ABSTRACT
Objectives
The aim of this study is to investigate the effect of treatment choice on survival, transfusion needs and hospitalizations in patients > 64 years old with newly diagnosed acute myeloid leukaemia (AML).
Material and methods
This study retrospectively analysed patients over 64 years with AML diagnosed at a regional healthcare network in Switzerland between 2017 and 2020. Patients underwent four therapy groups: intensive chemotherapy (IC), hypomethylating agent in combination with the BCL2-Inhibitor venetoclax (HMA + VEN), hypomethylating agents alone (HMA) or best supportive care (BSC).
Results
Of 54 patients 12 (22%) were selected for IC, 13 (24%) for HMA + VEN, 17 (32%) for HMA and 12 (22%) for BSC. The median overall survival of the patients was 76 days, with a significant difference in the four therapy groups (IC 119 days, HMA + VEN 732 days, HMA monotherapy 73 days and BSC 12 days Log-Rank Test Chi2(2): p < 0.001). Patients with HMA + VEN spent significantly less time in the hospital 6.8 days/month compared to IC (19.5 days/month), HMA (20.5 days/month) and BSC (10.5 days/month) (p = 0.005). Transfusion needs were the highest in IC (7.0 RBC/month, 8.0 PC/month) (p = 0.023), whereas there was no difference between HMA + VEN (2.5 RBC/month, 3.2 PC/month), HMA monotherapy (5.3 RBC/month, 6.2 PC/month) and BSC (3.0 RBC/month, 1.4 PC/month).
Conclusion
Our real-world data demonstrate superior OS rates of HMA + VEN when compared to IC, HMC or BSC, with a favourable side effect profile with regard to transfusion needs or hospitalization days.
Abbreviations: AML, acute myeloid leukaemia; BCL2, B-cell leukaemia/lymphoma-2; BSC, best supportive care; CR, complete response; Cri, complete response with incomplete haematologic regeneration; FLT3, Fms Related Receptor Tyrosine Kinase 3; EKOS, Ethikkomission Ostschweiz; ELN, European Leukaemia Net; HMA, hypomethylating agent; IC, intensive chemotherapy; IDH, Isocitratdehydrogenase; LDAC, low-dose Cytarabine; NCCN, National Comprehensive Cancer Network; OS, overall survival; PC, platelet concentrate; RBC, red blood cell; RCT, randomized controlled trials; t-AML, therapy relative acute myeloid leukaemia'; VEN, venetoclax
1. Introduction
Acute myeloid leukaemia most commonly affects older adults, with a median age at diagnosis of 68 years [Citation1]. Incidence rates of AML in the United States are around 5.5 cases per 100,000 individuals aged <65 years and around 20 cases per 100,000 individuals aged ≥65 years [Citation1]. Despite recent therapeutic progress, the outcome of older patients is far from satisfactory. Age and performance status is a major factor limiting intensive therapy approach in many patients. In addition, comorbidity indices have been developed, which more closely predict clinical outcomes. With increasing age, the incidence of unfavourable (cyto)genetics rises, which is believed to further contribute to the poor outcome of older patients with AML [Citation2,Citation3]. This results in an estimated overall two-year survival rate of around 14% among those aged ≥65 years compared with around 42% in patients aged 50–64 years [Citation1].
For many years the treatment choice was between intensive chemotherapy, low-dose cytarabine (LDAC), and the best supportive care. But even in intensively treated fit patients remission rates lack behind the clinical outcome in the younger patient population [Citation4]. Since 2012 lower-intensity therapies, particularly hypomethylating agents (HMA) such as azacytidine or decitabine have been the mainstay of therapy for unfit AML patients who are poor candidates for intensive induction chemotherapy [Citation5,Citation6]. Notably, clinical outcome was getting close to survival rates observed for intensively treated patients [Citation7]. In 2017 first results of new combination therapy of HMA with venetoclax, a selective inhibitor of B-cell leukaemia/lymphoma-2 (BCL-2), showed promising results with significant activity in up-front treatment resulting in even higher complete response (CR)/complete response with incomplete haematologic regeneration (CRi) rates and a median overall survival of 14.7 months vs. 9.6 months compared to HMA monotherapy in older patients with AML [Citation8–10]. These survival rates are in the range of what is reported in intensively treated elderly fit patients, especially with an adverse cytogenetic risk profile [Citation11]. However, there is no prospective head-to-head comparison available addressing this question. How to choose between these therapy strategies for each individual patient remains a subject of discussion [Citation12–15]. The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology recommend for patients aged >60 years with acute myeloid leukaemia use performance status, adverse risk features (de novo AML without favourable cytogenetics or molecular markers; therapy-associated AML (t-AML); antecedent haematologic disorder) and comorbidities to select treatment options rather than just relying on patients’ chronological age [Citation16]. Bories et al. showed that not only patients’ characteristics and comorbidities influence the treatment decision but also the individual behavioural characteristics of the physician [Citation17].
