ABSTRACT
Objectives
To determine the impact of pretransplant measurable residual disease (pre-MRD) and the efficacy of maintenance therapy in t(8;21) acute myeloid leukemia (AML) patients after allogeneic hematopoietic cell transplantation (allo-HCT).
Methods
We retrospectively analyzed 100 t(8;21) AML patients who underwent allo-HCT between 2013 and 2022. 40 patients received pre-emptive therapy including immunosuppressant adjustment, azacitidine, and donor lymphocyte infusion (DLI) combined with chemotherapy. 23 patients received prophylactic therapy, including azacitidine or chidamide.
Results
Patients with a positive pre-MRD (pre-MRDpos) had a higher 3-year cumulative incidence of relapse (CIR) (25.90% [95% CI, 13.87%–39.70%] vs 5.00% [95% CI, 0.88%–15.01%]; P = 0.008). Pre-MRDpos patients were less likely to have a superior 3-year disease-free survival (DFS) (40.83% [95% CI, 20.80%–80.16%]) if their MRD was still positive at 28 days after transplantation (post-MRD28pos). The 3-year DFS and CIR were 53.17% (95% CI, 38.31% – 73.80%) and 34.87% (95% CI, 18.84% – 51.44%), respectively, for patients receiving pre-emptive interventions after molecular relapse. The 3-year DFS and CIR were 90.00% (95%CI, 77.77% – 100%) and 5.00% (95%CI, 0.31% – 21.10%), respectively, for high-risk patients receiving prophylactic therapy. In most patients, epigenetic-drug-induced adverse events were reversible with dose adjustment or temporary discontinuation.
Conclusion
Patients with pre-MRDpos and post-MRD28pos were more likely to have higher rates of relapse and inferior DFS, even after receiving pre-emptive interventions. Prophylactic therapy may be a better option for high-risk t(8;21) AML patients; however, this warrants further investigation.
Introduction
Acute myeloid leukemia (AML) carrying the t(8;21) chromosomal translocation, which corresponds to the RUNX1/RUNX1T1 fusion, is classified as a favorable-risk AML subset. For most t(8;21) AML patients, complete remission (CR) can be achieved because of their high sensitivity to standard induction chemotherapy. Moreover, cytarabine-based consolidation therapy can provide long-term remission. However, up to 40% of patients may still experience relapse [Citation1–3]. Allogeneic hematopoietic cell transplantation (allo-HCT) can improve the prognosis of high-risk t(8;21) AML patients [Citation4, Citation5]. However, as a disease with heterogeneous characteristics, nearly 15% – 20% of t(8;21) AML patients who undergo allo-HCT experience relapse with a dismal outcome [Citation6–8]. Therefore, it is urgent to identify patients at high risk of relapse and implement strategies to improve their survival.
The RUNX1/RUNX1T1 fusion gene expression, which is evaluated by quantitative real-time PCR (qRT-PCR), is a target of minimal residual disease (MRD) assessment and a powerful predictor of t(8;21) AML patient outcomes [Citation6, Citation9]. Studies have found that pretransplant (pre-)MRD may predict the relapse of patients with t(8;21) AML after allo-HCT [Citation10]. Hence, interventions based on pre-MRD might be a feasible option. However, the impact of pre-MRD on outcomes after allo-HCT remains controversial [Citation10, Citation11].
Broadly speaking, maintenance therapy includes pre-emptive therapy (based on MRD) and prophylactic therapy (not based on MRD) [Citation12]. Emerging studies have shown that high-risk AML patients may benefit from maintenance therapy after allo-HCT because it can induce graft versus leukemia (GVL) effect and eradicate persistent MRD [Citation13, Citation14]. Considering the risk of unnecessary exposure to treatment, pre-emptive interventions directed by MRD after allo-HCT are more common. However, no comparison has been made between the suitability of prophylactic and pre-emptive therapy for use in AML patients. Besides, studies reporting the utility of prophylactic and pre-emptive strategies in a specific disease setting, such as t(8;21) AML, are limited [Citation15]. Azacitidine (AZA), a hypomethylating agent, is commonly used as a maintenance drug in AML patients. Moreover, studies have demonstrated the feasibility of pre-emptive AZA use in patients with high-risk AML and myelodysplastic syndromes (MDS) [Citation16, Citation17]. As a novel histone deacetylase inhibitor, chidamide has been proven to inhibit AML cell proliferation and induce cell apoptosis [Citation18, Citation19]. A phase 2 trial reported that the double epigenetic priming regimen, which included chidamide, showed superior anti-leukemia activity. In our study, epigenetic drugs, including AZA and chidamide, were used as prophylactic agents. Therefore, we aimed to investigate the influence of pre-MRD on the outcomes of t(8;21) AML patients, in addition to evaluating the efficacy of maintenance therapy, including pre-emptive and prophylactic interventions, after allo-HCT.
