ABSTRACT
Autoimmune hemolytic anemia (AIHA) can be life-threatening, if hemoglobin (Hb) levels continue to decline after established treatments with glucocorticoids, rituximab, intravenous immunoglobulins, and plasmapheresis. Impaired regulatory T cells (Treg) are proposed to alleviate AIHA development through decreased binding of CTLA-4 to antigen-presenting cells. Abatacept is a fusion protein with a CTLA-4 domain and is approved for use in rheumatoid arthritis. It mimics the immunosuppressive CTLA-4 effect of Treg. Thus, application of abatacept in refractory AIHA might be reasonable. A 54-year-old woman with known AIHA was admitted to our clinic due to therapy-refractory hemoglobin decrease to 4.0 g/dl. Previously, multiple courses of glucocorticoids, rituximab, azathioprine, mycophenolate mofetil, cyclophosphamide, bortezomib, and a splenectomy failed to stop or stabilize hemoglobin levels and hemolysis. A new immunosuppressive therapy with cyclosporine was initiated and erythropoiesis was stimulated with darbepoetin alfa. Again, therapy failed even though we tried to support immunosuppressive therapy by reducing the amount of pathogenic antibody through plasmapheresis. We stopped the treatment with cyclosporine and applied abatacept instead. After seven days hemoglobin stabilized at 4.3 g/dl and no further red blood cells transfusions were necessary. About one month later hemolysis aggravated again and azathioprine was added to the ongoing abatacept treatment. Finally, the combination of abatacept and azathioprine led to a long-lasting increase of the Hb level above 11 g/dl six months later. Abatacept can be applied to overcome therapy refractory autoimmune hemolytic anemia but should be combined with an additional immunosuppressive medication such as azathioprine.
Introduction
Autoimmune hemolytic anemia (AIHA) is caused by autoantibodies against red blood cells (RBC). In most cases autoantibodies belong to the Immunoglobulin G (IgG) class, react with antigens of the Rh complex or glycophorins on the surface of RBC, and are active at body temperature (warm AIHA). AIHA can arise spontaneously or is associated with other conditions (e.g. viral infections, autoimmune disorders, lymphomas). Patients present with symptoms related to anemia, jaundice, and dark urine. Hemolytic autoantibodies can be proven with a direct antiglobulin test (DAT). Laboratory findings in hemolysis are low haptoglobin and high (indirect) bilirubin, lactate dehydrogenase (LDH), and reticulocyte count. Treatment of spontaneous AIHA consists of glucocorticoids with or without the CD20 antibody rituximab and may be accompanied by transfusion of RBC, stimulation of erythropoiesis with erythropoietin, intravenous immunoglobulins (IVIG), and removal of pathological autoantibodies through plasmapheresis. In two randomized trials AIHA response rates after one year were 75% and 75% in the combination arm with rituximab and a glucocorticoid vs. 36% and 31% in the monotherapy arm with a glucocorticoid [Citation1,Citation2]. Treatment of persistent AIHA is a matter of debate, and many different immunosuppressive or cytotoxic agents (e.g. cyclophosphamide, mycophenolate mofetil, azathioprine, etc.) have been reported to be effective [Citation3–5]. However, randomized trials with a direct comparison of these agents are lacking. This is especially true for treatment beyond the second line. A new approach to treating AIHA could be the CTLA-4 agonist abatacept. CTLA-4 is expressed on regulatory T cells and activated T cells. It binds and diminishes CD86 and CD80 on the surface of antigen presenting cells (APC). In turn, this leads to reduced binding and stimulation of T cells via CD28 to CD80/CD86 on APC [Citation6]. Subsequently, the immune response is altered.
Case report
A-54-year-old woman with treatment-refractory AIHA was assigned to our clinic with a Hb of 4.0 g/dl, LDH 468 U/l, total bilirubin 4.06 mg/dl, and undetectable haptoglobin. As a wide range of different therapies including prednisolone/dexamethasone, rituximab, azathioprine, mycophenolate mofetil, cyclophosphamide, bortezomib, and a splenectomy had not stopped decreasing Hb levels, the treating hematologist referred the patient to our hospital. We started a new immunosuppressive therapy with cyclosporine (Sandimmun® 3 × 150 mg, Novartis, Basel, Switzerland) and stimulated erythropoiesis with darbepoetin alfa (Aranesp® 500 µg two times a week, Amgen, Thousand Oaks, CA). Underlying diseases or trigger factors were excluded by CT scan, bone marrow examination, screening for vasculitis associated antibodies, and PCR for Cytomegalovirus, Epstein–Barr virus, Parvo B19 virus, Herpes simplex virus 1 and 2, and Varicella zoster. Paroxysmal nocturnal hemoglobinuria was excluded by flow cytometry and drug-dependent antibody testing was negative. AIHA was confirmed by repeated DAT with positivity for IgG and C3d.