2. Methods
2.1. Patients and disease characteristics
We retrospectively analysed n = 54 older patients with AML treated at the healthcare network of the cantonal hospital St. Gallen in Switzerland from January, 1 2017 to October, 1 2020 to assess for survival outcome and quality of life parameters according to the treatment chosen. All patients were >64 years and had a confirmed diagnosis of AML according to World Health Organization 2016 criteria [Citation18]. Patients were identified using the institutional registry. Patient- and disease-specific variables, date of diagnosis, treatment and date of death were collected from the clinical information system covering the entire local network. The day of bone marrow biopsy was used as the date of diagnosis, alternatively, if bone marrow examination was not performed, the date of peripheral blood blast count reaching or exceeding 20%, respectively the date of flow cytometry analysis, was used. Prognostic groups were categorized according to the European Leukemia Net (ELN) 2017 guidelines [Citation19]. This retrospective data analysis was approved by the local ethics committee (Ethikkomission Ostschweiz).
2.2. Therapy regimens
We defined four treatment groups before data analysis. Patients in the intensive chemotherapy group (n = 12) received standard induction therapy (7 + 3) in curative intention with cytarabine (200 mg/m2 d1–7) and daunorubicin (60 mg/m2 d1–3) (n = 11) or liposomal daunorubicin/cytarabine in a 1:5 formulation (CPX 351 100 U/m2 d1, 3, 5) (n = 1) for two cycles. Three patients died before cycle two. CPX was chosen in the case of secondary AML and treatment started after the 1st of January 2020, as CPX was not available before. Induction was followed by risk-adapted consolidation therapy either with intermediate-dose cytarabine (ELN favourable risk) or an allogeneic haematopoietic stem cell transplantation concept (ELN intermediate/high risk). A reduced-intensity conditioning regimen was chosen for all patients by the transplant centre.
Patients undergoing monotherapy with a hypomethylating agent received either decitabine (DEC) 20 mg/m2 days 1–5 intravenously (n = 13) or azacytidine (AZA) 100 mg/m2 days 1–5 subcutaneously (n = 4) per 28-day treatment cycles. It has been shown that the 5-day AZA regimen is comparable in efficacy and safety to the original 7-day treatment regimen [Citation20]. The patients received on average 5.3 cycles of HMA (range 1–26 cycles). Most of these patients were treated before the availability of Venetoclax in Switzerland or due to rejected cost approval by the insurance companies.
The third group consisted of patients undergoing an HMA + venetoclax combination strategy (n = 13). HMA was administered in the same dosage as in the monotherapy setting. As recommended, a short dose ramp-up was performed for venetoclax in the first cycle to achieve an end dose of 400 mg daily.
To note, all patients started with HMA monotherapy due to the necessity to apply for cost credit for venetoclax by health insurance before the start of treatment. By experience time to response is expected within 60 days.
We therefore defined two categories of patients undergoing HMA + VEN: Patients starting the combination therapy within the 60 days limit – and patients starting later than day 60, which we defined as a cross-over situation. Cross-over situations were mainly due to insufficient response on HMA monotherapy or by choice of the treating physician as there was new rising evidence of treatment efficacy of venetoclax during that time.
Last, n = 12 frail patients were treated with the best supportive care only. In this cohort cytoreductive treatment with hydroxyurea was administered if appropriate (n = 2).
2.3. Hospitalizations
As a parameter for health economics and quality of life hospitalization rate were calculated. All hospitalization events from the day of diagnosis until death or the last day of follow-up were collected from the electronic records of the local health network. In addition, total hospitalization days were determined. All hospitalizations were included irrespective of the cause of hospitalization.