Materials and methods
Patients and treatment details
This retrospective study included 100 patients with t(8;21) AML, aged 14 years or older, who underwent their first allo-HCT at the Institute of Hematology & Blood Diseases Hospital from January 2013 to April 2022. All patients achieved CR after induction therapy. All patients received the myeloablative conditioning regimen (MAC) based on busulfan (BU) and cyclophosphamide (CY).
The characteristics of the patients are shown in . The study was approved by the Medical Ethics Committee of the Institute of Hematology & Blood Diseases Hospital and all patients or their guardians provided informed consent before participation.
Table 1. The characteristics of patients.
Maintenance therapy
The reasons for initiating prophylactic therapy were: (1) a positive pre-MRD status (pre-MRDpos); (2) relapse before transplantation; (3) and requiring > 1 course of induction therapy to achieve CR; these were considered risk factors for relapse after transplantation according to published reports [Citation8–10]. Patients qualifying for prophylactic therapy had at least one of the three risk factors. The decision to treat patients with prophylactic donor lymphocyte infusion (DLI, n = 3) or epigenetic drugs (n = 20), was based on the patient’s condition, accessibility of donors, and physician’s preference. The epigenetic drugs used were AZA (n = 12) and chidamide (n = 8). All patients experiencing molecular relapse initially underwent immunosuppressant adjustment, and other pre-emptive agents based on their RUNX1/RUNX1T1 transcript levels and the physician and patient preferences. 14 of the patients used only immunotherapeutic interventions. Other pre-emptive agents included AZA alone (n = 12), AZA or chemotherapy based on cytarabine with DLI (n = 14).
AZA was administered by subcutaneous injection for 5 days every 4 weeks at a dose of 50 mg/m2 per day when the platelet count was > 50 × 109/L without transfusion for at least 7 days. Chidamide was administrated 3–5 times a week orally at an initial dosage of 5 mg per day approximately 30 min after a meal when the platelet count was > 20 × 109/L without transfusion for at least 7 days. The dosage could be gradually increased to 10 mg per day 5 times a week when the platelet count was > 50 × 109/L, which lasted for at least 1 year after transplantation. DLI was performed as previously described [Citation20]. For the pre-emptive interventions, DLI was combined with chemotherapy.
The exclusion criteria for maintenance therapy were as follows: (1) active graft versus host disease (GVHD); (2) active and uncontrolled infections; (3) hematologic relapse; and (4) organ failure [Citation15].
MRD monitoring and samples
MRD was monitored using qRT-PCR to quantify transcript levels of RUNX1/RUNX1T1 expression; the results were expressed as the percent ratio of RUNX1/RUNX1T1 to ABL1 transcript levels × 100. The sensitivity of this assay was 0.001%. Therefore, a positive MRD status (MRDpos) was defined as a transcript ratio > 0.001%, and a negative MRD status (MRDneg) was defined as a transcript ratio ≤ 0.001%, on the condition that at least 10,000 copies of the ABL1 control gene had been amplified [Citation11]. Samples were collected from the bone marrow. MRD was detected in all the patients 30 days before the day of transplantation, which was defined as the pre-MRD. After allo-HCT, bone marrow samples were collected serially at 0, 14, 28, and 42 days, and 2, 3, 4.5, 6, 9, 12, 18, and 24 months to monitor MRD levels [Citation6, Citation9]. When patients showed signs of relapse, MRD was measured immediately and may shorten the time interval to measure the RUNX1-RUNX1T1 transcript levels later.
