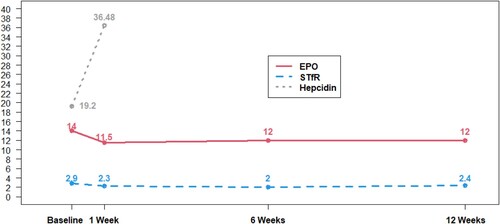
To gain insight into IV iron-mediated erythropoiesis, we looked at markers of erythropoiesis assessing STfR and EPO over the course of treatment. EPO levels were slightly lower following IV iron treatment, falling from 14 to 11.5 mU/mL 1 week after the first dose of IV iron, a median change of −3.5 (p = 0.017). However, the decrease in EPO did not remain statistically significant at 6 and 12 weeks (−2.0, p = 0.4) ( and ).
Figure 3. Median values of STfR, EPO, and hepcidin over time with iron therapy. Pooled changes in STfR (mg/L), EPO (mU/L), and Hepcidin (ng/mL) with IV iron therapy. Week 1 corresponds to the second of five IV iron treatments; week 6 is one week after completion of all five IV iron treatments.
Though Hb increased, inflammation (as measured by hsCRP) did not change over time arguing against inflammation as a major driver of iron-restricted erythropoiesis in patients such as these.
Iron trafficking
After at least one dose of IV iron, iron loading was identified by not only increased ferritin and serum iron, but also through a rise in hepcidin and decline in STfR ( and ). We interpret the small decline in serum EPO to further reflect a physiologic response to iron loading.
Iron repletion persisted when assessed at the end of study, 12 weeks after initiation of IV iron (7 weeks after the last IV iron dose) relative to baseline, likely promoting the ongoing erythropoietic response from week 6 to week 12, after completion of IV iron.
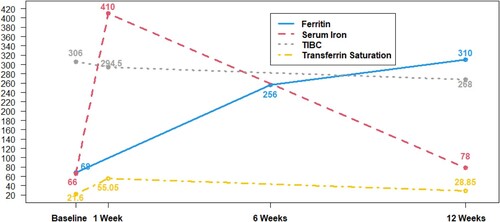
The sampling inconsistency before and after the second dose of IV iron serendipitously offered additional insight into serum iron kinetics. Relative to baseline, serum iron immediately before IV iron dose 2, and thus one week after IV iron dose 1, showed no change from baseline (). As expected, serum iron spiked immediately after treatment; the six patients sampled 30 min after IV iron dose 2, experienced a median increase in serum iron of 338.5 mcg/dL (p = 0.031).
Figure 4. Median values of iron indices over time with IV iron therapy. Changes in iron indices over time with IV iron therapy. Ferritin (ng/mL), Serum Iron (mcg/dL), TIBC (mcg/dL), Transferrin Saturation (%). Week 1 corresponds to the second of five IV iron treatments; week 6 is one week after completion of all five IV iron treatments.
Discussion
We sought to characterize markers of iron homeostasis among older anemic adults treated on a prospective trial utilizing IV iron for borderline low to normal iron stores, measured by serum ferritin of 20–200 ng/mL inclusive, but otherwise unexplained anemia [Citation22]. We again documented IV iron induced a moderate erythroid response, documented by hemoglobin rise of 0.5 g/dL at week 6 and a 0.7 g/dL Hb increase by week 12 relative to baseline values. In the original report, we described a Hb rise of 0.39 g/dL for IV iron as opposed to a slight decline in the control patients. Occult iron deficiency and/or iron-restricted erythropoiesis thus may be a targetable subset of older adults.
The biochemical markers offer insights related to IV iron therapy in this population. The finding of a transferrin saturation of >20% in the majority of subjects, normal STfR, lack of elevation in the STfR index and serum erythropoietin, all point away from frank iron deficiency anemia [Citation11,Citation30]. We also effectively excluded significant inflammation through exclusion criteria further evidenced by normal CRP levels. Most compelling, serum hepcidin values in the range of 19–24 ng/mL at baseline mirrored another recent study among older iron-replete men with low testosterone rather than suppressed hepcidin expected in iron deficiency [Citation32]. Further, one study found hepcidin values >20 ng/mL predicted poor responsive to oral iron therapy among patients with ferritin <300 ng/mL yet IV iron could overcome oral iron resistance likely explained by hepcidin suppression of iron absorption [Citation33].