2.4. Blood transfusions
A number of transfused red cell- and platelet concentrates per patient were collected from the local blood bank repository from the day of diagnosis to the last follow-up.
2.5. Statistics
Fisher’s exact test and Kruskal–Wallis equality-of-populations rank tests were used to assess potential differences in baseline characteristics.
Nonparametric Kaplan-Meier survivor function was employed to assess the overall survival of patients. Differences in overall survival were computed using nonparametric log-rank tests. Univariate Cox proportional hazard models were applied to further explore associations between therapy start, therapy duration, and overall survival. Hazard ratios (HR) with corresponding 95% confidence intervals (CI) are reported.
For the analyses of secondary endpoints, i.e. number of erythrocyte transfusions, number of platelet transfusions, number of hospitalization days, and number of hospitalization events, we employed generalized linear models of the negative binomial family with time at risk as exposure variable and robust standard errors. Incidence rate ratios (IRR) are reported with corresponding 95% confidence intervals. Statistical significance was established at P < 0.05. Stata Version 15.1 (Stata Corp, College Station, TX, USA) was used for statistical analyses.
3. Results
3.1. Baseline characteristics
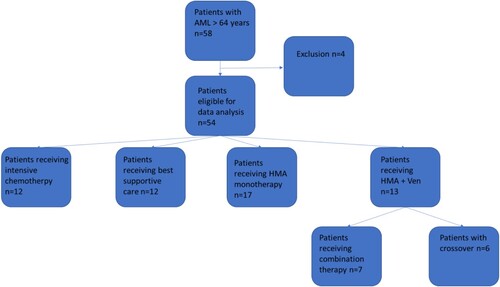
A total of 58 patients older than 64 years were diagnosed with AML between January 2017 and October 2020. 54 patients were eligible for data analysis and four patients could not be included due to the lack of consent.
In total 42.6% were female and the median age was 76 years. 22% (n = 12) of the patients received intensive chemotherapy, 24% (n = 13) received combination therapy of HMA and venetoclax, 32% (n = 17) received hypomethylating agent monotherapy, and 22% (n = 12) received BSC (). Patients receiving IC tended to be younger (median age 67 years) than patients receiving HMA or HMA + VEN (median 77 years in both groups), and patients receiving BSC tend to be the oldest group (median age 80 years). In total 22.2% were secondary or therapy-associated AML, a subgroup of AML Patients with poor prognosis [Citation21]. According to the European leukaemia net (ELN) 2017 risk stratification [Citation19] the four subgroups that received disease-directed therapy showed a heterogenous distribution with significant differences with the worst risks in the combination group of HMA + VEN, intermediary in the HMA and the BSC group and the best risks in the chemotherapy group (p = 0.031) ( and ). Details of the used next-generation sequencing are available in the supplementary material.
Table 1. Patient characteristics.
Dosage adjustments of HMA and/or venetoclax were necessary for four of the patients due to side effects, in particular cytopenias, acc. to VIALE trial recommendations ().
Table 2. Patient characteristics according to ELN 2017 Rik stratification, molecular and cytogenetic abnormalities.
3.2. Overall survival (OS)
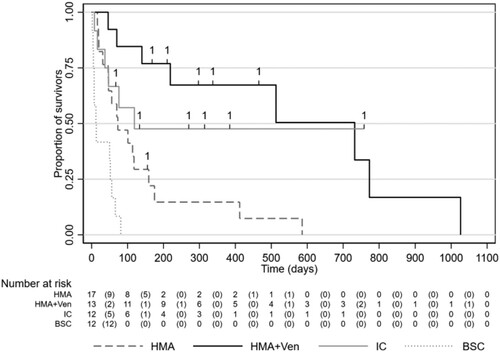
We followed 54 AML patients who were 9754 days at risk. Patients remained in the sample until they died or were censored. The median survival of the entire study population was 76 days (IQR 37–513 days). Patients treated with IC had a median survival of 119 days, HMA + VEN of 732 days, HMA monotherapy of 73 days and BSC of 12 days.