Definitions
CR was defined as the presence of < 5% blasts in the bone marrow with the recovery of peripheral cell counts and no signs of extramedullary disease. Relapse was defined as the presence of > 5% blasts in the bone marrow, blasts in peripheral blood, and/or extramedullary disease. Molecular relapse after transplantation was defined as the transition from MRDneg to MRDpos or an increase in MRD copy numbers > 1 log10 between any two positive samples [Citation2]. Complete molecular remission (CMR) after transplantation was defined as a transcript ratio ≤ 0.001% [Citation21]. The last follow-up date was July 31, 2022. The median follow-up time was 32.67 (interquartile range [IQR], 17.57–58.53) months.
Objectives and statistical analysis
The main objective was relapse rate. The secondary objectives were disease-free survival (DFS) and overall survival (OS). The severity of adverse events was graded according to the National Cancer Institute Common Toxicity Criteria (CTCAE, version 5.0). Acute (a)GVHD was graded according to the MAGIC criteria. The cumulative incidence of relapse (CIR) was calculated by competing risk analysis, taking into account competing risks. The Gray’s test was used to examine differences between groups. The events for DFS included death and hematological relapse. The probability of OS and DFS was calculated using the Kaplan – Meier method, and differences between groups were tested using the log-rank test. The Mann – Whitney test was used for continuous variables and the χ2 test or Fisher exact test were used for categorical variables. Statistical analyses were performed using R software (version 4.1.3) and SPSS 20. P-values < 0.05 were considered as a measure of statistical significance.
Results
Patient characteristics
The characteristics of 100 patients with t(8;21) AML who received allo-HCT in our center between 2013 and 2022 are shown in . The RUNX1/RUNX1T1 transcript levels at diagnosis were assessed in 62 of 100 (62.0%) patients and the median level was 209% (IQR, 131.0% – 323.3%). 73 were alive by the end of the study, which had a median follow-up time of 32.67 (IQR, 17.57–58.53) months; 12 of the patients died from relapse, and 15 died because of treatment-related complications. 15 patients relapsed with a median relapse time of 8.53 (IQR, 3.27–20.30) months. The CIR at 3 years was 16.40% (95% confidence interval [CI], 9.28% – 25.28%). The probability of OS at 3 years was 70.97% (95% CI, 61.85% – 81.43%) and DFS was 68.04% (95% CI, 58.70% – 78.86%).
The prognostic value of pre-MRD
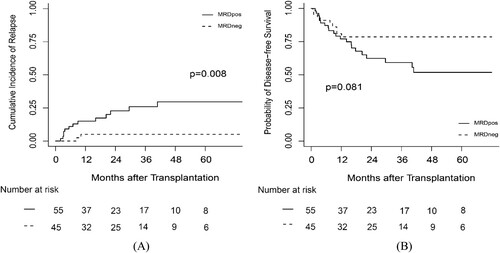
55 patients had pre-MRDpos and 45 patients had negative pre-MRD (pre-MRDneg). The risk factors that influenced patient outcomes are listed in Supplementary Table 1. The 3-year CIR was 25.90% (95% CI, 13.87% – 39.70%) and 5.00% (95% CI, 0.88% – 15.01%) for pre-MRDpos and pre-MRDneg patients, respectively (P = 0.008; (a)). Pre-MRDpos patients had a 3-year DFS of 59.27% (95% CI, 46.27% – 75.94%), which was slightly lower than that of the pre-MRDneg patients, which was 78.59% (95% CI, 67.07% – 92.10%; P = 0.081) ((b)). The 3-year OS was 78.59% (95% CI, 67.07% – 92.10%) and 64.66% (95% CI, 51.90% – 80.57%) for the pre-MRDneg and pre-MRDpos patients, respectively (P = 0.240).
Figure 1. The probability of (A) CIR and (B) DFS according to MRD status at transplantation. MRD: measurable residual disease.
In 97 of 100 (97.0%) patients, the RUNX1/RUNX1T1 transcripts were detected at around 28 days after transplantation (post-MRD28); of the 54 pre-MRDpos patients, 38 (70.4%) achieved negative post-MRD28 but 16 (29.6%) failed. As shown in (a), the 3-year CIR of pre-MRDpos and post-MRD28 patients was 37.29% (95% CI, 11.38% – 63.92%) and that of pre-MRDpos but post-MRD28neg patients was 21.37% (95% CI, 8.91% – 37.35%; P = 0.208). Irrespective of whether the patients’ post-MRD28 was positive or not, the 3-year CIR for pre-MRDpos patients was inferior to that of pre-MRDneg patients (P = 0.002 for post-MRD28pos; P = 0.044 for post-MRD28neg).