Iron markers confirmed biochemical iron loading early after 1–2 doses of IV iron; hepcidin rose early, STfR and iron saturation fell, and ferritin rose. We believe the changes in biochemical iron markers represent iron loading that promotes erythropoiesis. The initial suppression of erythropoietin, quantitatively small, requires further study. Because of the early response to IV iron seen in the iron markers, these clinically relevant markers could be studied in larger cohorts to predict erythroid response in the treatment of UAE with IV iron.
The modest clinical response to IV iron suggests Hb rise may be capped either due to correction of anemia in some patients or more likely the presence of other mechanisms underlying the unexplained anemia. The response to IV iron further supports a role of impaired iron trafficking in some older anemic adults, a similar phenotype to iron-restricted erythropoiesis in anemia of chronic disease [Citation34]. Other studies of patients with conditions co-occurring with dysregulated inflammation, such as congestive heart failure or chronic kidney disease, have likewise shown Hb response to IV iron in patients with low to normal iron stores based on ferritin, as well as achieving functional improvements [Citation21,Citation35].
Limitations lie in the small sample size related to the extensive entry criteria which hinders generalizability. The trial accrued poorly due to the combination of extensive entry criteria, challenges finding sites managing such patients with research infrastructure and ability to administer an experimental agent, and/or lack of patient or physician interest [Citation22]. For that reason, we maximized the sample size by a cross-over design allowing treatment of the control group and pooling sample results. However, this approach limits the analysis to longitudinal change scores rather than comparison to control compounded by lack of ‘new’ baseline values at cross-over. The proportion of older anemic adults that meet the criteria for this study is not known which hinders generalizability. However, UAE subjects with ferritin 20 ng/mL or above and those with ferritin <200 ng/mL without other etiologies would have been eligible so our study may apply to a substantial proportion of older anemic patients [Citation1,Citation5,Citation6]. Finally, while many IV iron preparations exist, our results only directly apply to IV iron. Whether oral iron would induce similar changes in iron parameters or erythropoiesis is not known.
In conclusion, a subset of older adults characterized as UAE may have iron-restricted erythropoiesis responsive to IV iron. Iron loading is seen as early as one dose of IV iron with continued hemoglobin rise at 12 weeks. Larger studies will be needed to confirm hemoglobin response to IV iron, characterize clinical benefits including functional change, and establish the safety profile in UAE with iron-restricted erythropoiesis.
Author’s contributions
Conception and Design of work – HJC, ASA, EP, JP,HB
Collection of data – HJC, ASA, EP, JP
Analysis and interpretation of data – HJC, ASA, JJY, SS, HB, JF
Initial Drafting of Manuscript – JJY, ASA, HJC
Review, Editing and Approval of final manuscript – all authors.
Ethics approval
The study was approved by the Institutional Review Board at each participating institution and conducted in accordance with the Declaration of Helsinki for biomedical research involving human subjects.
Informed consent
Written informed consent was provided by all subjects.
Supplemental Material
Download MS Word (23.2 KB)Acknowledgements
We wish to acknowledge the contributions of Dr Stanley Schrier who was integral to the conceptualization of this work but died before working on this manuscript, Dr Cindy Roy who was also involved in the initiation of this work but entered government administrative service and could not participate further, and MaryJo Wright MSci, for her technical assistance in the performance of the Hepcidin assays.
Disclosure statement
J. Fill is employed by Eli Lilly and Co. who performed the Hepcidin assays. No potential conflict of interest was reported by the other author(s).
Data availability statement
Available from the Duke Clinical Research Institute. Code Availability – Available from the Duke Clinical Research Institute.
Additional information
Funding
References
- Guralnik JM, et al. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263–2268.
- Ble A, et al. Renal function, erythropoietin, and anemia of older persons. Arch Intern Med. 2005;165(19):2222–2227.
- Artz AS, et al. Prevalence of anemia in skilled-nursing home residents. Arch Gerontol Geriatr. 2004;39(3):201–206.
- Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications, and management. Blood. 2018;131(5):505–514.
- Price EA, et al. Anemia in older persons: etiology and evaluation. Blood Cells Mol Dis. 2011;46(2):159–165.
