ABSTRACT
Introduction
Several observations have shown that patients with polycythemia have iron deficiency. Our objectives were to report the prevalence of iron deficiency and to evaluate the diagnostic performance of serum ferritin in polycythemia vera.
Patients and method
This is a retrospective descriptive and analytical study carried out in the internal medicine department of the Henri Mondor Hospital, Aurillac, France. The study involved 114 patients with polycythemia, followed in the department from January 1, 2010 to December 31, 2021. To evaluate the diagnostic performance, the JAK2 mutation was considered as the gold standard of diagnosis.
Results
Thirty-three patients had polycythemia vera and 76 patients had secondary polycythemia. The mean age of the patients was 61.79 years (±15.44) with a sex ratio of 4.43. The overall prevalence of iron deficiency was 21.05%. The prevalence was 53% in polycythemia vera group and 1.32% in secondary polycythemia group. The risk of iron deficiency was high in polycythemia vera (OR = 115; 95% CI [14.4-918.2], p < 0.0001) and the sensitivity and specificity of serum ferritin were 52.63% and 100% respectively.
Conclusion
Assessment of iron deficiency should be part of the initial evaluation of polycythemia. Iron deficiency had a high specificity during polycythemia vera.
Introduction
Polycythemia vera (PV) is a clonal proliferation of the erythroid lineage secondary to mutation of hematopoietic stem cells [Citation1]. Secondary polycythemia is often related to high erythropoietin (EPO) production, secondary to alteration of the hematopoietic cell environment [Citation2]. The main risk of polycythemia is related to thromboembolic events.
Observations have shown that patients with polycythemia have iron deficiency at the time of diagnosis [Citation3–5]. The paradoxical association between polycythemia and iron deficiency is mostly reported in PV. Therapeutic phlebotomy is one of the causes of iron deficiency. Other hypotheses have been described and some are discussed [Citation6].
Studies of iron deficiency during polycythemia are limited in the literature. Some authors have suggested that iron deficiency is specific to PV. Others have reported that iron deficiency presents a high risk of thrombosis [Citation7]. In order to better understand this association and to provide new elements, we conducted the current study. Our objectives were to report the prevalence of iron deficiency during polycythemia and to evaluate the diagnostic performance of serum ferritin in PV.
Patients and method
Characteristics of the study
This was a retrospective descriptive and analytical study conducted in the internal medicine department of Henri Mondor Hospital, Aurillac, France. The study involved patients over 18 years of age, registered and followed in the department for a period of 12 years, from January 1, 2010 to December 31, 2021.
Inclusion/exclusion criteria
We included patients diagnosed with polycythemia unrelated to dehydration and hypovolemia. Polycythemia was defined as elevated hemoglobin greater than 16.0 g/dL in women and 16.5 g/dL in men, or hematocrit greater than 48% and 49%, respectively. PV was defined according to the World Health Organization (WHO) criteria revised in 2016 [Citation8]. The diagnosis requires meeting either the three major criteria or the first two major criteria and the minor criterion. The absence of a JAK2 mutation with an elevated serum EPO level suggested secondary polycythemia.
We excluded patients with phlebotomy and those without iron deficiency assessment. The assessment of iron deficiency was serum ferritin and transferrin saturation coefficient (TSC). Iron deficiency was defined as a serum ferritin level less than 30 ng/mL or TSC less than 20% [Citation9].
Studied parameters
We collected demographic characteristics, body mass index (BMI), serum ferritin level, TSC, blood count with mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC), C-reactive protein (CRP), transaminases, lactate dehydrogenase (LDH) and EPO levels, isotopic measure of red blood cell volume, and janus kinase 2 (JAK2) mutation testing. In the PV group, we collected the result of bone marrow cytology, treatment, iron supplementation, and the latest available serum ferritin values.
Statistical analysis
Data were analyzed with Epi Info 7 software. Frequencies and means were calculated by descriptive statistics. Continuous variables were presented as means and categorical variables were expressed as frequencies. The Chi-square test was used in the univariate analysis. Values with p < 0.05 were accepted as statistically significant. In PV group, we calculated the sensitivity, specificity, positive and negative predictive values of serum ferritin and JAK2 mutation. We considered the JAK2 mutation as the gold standard for the diagnosis.