Figure 2. The probability of (A) CIR and (B) DFS according to MRD status at transplantation and 28 days after transplantation. ++: MRD status was positive at transplantation and at 28 days after transplantation. + -: MRD status was positive at transplantation but negative at 28 days after transplantation. neg: MRD status was negative at transplantation.
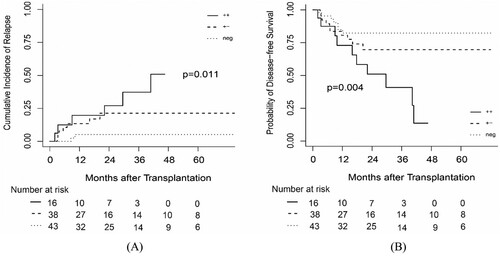
The 3-year DFS was 40.83% (95% CI, 20.80% – 80.16%) and 69.66% (95% CI, 55.21% – 87.90%) for the patients with pre-MRDpos and post-MRD28pos and patients with pre-MRDpos but post-MRD28neg, respectively (P = 0.041; (b)). The DFS of pre-MRDneg patients was superior to that of patients with pre-MRDpos and post-MRD28pos (P = 0.003). However, the DFS was comparable between pre-MRDneg patients and those with a pre-MRDpos but post-MRD28neg status (P = 0.267).
The 3-year OS of patients with pre-MRDpos and post-MRD28pos was 57.14% (95% CI, 36.30% – 89.94%) and that of patients with pre-MRDpos but post-MRD28neg was 70.23% (95% CI, 55.32% – 89.16%; P = 0.178). No significant difference was found between pre-MRDneg patients and post-MRD28pos (P = 0.065) or post-MRD28neg (P = 0.416) patients.
The pre-MRD status and molecular relapse after transplantation
During the follow-up period, 57 (58.8%) of patients had persistent CMR; 40 (41.2%) patients experienced molecular relapse with a median time of 2.42 months (IQR, 2.00–4.43). Of the 16 patients with pre-MRDpos and post-MRD28pos, 13 (81.3%) patients eventually experienced molecular relapse. 18 of 38 (47.4%) patients with pre-MRDpos but post-MRD28neg and 9 of 43 (20.9%) patients with pre-MRDneg experienced molecular relapse. Compared pre-MRDpos but post-MRD28neg patients, the pre-MRDpos and post-MRD28pos patients were more likely to experience molecular relapse after transplantation (P = 0.021). The pre-MRDneg patients were less likely to experience molecular relapse, compared to the post-MRD28pos (P < 0.001) or post-MRD28neg (P = 0.012) patients.
Pre-emptive and prophylactic therapy after allo-HCT
After molecular relapse, all patients received pre-emptive therapy, including adjustment of immunosuppressants, followed by other pre-emptive interventions (Supplementary Table 2). After pre-emptive therapy, 40.0% (16/40) of patients achieved MRDneg within 1 month and 75.0% (30/40) within 3 months. However, 13 (32.5%) patients eventually progressed to hematological relapse. The 3-year CIR was 34.87% (95% CI, 18.84% – 51.44%) for patients receiving pre-emptive interventions. The probability of DFS at 3 years was 53.17% (95% CI, 38.31% – 73.80%) and OS was 60.89% (95% CI, 46.13% – 80.37%). Besides, 14 patients were treated using only immunosuppressant adjustment; however, 3 (21.4%) of these patients eventually experienced hematological relapse. 10 of 26 (38.5%) patients who used additional pre-emptive interventions progressed to hematological relapse with a median progression time (from molecular relapse to hematological relapse) of 6.83 (IQR, 1.62–21.52) months.