- Artz AS, Thirman MJ. Unexplained anemia predominates despite an intensive evaluation in a racially diverse cohort of older adults from a referral anemia clinic. J Gerontol A Biol Sci Med Sci. 2011;66(8):925–932.
- Juarez-Cedillo T, et al. Prevalence of anemia and its impact on the state of frailty in elderly people living in the community: SADEM study. Ann Hematol. 2014;93(12):2057–2062.
- Migone De Amicis M, et al. Anemia in elderly hospitalized patients: prevalence and clinical impact. Intern Emerg Med. 2015;10(5):581–586.
- Artz AS. Anemia and the frail elderly. Semin Hematol. 2008;45(4):261–266.
- Rohrig G. Anemia in the frail, elderly patient. Clin Interv Aging. 2016;11:319–326.
- Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89(3):1052–1057.
- Tavenier J, Leng SX. Inflammatory pathways to anemia in the frail elderly. Clin Geriatr Med. 2019;35(3):339–348.
- Ferrucci L, et al. Unexplained anaemia in older persons is characterised by low erythropoietin and low levels of pro-inflammatory markers. Br J Haematol. 2007;136(6):849–855.
- Artz AS, et al. Unexplained anaemia in the elderly is characterised by features of low grade inflammation. Br J Haematol 2014;167(2):286–289.
- Makipour S, Kanapuru B, Ershler WB. Unexplained anemia in the elderly. Semin Hematol. 2008;45(4):250–254.
- Pang WW, Schrier SL, Weissman IL. Age-associated changes in human hematopoietic stem cells. Semin Hematol 2017;54(1):39–42.
- Sriram S, Xenocostas A, Lazo-Langner A. Erythropoietin in anemia of unknown etiology: a systematic review and meta-analysis. Hematology. 2016;21(4):234–240.
- Gowanlock Z, et al. Erythropoietin levels in elderly patients with anemia of unknown etiology. PLoS One. 2016;11(6):e0157279.
- Guyatt GH, et al. Laboratory diagnosis of iron-deficiency anemia. J Gen Intern Med. 1992;7(2):145–153.
- Joosten E, et al. Serum transferrin receptor in the evaluation of the iron status in elderly hospitalized patients with anemia. Am J Hematol. 2002;69(1):1–6.
- Anker SD, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361(25):2436–2448.
- Price E, et al. A prospective randomized wait list control trial of intravenous iron sucrose in older adults with unexplained anemia and serum ferritin 20–200ng/mL. Blood Cells Mol Dis. 2014;53(4):221–230.
- Ganz T, et al. Immunoassay for human serum hepcidin. Blood. 2008;112(10):4292–4297.
- Ganz, T., Anemia of Inflammation. N Engl J Med, 2019. 381(12): p. 1148–1157.
- Kautz L, et al. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–684.
- Yoon D, et al. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem. 2006;281(35):25703–25711.
- Cazzola M, et al. Defective iron supply for erythropoiesis and adequate endogenous erythropoietin production in the anemia associated with systemic-onset juvenile chronic arthritis. Blood. 1996;87(11):4824–4830.
- Suominen P, et al. Single values of serum transferrin receptor and transferrin receptor ferritin index can be used to detect true and functional iron deficiency in rheumatoid arthritis patients with anemia. Arthritis Rheum. 2000;43(5):1016–1020.
- Huebers HA, et al. Intact transferrin receptors in human plasma and their relation to erythropoiesis. Blood. 1990;75(1):102–107.
- Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta. 2003;329(1–2):9–22.
- Butterfield AM, et al. A dual-monoclonal sandwich ELISA specific for hepcidin-25. Clin Chem. 2010;56(11):1725–1732.
- Artz AS, et al. Markers of iron flux during testosterone-mediated erythropoiesis in older Men with unexplained or iron-deficiency anemia. J Clin Endocrinol Metab. 2020;105(11):3396–3403.
- Onken JE, et al. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion. 2014;54(2):306–315.
- Ganz T. Anemia of chronic disease. In: K. Kaushansky, et al., editors. Williams hematology, 10th ed. New York: McGraw-Hill Education; 2021.
- Qunibi WY, et al. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant. 2011;26(5):1599–1607.