Results
One hundred and fourteen patients were included. PV was found in 33.33% and secondary polycythemia in 66.67% of cases.
Demographic characteristics
The demographic characteristics of the patients are represented in . The mean age of patients was 61.79 years (± 15.44) with extremes of 19 and 89 years and the sex ratio was 4.43. In the PV group, the mean age was 65.13 years (± 16.5) and the sex ratio was 1.71. In the secondary polycythemia group, the mean age was 60.11 (± 14.7) and the sex ratio was 9.86.
Table 1. Demographic characteristics of patients.
Biological characteristics
shows the biological characteristics of the patients. Mean hemoglobin level was 17.89 g/dL and hematocrit level was 53.68%. The mean serum ferritin level was 223.72 ng/mL with extremes of 1.6 and 977 µg/L. The mean TSC was 32.78% with extremes of 5.8% and 56.5%. Isotopic measure of red blood cell volume was performed in 11 patients and the mean value was 125.64%. In the PV group, the JAK2 mutation was found in 84.21% of cases and all were VF617F. Seven patients had bone marrow cytology: five cases of erythroid hyperplasia and myelofibrosis, one case of global hyperplasia and one case of myelodysplasia.
Table 2. Biological characteristics of patients.
Prevalence of iron deficiency
Twenty-four patients had iron deficiency (21.05%). The prevalence of iron deficiency was 60.53% in the PV group versus 1.32% in the secondary polycythemia group. The risk of iron deficiency was significantly elevated in the PV group (). The sensitivity and specificity of serum ferritin were 52.63% and 100%, respectively ().
Table 3. Iron deficiency prevalence and risk assessment using Chi-square test.
Table 4. Diagnostic performance of JAK2 mutation and serum ferritin in polycythemia vera.
Iron deficiency and thrombosis
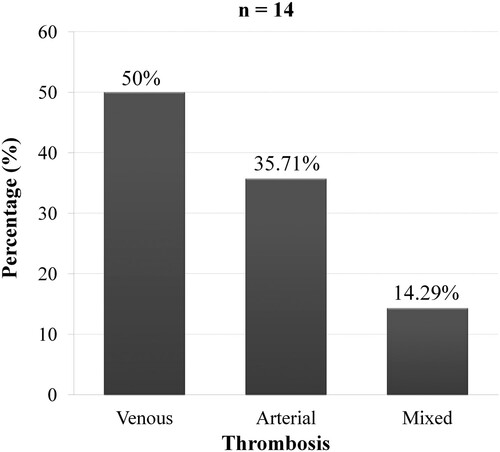
In the PV group, 14 of 38 patients (36.84%) had thrombosis. The proportion of thrombosis was 43.48% in patients with iron deficiency (p = 0.241, OR = 2.11; CI95% [0.51 − 8.66]). Arterial thrombosis was found in 50% of cases ().
Treatment
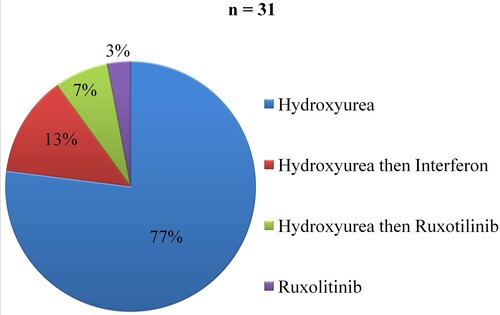
In the PV group, 81.58% of cases had received pharmacological treatment. Hydroxyurea alone was used in 77% and Ruxotulinib alone in 3% of cases. Hydroxyurea was relayed by Interferon in 13% and Ruxotilinib in 7% of cases (). The mean duration of treatment was 36.36 months (± 23.43) with a minimum of five months and a maximum of 108 months. Phlebotomy was performed in 55.26% of cases. Two patients (5.26%) had received iron supplementation.
During follow-up, four patients had a serum ferritin control. Ferritin levels were increased in three patients after treatment of PV ().
Table 5. Evolution of serum ferritin in four patients with polycythemia vera.