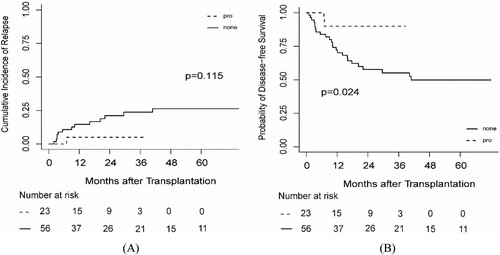
Prophylactic therapy was performed in high-risk patients, meaning that these patients received the therapy when their MRD levels did not meet the standard for molecular relapse. 23 patients received prophylactic therapy with a median initial time of 3.07 (IQR, 2.03–5.17) months after transplantation. 3 of 23 (13.04%) patients experienced molecular relapse and all of them were treated using pre-emptive intervention; however, one (4.3%) patient progressed to hematological relapse (Supplementary Table 2). The 3-year CIR was 5.00% (95%CI, 0.31% – 21.10%). The probability of DFS at 3 years was 90.00% (95%CI, 77.77% – 100%) and the 3-year OS was 89.06% (95%CI, 75.77% – 100%). We also compared patients receiving prophylactic treatment with patients who had at least one of the three risk factors, but were not treated using prophylactic therapy (n = 56). The probability of OS and CIR at 3 years was comparable between the two groups (P = 0.064 and P = 0.115, respectively) ((a)). However, the 3-year DFS was superior for patients that received prophylactic therapy (P = 0.024) ((b)).
Among the patients treated with prophylactic therapy, three patients received DLI; two of these patients experienced grade II aGVHD, which was improved using intensive immunosuppressive treatment. None of the remaining 20 patients who received AZA or chidamide developed grade II-IV aGVHD after prophylactic treatment; although four of the patients experienced grade II aGVHD before prophylactic therapy initiation. 9 (39.13%) patients developed chronic GVHD. As shown in , the most common adverse effect after receiving epigenetic drugs was hematological toxicities with thrombocytopenia (all grades, 85.0%), neutropenia (all grades, 80.0%), and anemia (all grades, 40.0%). None of the patients discontinued maintenance therapy after adequate supportive measures despite experiencing hematological toxicity. Three (15.0%) patients developed an infection, and one patient discontinued chidamide treatment. A further three patients experienced molecular relapse and stopped prophylactic therapy.
Table 2. Adverse effects after prophylacatic therapy (exclude DLI)
Discussion
A number of studies have reported the clinical utility of MRD monitoring (of RUNX1/RUNX1T1 fusion transcript by qRT-PCR) to predict the outcomes of patients with t(8;21) AML after allo-HCT [Citation8, Citation9]. In this retrospective study of allo-HCT in t(8;21) AML patients to achieve CR (CR1 or CR2), we found that the pre-MRDpos patients had a higher rate of relapse, which has been previously demonstrated [Citation10, Citation22]. However, we also found that pre-MRDpos patients were more likely to exhibit inferior DFS if their post-MRD28 was still positive. Besides, we evaluated the efficacy of pre-emptive therapy in patients with molecular relapse and found that it could delay but not prevent relapse. Finally, we reported that prophylactic therapy could improve the outcomes of patients at high risk of relapse and was well tolerated.
Since t(8;21) AML is a favorable-risk form of AML, allo-HCT is the first-line treatment option only for high-risk patients [Citation5]. Hence, the reliability of MRD monitoring to predict relapse after allo-HCT needed to be supposed with more evidence. Besides, whether pre-MRD could predict patient outcomes after transplantation was under debate. A study of 631 t(8;21) AML patients showed that pre-MRDneg status was significantly associated with a lower incidence of relapse for t(8;21) AML patients undergoing allo-HCT during CR2 [Citation10]. A study that measured RUNX1/RUNX1T1 fusion transcript levels in 50 t(8;21) AML patients showed that MRD at transplantation was not associated with an increased relapse rate but was related to inferior DFS and OS [Citation11]. Wang et al. reported that the pre-MRD could not predict the risk of relapse, but that MRD at the early phase after allo-HCT was an independent risk factor for relapse [Citation8]. In our study, besides pre-MRD, we also evaluated the impact of post-MRD28, and found that pre-MRDpos and post-MRD28pos patients had a higher incidence of relapse and inferior DFS.
