ABSTRACT
Background:
China lacks a standard for reference intervals (RIs) of complete blood cell (CBC) counts in newborns. This study aimed to determine local haematological RIs for newborns.
Methods:
This prospective study was conducted from January 2020 to December 2020 in Nanjing Maternity and Child Health Care Hospital. We collected capillary blood specimens from 497 healthy newborns aged 5–28 days. We calculated the RIs as nonparametric 2.5th to 97.5th percentiles and 90% confidence intervals following the EP28-A3c guideline. We validated the RIs in another 20 specimens from healthy newborns.
Results:
The RIs for the 18 CBC parameters were: white blood cell count 7.17–15.69 × 109/L; monocytes# 0.52–1.66 × 109/L; Mono% 5.7–14.1; eosinophils# 0.16–1.08 × 109/L; Eos% 1.5–8.9; Lymphocytes# 4.04–8.08 × 109/L; Lymph% 40.1–67.8; Neutrophils# 1.59–6.41 × 109/L; Neut% 18.1–46.7; Basophils# 0.00–0.04 × 109/L; Baso% 0.0–0.4; red blood cell count 3.38–5.89 × 1012/L; haemoglobin (Hb) 116–198 g/L; Hematocrit % 35.2–61.2; mean corpuscular Hb (MCH) 30.9–36.3 pg/L; MCH concentration 310–341 g/L; mean corpuscular volume 94.5–112.2 fL; and platelets 210–610 × 109/L. The RIs had a conformity rate of 90%–100% in the validation specimens.
Conclusion:
We established region-specific RIs for CBC parameters in healthy newborns to help diagnose haematological disease in neonates.
1 Introduction
The complete blood count (CBC) is a vital test for assessing the haematological status of newborns, especially in relation to anaemia. Anaemia is a prevalent condition in infants that can impair their growth and development. It is caused mainly by iron deficiency, which can be detected by measuring the reticulocyte haemoglobin (Hb) equivalent. Ringoringo et al. demonstrated this parameter to be a reliable indicator of iron-deficiency anaemia in infants aged 1–4 months and 9–11 months [Citation1, Citation2]. Therefore, establishing the CBC reference interval (RI) for newborns can facilitate the diagnosis and management of anaemia and other haematological disorders.
However, the CBC RI for infants aged 0–7 days has been reported in only a few studies, and there is a lack of data concerning older infants [Citation3]. Moreover, CBC RIs may vary according to geographical location, ethnicity and method of measurement. Thus, there is a need for more comprehensive and updated studies on CBC RIs for newborns in different regions and populations.
Establishing RIs among healthy people is extremely helpful for detecting different haematological diseases. According to the Clinical Laboratory Standards Institute (CLSI) guidelines, RIs are most commonly defined as the middle 95% of laboratory findings predicted in a healthy population, in which 2.5% of the results in the lower and upper ranges are outside of the RIs [Citation4, Citation5]. The accurate interpretation of findings within the correct RIs is necessary for patients to minimize the risk of illness. In addition, the correct RIs may help boost recovery rates and constantly improve the monitoring and treatment of illnesses [Citation6]. Therefore, RIs are one of the most significant responsibilities in a laboratory since up to 80% of medical decisions are made based on test findings [Citation7]. The standard approach for validating RIs suggested by the CLSI’s EP28-A3c: Sample Preparation for Elemental Analysis for clinical laboratories requires a minimum of 20 samples from a healthy population [Citation8]. In addition, the International Organization for Standardization’s ISO15189 standard for clinical laboratories mandates that every laboratory should regularly assess its RIs [Citation9].
Because of the absence of RIs for infants up to 28 days of age from the health industry standard WS/T 779–2021 Reference Intervals for Blood Cell Analysis in Children, there are no defined haematological RIs for neonates in China. Although the Pediatric Reference Intervals in China project attempts to standardize paediatric RIs of CBCs across China, analyse nationally representative data and define age-specific and gender-specific RIs, it does not include children in the neonatal period up to 28 days of age [Citation10]. In most Chinese hospitals, instead of establishing RIs directly from a healthy population, most clinical laboratories obtain RIs from various sources, such as publications, textbooks and multicentre studies [Citation11, Citation12]. In our laboratory, the RIs for babies up to 28 days of age are derived from textbooks. However, the haematological parameters of neonates are different from those of infants or adolescents since they alter during growth and development, underscoring the necessity of age-specific RIs [Citation13]. Most notably, the variance in RIs might be attributed to geographical location; consequently, locally established haematological RIs among infants are crucial for physicians to appropriately identify specific blood-related abnormalities and provide prompt treatment to newborns when required [Citation7].
Skin puncture is widely used in children, particularly newborns, due to its advantages of minimal invasiveness. In clinical laboratories, skin punctures can provide small but adequate amounts of capillary blood for CBC testing [Citation14, Citation15]. Therefore, the current study aimed to establish haematological RIs using the capillary blood of healthy newborns and validate them in another cohort of healthy neonates, thereby providing a basis for clinicians to screen and identify haematological diseases among newborns.
2 Materials and methods
2.1 Ethics statement
This prospective study was obtained data from the laboratory department of Nanjing Maternity and Child Health Care Hospital. This study was approved by the hospital’s ethics committee, and the parents of the newborns who participated in the study signed informed consent forms.
2.2 Study context and population
This study was conducted between January 2020 and December 2020 in Nanjing Maternity and Child Health Care Hospital, China. Data were obtained from the laboratory department of the hospital.