Significant differences in OS were observed with a significant prolonged survival of the HMA + VEN group compared to HMA monotherapy (Log-Rank Test Chi2(2): p = 0.004). There was no statistically significant difference in survival between IC and HMA (Log-Rank Test Chi2(1): p = 0.111) and between IC and HMA + VEN (Log-Rank Test Chi2(1): p = 0.545). Overall survival was significantly lower in BSC patients (12 days) as compared to patients with HMA, HMA + VEN or IC (Log-Rank Test Chi2(1); p = 0.001, p = 0.000, and p = 0.001, respectively) (). The causes of death did not differ between the groups (for details, see supplementary material).
Figure 2. Overall survival in groups IC = Intensive chemotherapy; HMA = Hypomethylating agents alone; HMA + VEN = Hypomethylating agent in combination with a BCL2-Inhibitor venetoclax: BSC = best supportive care.
We further explored the effect of the onset of venetoclax therapy in HMA + VEN patients (n = 13, 4,986 days at risk). Univariate Cox regression analysis with the number of venetoclax therapy days as a predictor found that the number of venetoclax therapy days was negatively associated with the risk of death (Hazard Ratio:0.990, 95% CI: 0.981–0.999, p = 0.022).
Noteworthy, in the nonparametric model, survival in the cross-over treatment group (therapy starts with VEN later than 60 days after the start of HMA) did not differ significantly (Log-Rank Test Chi2(1): p = 0.193) compared to the cohort with a timely start of HMA in combination with VEN. However, a univariate Cox model using the time delay from the start of HMA therapy until the day of treatment start with venetoclax as a predictor for survival, we found a trend that early initiation of venetoclax associated with longer overall survival whereas, this finding was not significant (Hazard Ratio:0.991, 95% CI: 0.982–1.001; p = 0.094).
3.3. Hospital stays
Hospitalization entries and total days in the hospital were calculated using time at risk as an exposure variable. Together, Patients in our cohort were hospitalized 1.6 times per month on average (SD = 2.6; MD = 0.8; RNG: 0–15) and spent 17 days per month on average in the hospital.
In detail, the hospitalization rate for IC was 0.7 times per month, HMA + VEN 0.5 times per month, HMA 1.2 times per month and BSC 2.3 times per month.
Patients treated with IC spent 19.5 days per month in the hospital, the HMA + VEN cohort 6.8 days per month, the HMA cohort 20.5 days per month and patients treated with BSC 19 days per month.
Of note, patients with HMA or IC spent three times more days in the hospital than patients treated with HMA + VEN (IRR = 3.0; 95% CI: 1.4–6.5; p = 0.005 and IRR = 2.9; 95% CI: 1.7–4.7; p = 0.000). In addition, patients with HMA therapy were hospitalized 2.3 times more often per month than patients with HMA + VEN (IRR = 2.3; 95% CI: 1.1–4.7; p = 0.026).
3.4. Transfusion needs
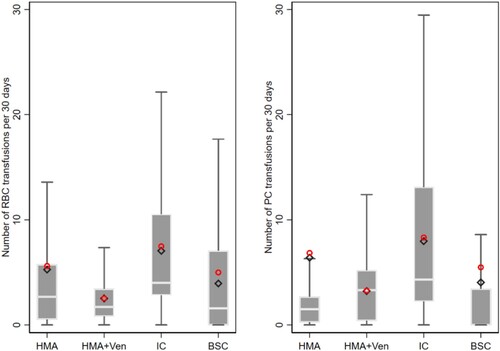
Transfusion needs were calculated using time at risk as an exposure variable. Patients undergoing IC received 7.0 RBC/month and 8.0 PC/month, HMA + VEN-treated patients 2.5 RBC/month and 3.2 PC/month, VEN 5.3 RBC/month and 6.2 PC/month and BSC 3.9 RBC/month and 4.0 PC/month.
The highest transfusion requirements had patients undergoing intensive chemotherapy with 2.8 times more red blood cell concentrates and 2.5 times more platelet concentrates as HMA + VEN-treated patients (95% CI: 1.3–6.1; p = 0.010 and 95% CI: 1.1–5.4; p = 0.023) or patients on HMA monotherapy (95% CI: 0.1–10.4; p = 0.045 and 95% CI: −2.8–15.6; p = 0.174), which did not differ significantly in transfusion requirements of RBC und PC in comparison to the HMA + VEN cohort ().