The use of maintenance therapy in patients with AML has been reported; however, few studies have demonstrated whether maintenance therapy is effective and safe to use in the context of a specific disease, such as t(8;21) AML [Citation12, Citation15, Citation23]. Besides, prophylactic therapy may result in overtreatment and subject patients to adverse long-term and late effects. Hence, the use of maintenance therapy for t(8;21) AML is typically aimed at pre-emptive interventions and it remains less clear whether maintenance therapy is effective in this context. To the best of our knowledge, this study was the first to report the success of prophylactic therapy in t(8;21) AML after allo-HCT. A case report previously showed that the combination of low-dose PD-1 blockade and AZA prevented the progression from molecular relapse to hematological relapse for a patient with t(8;21) AML [Citation24]. Recently, a published study showed that patients with low RUNX1/RUNX1T1 transcript burdens after transplantation might benefit from pre-emptive interferon (IFN)-α therapy, whereas patients with high RUNX1/RUNX1T1 transcript levels failed to benefit from pre-emptive IFN-α or DLI therapy [Citation15]. Pre-emptive AZA treatment of patients with MDS or AML failed to prevent imminent hematological relapse but relapse was delayed until a median of 231 (range, 56–558) days after MRD was detected [Citation16], which was similar to our results.
Considering that the RUNX1/RUNX1T1 fusion transcripts levels after transplantation played an important role in relapse and that the pre-MRDpos status had an impact on MRD in the early phase after transplantation, we believe that prophylactic therapy should be taken to reduce the burdens of the RUNX1/RUNX1T1 fusion transcript for high-risk patients, especially for patients with pre-MRDpos and post-MRD28pos. Our initial prophylactic therapy results, which showed that t(8;21) AML patients had a superior DFS and low incidence of relapse, were promising. However, the optimal dosage and timing of prophylactic therapy and which t(8;21) AML patients could benefit more from prophylactic therapy still need to be addressed [Citation12]. In a phase 3 randomized controlled trial, the use of AZA (administered at a dose of 32 mg/m2) as a maintenance therapy in the post-transplant setting did not significantly improve relapse-free survival in patients with high-risk AML or MDS after allo-HCT [Citation23]. Hence, the dose of AZA was increased to 50 mg/m2 in this study. Whether prophylactic therapy is safe and effective in patients with t(8;21) AML must be confirmed with additional studies.
Our study had several limitations. The numer of patients who received maintenance therapy was relatively small. Thus, larger randomized controlled trials are needed to confirm the utility of prophylactic therapy for t(8;21) AML patients. Besides, the maintenance strategies included in this study varied considerably, from immunotherapy to targeted drugs. Therefore, the efficacy of different targeted therapy regimens should be compared in future studies.
To summarize, pre-MRDpos t(8;21) AML patients experienced a higher rate of hematological relapse. Moreover, the DFS at 3 years was inferior for patients with pre-MRDpos and post-MRD28pos status. Pre-emptive therapy failed to prevent forthcoming hematological relapse after molecular relapse. Therefore, prophylactic therapy maybe a better option for high-risk t(8;21) AML patients.
Author contributions
WWG performed the research and drafted the manuscript. WWG and XL analyzed the data. WWG, XL, MYW, and JL collected data. YGC, YWZ, XC, RLZ, YH, QLM, WHZ, DLY, JLW, and AMP obtained clinical information. MZH, SZF, and ELJ designed the study. ELJ supervised the study. All authors contributed to the article and approved the submitted version.
Geolocation information
Tianjin, China
Acknowledgments and fundings
The study was supported by the Haihe Laboratory of Cell Ecosystem Innovation Fund (No. HH22KYZX0034), the National Nature Science Foundation of China (No. 82170217, No. 82070192 and No. 81670171), Fundamental Research Funds for the central universities (No. 3332020052), CAMS Innovation Fund for Medical Sciences (No. 2021-I2M-1-073), and Key Project of Tianjin Natural Science Foundation (No. 20JCZDJC00410).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The raw data of this article can be available by all authors.
Additional information
Funding
References
- Prébet T, Boissel N, Reutenauer S, et al. Acute myeloid leukemia with translocation (8;21) or inversion (16) in elderly patients treated with conventional chemotherapy: a collaborative study of the French CBF-AML intergroup. J Clin Oncol. 2009;27(28):4747–53.
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345–77.
- Beghini A. Core binding factor leukemia: chromatin remodeling moves towards oncogenic transcription. Cancers (Basel). 2019;11(12.
- Kayser S, Kramer M, Martínez-Cuadrón D, et al. Characteristics and outcome of patients with core-binding factor acute myeloid leukemia and FLT3-ITD: results from an international collaborative study. Haematologica. 2022;107(4):836–43.