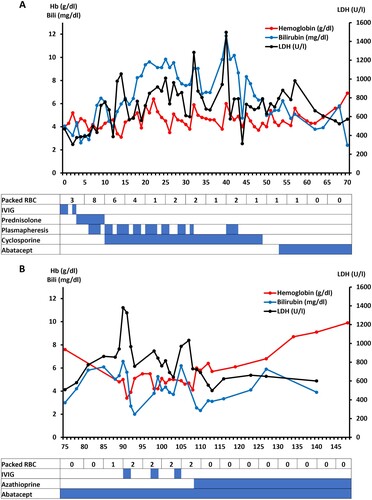
Cyclosporine along with the supportive therapies, such as darbepoetin alfa, plasmapheresis, intravenous immunoglobulins (IVIG) turned out to be insufficient to stabilize hemoglobin levels sustainable. Blood transfusions were often mandatory. Treatment attempts and their impact on hemolysis (that is, LDH and bilirubin) and Hb levels during hospitalization are specified in .
Figure 1. The treatment components of AIHA during the first hospital stay (days 0–71) (A) of the patient are listed in the bottom table and the corresponding effects on the hemoglobin (Hb) values (red line) and hemolysis activity (i.e. LDH (black) and total bilirubin (blue)) are depicted above. In (B) the course of AIHA is demonstrated after the first hospital release of the patient until day 148. The last RBC transfusion was on day 108. Abbreviations: RBC-Red blood cells; IVIG-Intravenous immunoglobulin; LDH-Lactate dehydrogenase.
In this desperate situation, we stopped cyclosporine therapy and applied a new immunosuppressive therapy with abatacept (Orencia® 500 mg every two weeks Bristol-Meyers Squibb, New York City, NY) on day 54. Abatacept mimics CTLA-4 and has been used successfully in case series to treat cyclosporine-resistant AIHA in children after allogeneic stem cell transplantation [Citation7].
After the first dose of abatacept was administered, hemoglobin still decreased to 4.3 g/dl until day 61 (7 days after start) but then increased without transfusion to 6.9 g/dl on day 70. Subsequently, the patient was released from the hospital after 71 days.
However, this was only a temporary success, as on day 89 the patient was readmitted again to our department with decreasing Hb levels (4.8 g/dl) and severe hemolysis (LDH 940 U/l, total bilirubin 5.33 mg/dl). Because the patient had a high temperature, empiric antibiotic therapy with piperacillin-tazobactam was initiated. Subsequently, the infection parameters improved, but AIHA did not, and red blood cell transfusions were again necessary again (shown in ). We combined abatacept with the immunosuppressive purine analogue azathioprine (Azathioprin Stada®, Bad Vilbel, Germany) 100 mg daily. After four days, the hemoglobin levels increased without additional red blood cell transfusions and the patient was released from the hospital. This time, the treatment effects remained constant and resulted in increased Hb levels (9.9 g/dl on day 148) about 40 days after starting the combined therapy of abatacept and azathioprine. After six months of combination therapy Hb levels still remain above 11 g/dl.
Discussion
Here we present a case of a life-threatening refractory AIHA which finally responded to an abatacept based therapy. The patient stayed in the hospital for 71 days, had Hb levels below 5 g/dl for 46 days and received 32 units of packed RBC at the first stay. Of note, abatacept alone was insufficient to increase Hb over a longer period of time. Finally, the combination of azathioprine and abatacept achieved long-lasting effects. This corresponds with the observations in refractory rheumatoid arthritis, where abatacept is combined with other disease-modifying antirheumatic drugs [Citation8,Citation9]. Previously, Hess et al. [Citation7] reported of three pediatric patients with AIHA after allogeneic transplantation who responded to abatacept treatment. They postulated indirect activation and induction of Treg through dendritic cells [Citation7] and referred to Fallarino et al. [Citation10] CTLA-4 is essential for the suppressive function of Treg [Citation11] whereas the importance of Treg for AIHA is still debated [Citation12–15].
It is known that CTLA-4 polymorphisms are associated with the occurrence of AIHA [Citation16] and approximately 28% of patients with CTLA-4 mutations present with AIHA [Citation17]. Dhunputh et al. reported about the use of abatacept in children with autoimmune cytopenia due to defects in the CTLA-4 pathway [Citation18]. On the other hand, AIHA is a possible side effect of CTLA-4 inhibitors used for cancer therapy [Citation19]. This indicates a role of CTLA-4 in the pathogenesis of AIHA and the need for clinical trials with abatacept in AIHA. However, we acknowledge that there are alternatives for abatacept in special situations (e.g. alemtuzumab, ibrutinib, venetoclax, ofatumumab, fostamatinib) with specific advantages and disadvantages [Citation20–24].