Figure 3. Boxplots show the number of respective transfusions per 30 days. The grey box shows the interquartile range (25th–75th percentile). The horizontal white line within the box shows the median. Whiskers show upper and lower adjacent values which are not yet considered outliers. The red circle shows the arithmetic mean and the black diamond shows the predicted mean from respective negative binomial model estimates. IC = Intensive chemotherapy; HMA = Hypomethylating agents alone; HMA + VEN = Hypomethylating agent in combination with a BCL2-Inhibitor venetoclax: BSC = best supportive care.
4. Discussion
After decades of a standstill of progress in AML therapy, especifically in the older population, the last years were characterized by the introduction of novel therapeutics, namely, hypomethylating agents and the BCL-2 inhibitor venetoclax, which significantly improved survival outcomes. In addition, with the advent of HMA+/-VEN, it is more and more questionable whether elder patients still should undergo intensive chemotherapy approaches [Citation22] or better profit from less-intensive approaches.
This retrospective single-institution, multi-site analysis addressed survival outcomes of 54 patients with AML older than 64 years in a real-world setting. Despite the progress that was made, we demonstrate that AML remains a fatal disease for the majority of older patients with high imminent mortality, which is in line with previous reports [Citation23].
First, a significant portion of patients (22%) did not receive any kind of anti-disease treatment due to poor conditions or personal decisions. It is not surprising that these patients died within weeks.
Only another 22% of patients were considered fit enough to undergo an intensive chemotherapy approach for a cure.
Patients treated with IC tended to be younger, which is consistent with previously reported results that older patients are less likely to receive chemotherapy [Citation24]. Furthermore, the group of intensive chemotherapy showed the most favourable ELN-risk stratification. This is consistent with NCCN guidelines, which recommend to integrating not only age and performance status but also ELN-risk stratification into treatment choice.
In our cohort only three patients had long-term survival, which included a consolidating allogeneic stem cell transplantation strategy. However, the chance of long-term survival goes hand in hand with longer hospitalization rates and the risk of a higher need for transfusions. Although different scores have been developed to assess the individual risks and benefits of intensive therapy for older patients, this decision is still a challenge [Citation25].
Notably, overall survival rates of the IC arm did not differ from HMA and HMA + VEN, whereas transfusion needs and hospitalization rates and days were significantly lower, arguing for a valuable therapeutic alternative of HMA-based therapies in the elder population.
A direct comparison of the combination strategy of venetoclax plus HMA versus HMA monotherapy showed a clear advantage of the combination strategy with regard to survival and hospitalization rates and transfusion needs, which confirms HMA + VEN as the standard of care in a real-world setting.
It has to be noted that the OS of the HMA monotherapy group was surprisingly short with a median OS of only 73 days, which is less than expected when compared to previous reports [Citation6]. There are several possible explanations for this: Phase III trials have a highly selected patient population, which is not reflected in a real-world scenario, as described in our study. In addition, our study population was not well balanced with regard to risk stratification and disease specifications: 41.2% of patients in the HMA cohort have high-risk features according to the ELN 2017 risk score. In addition, the proportion of secondary and therapy-related AML was highest in the HMA cohort (41%) compared to the HMA + VEN (23%) or the IC (17%) cohorts. Last, it is likely that some bias regarding patient fitness may have obscured the outcome, as physicians tend to treat fit patients more intensively (IC or VEN + HMA) compared to more unfit patients (HMA, BSC). Unfortunately, performance status data were not available for our patient population. This assumption, however, may as well explain why the combination of HMA and venetoclax in our real-world setting was remarkably better with an OS of 24 months compared to 14.7 months in the phase III VIALE trial.
Before the start of venetoclax, an insurance agreement on costs had to be obtained, which may have significantly delayed the therapy start of the HMA + VEN combination. Notably, a subgroup analysis of the cross-over cohort, which was defined as the patient population starting VEN later than 60 days after the start of HMA, with the patient population starting VEN within 60 days after the start of HMA did reveal a trend that early initiation of venetoclax associates with longer overall survival. Whereas this finding closely failed formal statistical significance and needs to be confirmed in larger patient populations, this information points to an ethical problem that insurance coverage may be a survival risk factor and this issue will need to be addressed by professional societies.
Importantly, our data show that the combination of HMA + VEN does not come at the price of more hospitalizations or a higher need for transfusions. In comparison to the IC or HMA cohorts, patients in the combination group spent a third of the time in the hospital per month life. The transfusion requirement per month life for erythrocyte concentrates and platelet concentrates was comparable with the HMA monotherapy but significantly lower than in the intensive chemotherapy group.