Discussion
Polycythemia
The annual incidence of PV ranges from 0.4 to 2.8 cases per 100,000 population [Citation10]. The incidence rate increases with age and men are the most affected [Citation11]. PV is a BCR-ABL negative myeloproliferative neoplasia, characterized by a mutation of hematopoietic stem cells [Citation1]. The JAK2 mutation in exon 14 or JAK2V617F accounts for 95-99% of cases and 1-5% are mutations in exon 12 [Citation12,Citation13]. In our study, the JAK2V617F mutation was found in 84.21% of cases.
Secondary polycythemia is characterized by elevated erythrocyte mass secondary to certain situations: hypoxia, malignant tumors (hepatocellular carcinoma, renal cancer, parathyroid carcinoma) and benign tumors (renal cyst, uterine leiomyoma, meningioma) [Citation14]. Respiratory failure is the main cause of secondary polycythemia [Citation2].
The diagnostic criteria for distinguishing PV from secondary polycythemia have been studied repeatedly. The diagnostic criteria established by the Polycythemia Vera Study Group have been criticized by several authors [Citation15–18]. However, bone marrow examination, clinical exclusion of secondary polycythemia, JAK2 mutation and EPO testing are the most accepted criteria.
Iron deficiency
Iron deficiency is the leading cause of anemia, making iron deficiency anemia a major health problem. Iron deficiency anemia is most common in children, pregnant women and women of childbearing age. In the elderly, iron deficiency accounts for 30% of anemia cases and is often difficult to treat [Citation19]. There are two types of iron deficiency. Absolute iron deficiency is the reduction of iron stores in macrophages and hepatocytes. It can occur with increased iron requirements, decreased iron intake, malabsorption and chronic bleeding. Functional iron deficiency is secondary to mobilization of iron stores in the circulation as a result of chronic inflammation or infection [Citation9].
Serum ferritin is the most commonly used test for diagnosing iron deficiency. A ferritin threshold of <30 ng/mL has a sensitivity of 92% and specificity of 98% [Citation9]. In the presence of inflammation, a TSC <20% can be used [Citation20]. Other tests have been proposed, such as cross-linked hemoglobin, soluble transferrin receptor and hepcidin level [Citation9].
Iron homeostasis is mainly controlled by hepcidin. Hepcidin is a peptide produced by hepatocytes, playing a role in intestinal absorption and transport of iron [Citation21]. Hepcidin reduces the utilization of iron in cells and prevents its accumulation in tissues. Hepcidin expression is elevated in iron overload, inflammation and infection and decreased in iron deficiency, hypoxia or high erythropoiesis activity.
Iron deficiency and polycythemia
In recent decades, the association between polycythemia and iron deficiency is frequently discussed. Iron deficiency may have an impact on the diagnostic management of polycythemia. The clinical signs of iron deficiency may resemble the symptomatology of polycythemia. Polycythemia may be masked by microcytosis or anemia, as reported in several cases [Citation22,Citation23]. The diagnosis may be delayed, increasing morbidity and mortality. Iron deficiency has been reported mainly in patients with JAK2 mutation [Citation6]. In the current study, the prevalence of iron deficiency was high (60.53%) in the group PV and the risk was also high (OR = 115; CI95% [14.4 − 918.2]). Assuming that iron deficiency is specific to PV, our study showed that its specificity was 100% despite the prevalence of 60.53%.
Apart from phlebotomy, iron deficiency occurring in PV results from several factors. Hematopoietic activity causes significant iron utilization in hemoglobin synthesis. The role of hepcidin has been suggested by some authors, reporting high hepcidin levels during PV. This hypothesis remains inconclusive for other authors [Citation6,Citation24]. Physiological hypoxia at the intestinal lumen promotes iron absorption. During polycythemia, reduced hypoxia inhibits iron absorption [Citation25]. Iron deficiency can be explained by an aberrant iron restriction response [Citation26]. The usual causes of iron deficiency can occur, such as digestive bleeding.
Complications of PV are mainly related to thromboembolic events (41%). Authors have shown that the presence of iron deficiency is associated with an increased risk of thrombosis [Citation7]. In our study, this association was not significant.
Treatment
In PV, the main goal of treatment is to keep the hematocrit level below 45% to reduce the risk of thrombosis [Citation27]. Patients under 60 years of age with a low risk of thrombosis are often treated with phlebotomy alone. Cytoreductive therapy is indicated in cases of repeated phlebotomy or high risk of thrombosis. Hydroxyurea is the first choice because of its good tolerance and lower toxicity. Interferon alpha, Ruxolitinib and Busulfan are second-line agents, indicated in case of failure or intolerance to Hydroxyurea [Citation28].