To our knowledge, this is the first report of AIHA without previous allogeneic stem cell transplantation and without genetic defect in the CTLA-4 pathway that has been successfully treated with abatacept.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Birgens H, Frederiksen H, Hasselbalch HC, et al. A phase III randomized trial comparing glucocorticoid monotherapy versus glucocorticoid and rituximab in patients with autoimmune haemolytic anaemia. Br J Haematol. 2013;163:393–399.
- Michel M, Terriou L, Roudot-Thoraval F, et al. A randomized and double-blind controlled trial evaluating the safety and efficacy of rituximab for warm auto-immune hemolytic anemia in adults (the RAIHA study). Am J Hematol. 2017;92:23–27.
- Kotb R, Pinganaud C, Trichet C, et al. Efficacy of mycophenolate mofetil in adult refractory auto-immune cytopenias: a single center preliminary study. Eur J Haematol. 2005;75:60–64.
- Moyo VM, Smith D, Brodsky I, et al. High-dose cyclophosphamide for refractory autoimmune hemolytic anemia. Blood. 2002;100:704–706.
- Hantaweepant C, Pairattanakorn P, Karaketklang K, et al. Efficacy and safety of second-line treatment in Thai patients with primary warm-type autoimmune hemolytic anemia. Hematology. 2019;24:720–726.
- Walker LSK, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852–863.
- Hess J, Su L, Nizzi F, et al. Successful treatment of severe refractory autoimmune hemolytic anemia after hematopoietic stem cell transplant with abatacept. Transfusion. 2018;58:2122–2127.
- Genovese MC, Becker J-C, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114–1123.
- Kremer JM, Westhovens R, Leon M, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915.
- Fallarino F, Grohmann U, Hwang KW, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212.
- Wing K, Onishi Y, Prieto-Martin P. CTLA-4 control over Foxp3+ regulatory T cell function. Science [Internet]. 2008 [cited 2022 Feb 6]; Available from: https://www.science.org/doi/abs/10.1126science.1160062.
- Mqadmi A, Zheng X, Yazdanbakhsh K. CD4 + CD25 + regulatory t cells control induction of autoimmune hemolytic anemia. Blood. 2005;105:3746–3748.
- Richards AL, Kapp LM, Wang X, et al. Regulatory T cells are dispensable for tolerance to RBC antigens. Front Immunol. 2016;7:348.
- Ward FJ, Hall AM, Cairns LS, et al. Clonal regulatory T cells specific for a red blood cell autoantigen in human autoimmune hemolytic anemia. Blood. 2008;111:680–687.
- Ahmad E, Elgohary T, Ibrahim H. Naturally occurring regulatory T cells and interleukins 10 and 12 in the pathogenesis of idiopathic warm autoimmune hemolytic anemia. J Investig Allergol Clin Immunol. 2011;21:8.
- Pavkovic M, Georgievski B, Cevreska L, et al. CTLA-4 exon 1 polymorphism in patients with autoimmune blood disorders. Am J Hematol. 2003;72:147–149.
- Schubert D, Bode C, Kenefeck R, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20:1410–1416.
- Dhunputh C, Ducassou S, Fernandes H, et al. Abatacept is useful in autoimmune cytopenia with immunopathologic manifestations caused by CTLA-4 defects. Blood. 2022;139:300–304.
- Tanios GE, Doley PB, Munker R. Autoimmune hemolytic anemia associated with the use of immune checkpoint inhibitors for cancer: 68 cases from the Food and Drug Administration database and review. Eur J Haematol. 2019;102:157–162.
- Galindo-Navarro P, Delgado-García A, Rodríguez-Gil MA. Venetoclax for treating refractory autoimmune hemolytic anemia in chronic lymphocytic leukemia: report of two cases in Spain. Haematologica [Internet]. 2020 [cited 2023 Apr 10]; Available from: https://haematologica.org/article/view/haematol.2022.281850.
- Karlsson C, Hansson L, Celsing F, et al. Treatment of severe refractory autoimmune hemolytic anemia in B-cell chronic lymphocytic leukemia with alemtuzumab (humanized CD52 monoclonal antibody). Leukemia. 2007;21:511–514.
- Nader K, Patel M, Ferber A. Ofatumumab in rituximab-refractory autoimmune hemolytic anemia associated with chronic lymphocytic leukemia: a case report and review of literature. Clin Lymphoma Myeloma Leuk. 2013;13:511–513.
- Montillo M, O’Brien S, Tedeschi A, et al. Ibrutinib in previously treated chronic lymphocytic leukemia patients with autoimmune cytopenias in the RESONATE study. Blood Cancer J. 2017;7:e524.
- Kuter DJ, Rogers KA, Boxer MA, et al. Fostamatinib for the treatment of warm antibody autoimmune hemolytic anemia: phase 2, multicenter, open-label study. Am J Hematol. 2022;97:691–699.