- Zhu HH, Zhang XH, Qin YZ, et al. MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: results from the AML05 multicenter trial. Blood. 2013;121(20):4056–62.
- Guo WW, Liu X, Pang AM, et al. [Analysis of risk factors of relapse after allogeneic hematopoietic stem cell transplantation in patients with t (8;21) acute myeloid leukemia]. Zhonghua Xue Ye Xue Za Zhi. 2021;42(12):998–1004.
- Qin Y-Z, Wang Y, Xu L-P, et al. The dynamics of RUNX1-RUNX1T1 transcript levels after allogeneic hematopoietic stem cell transplantation predict relapse in patients with t(8;21) acute myeloid leukemia. J Hematol Oncol. 2017;10(1):44.
- Wang Y, Wu DP, Liu QF, et al. In adults with t(8;21)AML, posttransplant RUNX1/RUNX1T1-based MRD monitoring, rather than c-KIT mutations, allows further risk stratification. Blood. 2014;124(12):1880–6.
- Qin YZ, Wang Y, Xu LP, et al. The dynamics of RUNX1-RUNX1T1 transcript levels after allogeneic hematopoietic stem cell transplantation predict relapse in patients with t(8;21) acute myeloid leukemia. J Hematol Oncol. 2017;10(1):44.
- Konuma T, Kondo T, Masuko M, et al. Prognostic value of measurable residual disease at allogeneic transplantation for adults with core binding factor acute myeloid leukemia in complete remission. Bone Marrow Transplant. 2021;56(11):2779–87.
- Yalniz FF, Patel KP, Bashir Q, et al. Significance of minimal residual disease monitoring by real-time quantitative polymerase chain reaction in core binding factor acute myeloid leukemia for transplantation outcomes. Cancer. 2020;126(10):2183–92.
- Xuan L, Liu Q. Maintenance therapy in acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. 2021;14(1):4.
- Lee CJ, Savani BN, Mohty M, et al. Post-remission strategies for the prevention of relapse following allogeneic hematopoietic cell transplantation for high-risk acute myeloid leukemia: expert review from the acute leukemia working party of the European society for blood and marrow transplantation. Bone Marrow Transplant. 2019;54(4):519–30.
- Wang Y, Liu QF, Wu DP, et al. Impact of prophylactic/preemptive donor lymphocyte infusion and intensified conditioning for relapsed/refractory leukemia: a real-world study. Sci China Life Sci. 2020;63(10):1552–64.
- Fan S, Shen MZ, Zhang XH, et al. Preemptive immunotherapy for minimal residual disease in patients With t(8;21) acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. Front Oncol. 2021;11(773394.
- Platzbecker U, Wermke M, Radke J, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. 2012;26(3):381–9.
- Platzbecker U, Middeke JM, Sockel K, et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): an open-label, multicentre, phase 2 trial. Lancet Oncol. 2018;19(12):1668–79.
- Lin L, Que Y, Lu P, et al. Chidamide inhibits acute myeloid leukemia cell proliferation by lncRNA VPS9D1-AS1 downregulation via MEK/ERK signaling pathway. Front Pharmacol. 2020;11:569651.
- Li Z, Zhang J, Zhou M, et al. Epigenetic therapy with chidamide alone or combined with 5-azacitidine exerts antitumour effects on acute myeloid leukaemia cells in vitro. Oncol Rep. 2022;47:4.
- Yan CH, Liu DH, Liu KY, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood. 2012;119(14):3256–62.
- Willekens C, Blanchet O, Renneville A, et al. Prospective long-term minimal residual disease monitoring using RQ-PCR in RUNX1-RUNX1T1-positive acute myeloid leukemia: results of the French CBF-2006 trial. Haematologica. 2016;101(3):328–35.
- Halaburda K, Labopin M, Mailhol A, et al. Allogeneic stem cell transplantation in second complete remission for core binding factor acute myeloid leukemia: a study from the acute leukemia working party of the European society for blood and marrow transplantation. Haematologica. 2020;105(6):1723–30.
- Oran B, de Lima M, Garcia-Manero G, et al. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv. 2020;4(21):5580–8.
- Tang Y, Zhou Z, Yan H, et al. Case report: preemptive treatment With Low-dose PD-1 blockade and azacitidine for molecular relapsed acute myeloid leukemia With RUNX1-RUNX1T1 after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2022;13:810284.