The treatment of iron deficiency in PV is complex. Although suggested by some authors, iron supplementation is not recommended because it may increase red blood cell mass [Citation29]. According to recent studies, Ruxolitinib (JAK2 inhibitor) is able to maintain iron homeostasis and correct iron deficiency [Citation28]. Currently, hepcidin mimetics are in clinical trials and may induce erythropoiesis without causing iron deficiency [Citation30].
Study strengths and limitations
The study has several limitations. The number of patients is small. Only four patients had serum ferritin after treatment. This is not appropriate to conclude the evolution. Larger studies are needed to strengthen the specificity of iron deficiency in PV. It would be interesting to evaluate the signs of polycythemia associated with iron deficiency.
However, the current study showed that the prevalence and the risk of iron deficiency were high in PV. Iron deficiency was less sensitive but the specificity was 100%. To our knowledge, this is the first study to determine the prevalence of iron deficiency in polycythemia and to evaluate the diagnostic performance. Our approach emphasizes the routine performance of serum ferritin and CST in the initial evaluation of polycythemia. The mechanisms of iron deficiency are complex and multifactorial. Iron deficiency may mask the diagnosis of polycythemia due to microcytosis and anemia. Iron supplementation is not recommended.
Conclusion
The paradoxical association between polycythemia and iron deficiency represents a diagnostic and therapeutic impact. Iron deficiency is rather specific to PV and may mask the diagnosis of polycythemia. Assessment of iron deficiency should be part of the initial evaluation of polycythemia.
Ethics approval
This study respected the Helsinki principles for research and the ethical guidelines of our institution.
Author contributions
RMF Randrianarisoa: conceptualisation, methodology, data collection, original drafting, writing-reviewing and editing. H Ramanandafy: visualisation. A Mania: conceptualisation, methodology, writing-reviewing and editing. H Monjanel, S Trouillier: visualisation, validation.
Acknowledgements
Thanks to all the staff of the Internal Medicine Department of the Henri Mondor Aurillac Hospital. Thanks to P. Vernet, F. Mondillon, M. Barrière and M. Turot for their support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data used in the study can be requested from the corresponding author.
Additional information
Funding
References
- Barbui T, Thiele J, Gisslinger H, et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018;8(2):15), doi:10.1038/s41408-018-0054-y.
- Kremyanskaya M, Mascarenhas J, Hoffman R. Why does my patient have erythrocytosis? Hematol Oncol Clin North Am. 2012;26(2):267–283. doi:10.1016/j.hoc.2012.02.011.
- Brodsky I, Kahn SB, Brady LW. Polycythaemia vera: differential diagnosis by ferrokinetic studies and treatment with busulphan (myleran*). Brit J Haemat. 1968;14(4):351–361. doi:10.1111/j.1365-2141.1968.tb06987.x.
- Thiele J, Kvasnicka HM, Muehlhausen K, et al. Polycythemia rubra vera versus secondary polycythemias. A clinicopathological evaluation of distinctive features in 199 patients. Pathol Res Pract. 2001;197(2):77–84. doi:10.1078/0344-0338-5710013.
- Gianelli U, Iurlo A, Vener C, et al. The significance of bone marrow biopsy and JAK2 V617F mutation in the differential diagnosis between the “early” prepolycythemic phase of polycythemia vera and essential thrombocythemia. Am J Clin Pathol. 2008;130(3):336–342. doi:10.1309/6BQ5K8LHVYAKUAF4.
- Ginzburg YZ, Feola M, Zimran E, et al. Dysregulated iron metabolism in polycythemia vera: etiology and consequences. Leukemia. 2018;32(10):2105–2116. doi:10.1038/s41375-018-0207-9.
- Hung SH, Lin HC, Chung SD. Association between venous thromboembolism and iron-deficiency anemia. Blood Coagul Fibrinolysis. 2015;26(4):368–372. doi:10.1097/MBC.0000000000000249.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544.
- Ning S, Zeller MP. Management of iron deficiency. Hematology Am Soc Hematol Educ Program. 2019;2019(1):315–322. doi:10.1182/hematology.2019000034.