To summarize, our real-world analysis confirms that a combination of HMA + VEN can safely be administered in the elder population with a superior survival outcome compared to HMA. The role of intensive chemotherapy in elderly patients is effective in a subset of patients – but the algorithm that benefits from IC rather than HMA-based therapies will have to be clarified [Citation26]. In this context, real-world data will be important in particular for the population of older and comorbid patients who are often insufficiently represented in clinical trials. To the best of our knowledge, this is the first report to compare less intensive versus intensive therapy regimens in these patients. Our data provide additional information for clinical counselling of elderly AML patients on therapy decision-making in the landscape of emerging treatment options [Citation27].
Supplemental Material
Download MS Word (15.7 KB)Acknowledgements
Author contribution: All authors have made substantial contributions to the design and analysis of this work and drafting and appraising the manuscript. All authors have approved the final manuscript for submission and agree to be accountable for the work. Conception and design: Tabea Sutter, Thomas Lehmann. Data collection: Tabea Sutter. Analysis/interpretation: Tabea Sutter. Statistical analysis: Thomas Volken.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- National Cancer Institute Surveillance, E., and End Results Program. Cancer stat facts: acute myeloid leukemia (AML); Surveillance Research Program (SRP), Bethesda, 2019.
- Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485.
- Nagel G, Weber D, Fromm E, et al. Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO). Ann Hematol. 2017;96(12):1993–2003.
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152.
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–2677.
- Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–299.
- Sorror ML, Storer BE, Fathi AT, et al. Multisite 11-year experience of less-intensive vs intensive therapies in acute myeloid leukemia. Blood. 2021;138(5):387–400.
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629.
- Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106–1117.
- Bose P, Gandhi V, Konopleva M. Pathways and mechanisms of venetoclax resistance. Leuk Lymphoma. 2017;58(9):1–17.
- Itzykson R, Fournier E, Berthon C, et al. Genetic identification of patients with AML older than 60 years achieving long-term survival with intensive chemotherapy. Blood. 2021;138(7):507–519.
- Bell JA, Galaznik A, Huelin R, et al. Effectiveness and safety of therapeutic regimens for elderly patients with acute myeloid leukemia: a systematic literature review. Clin Lymphoma Myeloma Leuk. 2018;18(7):e303–e314.
- Michaelis LC, Klepin HD, Walter RB. Advancements in the management of medically less-fit and older adults with newly diagnosed acute myeloid leukemia. Expert Opin Pharmacother. 2018;19(8):865–882.
- Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97(12):1916–1924.
- Juliusson G, Antunovic P, Derolf Å, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish acute leukemia registry. Blood. 2009;113(18):4179–4187.
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(6):721–749.
- Bories P, Lamy S, Simand C, et al. Physician uncertainty aversion impacts medical decision making for older patients with acute myeloid leukemia: results of a national survey. Haematologica. 2018;103(12):2040–2048.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405.
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Passweg JR, Pabst T, Blum S, et al. Azacytidine for acute myeloid leukemia in elderly or frail patients: a phase II trial (SAKK 30/07). Leuk Lymphoma. 2014;55(1):87–91.
- Kayser S, Döhner K, Krauter J, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117(7):2137–2145.
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345–1377.
- Shallis RM, Wang R, Davidoff A, et al. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87.
- Sacks NC, Cyr PL, Louie AC, et al. Burden of acute myeloid leukemia among older, newly diagnosed patients: retrospective analysis of data from the 2010-2012 medicare limited data Set. Clin Ther. 2018;40(5):692–703.e2.
- Borlenghi E, Pagani C, Zappasodi P, et al. Validation of the “fitness criteria” for the treatment of older patients with acute myeloid leukemia: a multicenter study on a series of 699 patients by the network rete ematologica lombarda (REL). J Geriatr Oncol. 2021;12(4):550–556.
- Pollyea DA, Bixby D, Perl A, et al. NCCN guidelines insights: acute myeloid leukemia, version 2.2021. J Natl Compr Canc Netw. 2021;19(1):16–27.
- Short NJ, Konopleva M, Kadia TM, et al. Advances in the treatment of acute myeloid leukemia: new drugs and New challenges. Cancer Discov. 2020;10(4):506–525.