- Moulard O, Mehta J, Fryzek J, et al. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol. 2014;92(4):289–297. doi:10.1111/ejh.12256.
- Deadmond MA, Smith-Gagen JA. Changing incidence of myeloproliferative neoplasms: trends and subgroup risk profiles in the USA, 1973-2011. J Cancer Res Clin Oncol. 2015;141(12):2131–2138. doi:10.1007/s00432-015-1983-5.
- Marneth AE, Mullally A. The molecular genetics of myeloproliferative neoplasms. Cold Spring Harb Perspect Med. 2020;10(2):a034876. doi:10.1101/cshperspect.a034876.
- Tefferi A, Guglielmelli P, Lasho TL, et al. Mutation-enhanced international prognostic systems for essential thrombocythaemia and polycythaemia vera. Br J Haematol. 2020;189(2):291–302. doi:10.1111/bjh.16380.
- Tefferi A. Polycythemia vera: a comprehensive review and clinical recommendations. Mayo Clin Proc. 2003;78(2):174–194. doi:10.4065/78.2.174.
- Dickstein JI, Vardiman JW. Hematopathologic findings in the myeloproliferative disorders. Semin Oncol. 1995;22(4):355–373.
- Pearson TC. Diagnosis and classification of erythrocytoses and thrombocytoses. Baillieres Clin Haematol. 1998;11(4):695–720. doi:10.1016/S0950-3536(98)80035-8.
- Murphy S. Diagnostic criteria and prognosis in polycythemia vera and essential thrombocythemia. Semin Hematol. 1999;36(1 Suppl 2):9–13.
- Pearson TC, Messinezy M. The diagnostic criteria of polycythaemia rubra vera. Leuk Lymphoma. 1996;22(Suppl 1):87–93.
- Kassebaum NJ, GBD 2013 Anemia Collaborators 2016. The global burden of anemia. Hematol Oncol Clin North Am; 30(2):247–308. doi:10.1016/j.hoc.2015.11.002.
- Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. doi:10.1056/NEJMra041809.
- De Domenico I, Lo E, Ward DM, et al. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc Natl Acad Sci USA. 2009;106(10):3800–3805. doi:10.1073/pnas.0900453106.
- Kambali S, Taj A. Polycythemia vera masked due to severe iron deficiency anemia. Hematol Oncol Stem Cell Ther. 2018;11(1):38–40. doi:10.1016/j.hemonc.2016.08.007.
- Aladağ E, Aksu S, Demiroğlu H, et al. Unclassifiable non-CML classical myeloproliferative diseases with microcytosis: findings indicating diagnosis of polycythemia vera masked by iron deficiency. Turk J Med Sci. 2019;49(5):1560–1563. doi:10.3906/sag-1907-67.
- Albayrak C, Tarkun P, Birtaş Ateşoğlu E, et al. The role of hepcidin, GDF15, and mitoferrin-1 in iron metabolism of polycythemia vera and essential thrombocytosis patients. Turk J Med Sci. 2019;49(1):74–80. doi:10.3906/sag-1803-13.
- Schächner E, Ronen M, Pinkhas J, et al. Iron absorption in patients with polycythemia vera. Eur J Nucl Med. 1978;3(2):125–127. doi:10.1007/BF00251637.
- Khalil S, Delehanty L, Grado S, et al. Iron modulation of erythropoiesis is associated with Scribble-mediated control of the erythropoietin receptor. J Exp Med. 2018;215(2):661–679. doi:10.1084/jem.20170396.
- Marchioli R, Vannucchi AM, Barbui T. Treatment target in polycythemia vera. N Engl J Med. 2013;368(16):1554), doi:10.1056/NEJMc1301262.
- Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426–435. doi:10.1056/NEJMoa1409002.
- Salem HH, Van der Weyden MB, Young IF, et al. Pruritus and severe iron deficiency in polycythaemia vera. Br Med J (Clin Res Ed). 1982;285(6335):91–92. doi:10.1136/bmj.285.6335.91.
- Casu C, Nemeth E, Rivella S. Hepcidin agonists as therapeutic tools. Blood. 2018;131(16):1790–1794. doi:10.1182/blood-2017-11-737411.