Our group opted to obtain RIs for clinical usage from the available literature rather than create them directly from healthy infants. The recommendations of the National Committee for Clinical Laboratory Standards and CLSI standards specified the number of samples, with a minimum of 20 observations needed to calculate haematological RIs [Citation16]. Therefore, we estimated the out-of-range (OOR) value and used it to verify the RIs using 20 samples of capillary blood from healthy newborns. The percentage of measurements that exceed the RIs is known as the OOR, and it is only acceptable when the OOR is less than 10% [Citation8]. As a result, the RI calculations used in textbooks were incorrectfor our specific study.
Newborns were included in this study if they met the following criteria: birth at full term (37–42 weeks gestation) and full examination at birth, with a healthy outcome. The exclusion criteria were as follows: acute/chronic infectious diseases, allergic diseases, haematological diseases, malnutrition, congenital diseases, height and/or weight out of corresponding ranges and any drug/nutrient intake.
To validate the applicability of the established RIs, we collected another 20 capillary blood samples from healthy newborns who met the same inclusion and exclusion criteria as those stated above. There were four cases each in five age groups (5–10, 11–15, 16–20, 21–25 and 26–28 days). The sample size of each age group was equal and representative. The blood samples were analysed using the same methods as described above. The conformity rate was calculated as the percentage of samples that fell within the established RIs for each CBC parameter.
The study population consisted of healthy full-term newborns, aged 5–28 days old, who were admitted to the neonatal department of the hospital. The inclusion criteria were as follows: gestational age of between 37 and 42 weeks, birth weight between 2,500 and 4,000 g, head circumference between 33 and 37 cm, APGAR:Appearance, Pulse, Grimace, Activity, and Respiration score ≥8 at 1 and 5 min, Downe score ≤1 and no congenital anomalies or infectious diseases. The exclusion criteria were as follows: preterm or post-term birth, low or high birth weight, abnormal head circumference, low APGAR score, high Downe score and presence of congenital anomalies or infectious diseases.
The sample size was calculated using Cochran’s formula based on a birth rate of 10‰ for regions in southern China, a population of Nanjing city of about eight million people, a confidence interval of 95%, an error range of 5%, a design effect of 1.5% and a non-response rate of 10%. The calculated sample size was 492 cases. Therefore, the 497 cases collected in this study met the sample size requirements.
This study collected a total of 497 capillary blood samples from healthy newborns; of these, there were 102 cases (20.5%) in the 5–10-day age group, 101 cases (20.3%) in the 11–15-day age group, 99 cases (19.9%) in the 16–20-day age group, 98 cases (19.7%) in the 21–25-day age group and 97 cases (19.5%) in the 26–28-day age group. The sample size of each age group was similar and representative.
The haematological parameters of neonates in the first few days after birth are influenced by various factors, such as gestational age, birth weight, delivery mode, the timing of umbilical cord clamping, hypoxia and infection; these result in the measured values, making it challenging to determine a stable RI. Therefore, we followed the internationally accepted approach of choosing healthy neonates aged 5–28 days as the study population to minimize the interference of these factors and enhance the reliability and applicability of the RI.
2.3 Blood collection and laboratory analysis
The newborns were born at Nanjing Maternity and Child Health Care Hospital. All capillary blood was collected according to World Health Organization guidelines (i.e. the Procedures and Devices for the Collection of Diagnostic Capillary Blood Specimens; Approved Standard–Sixth Edition, GP42-A6 established by the CLSI) [Citation17], and fasting was not necessary. All capillary blood samples were collected in 1.5-mL containers filled with EDTA-K2:Ethylenediaminetetraacetic acid dipotassium salt (Gongdong Medical, Zhejiang, China). The samples were gently mixed by inverting them eight times [Citation18].
All samples were processed using Mindray BC5180 and BC7500 analysers (Mindray Medical Electronics Company, Shenzhen, China). Due to the updating of laboratory instruments, this study used a Mindray BC5180 analyser in the early stage and a Mindray BC7500 analyser in the later stage. To ensure the consistency of the results, we calibrated and compared the two instruments and found that there was no significant difference between them and that they could be used interchangeably. The diluent, dye solution, haemolysis agent and other reagents used in the entire process were from the same manufacturer.
Before the analysis, quality control records and performance verification data were collected for quality assessment. The analytical procedures were performed according to the manufacturer's instructions and laboratory protocols, including function checks, regular maintenance, calibration and internal quality control. The following 18 CBC parameters were evaluated: white blood cell (WBC), Monocytes#, Mono%, Eosinophils#, Eos%, Lymphocytes#, Lymph%, Neutrophils#, Neut%, Basophils#, Baso%, red blood cell (RBC) count, Hb, Hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular Hb (MCH), MCH concentration (MCHC) and platelets (PLT). Haematological RI calculations of the nonparametric 2.5th–97.5th percentiles were performed according to the CLSI EP28-A3c guidelines. Further validation was performed on the data of 20 healthy newborns.
Capillary blood was collected from the heel of each newborn using a special heel blood collector. The blood volume was 1.5 mL. The blood collection time was any day within 5–28 days after birth, and no special preparation was required before blood collection. The newborns were in a quiet or mildly crying state during blood collection. This blood volume was approved by the CLINICAL PRACTICE AND ETHICS COMMITTEE as its withdrawal does not cause adverse effects in newborns.
2.4 Statistical analysis
Quantile–quantile plots and histograms were used to assess the normality of each partition, while the Box–Cox transformation method was used if necessary. Tukey’s test was applied to remove outliers in normally distributed and appropriately skewed partitions [Citation19, Citation20]. A Mann-Whitney U test was employed to examine whether gender differences existed among these haematological parameters. The lower and upper limits were defined as the 2.5th and 97.5th percentiles of each RI, and 90% confidence intervals (CIs) were calculated using the nonparametric method for the upper and lower limits [Citation21]. Pearson’s correlation coefficients were used to determine the correlation between CBC parameters and age. All the analyses used SPSS software (version 25.0, IBM Corp. Silicon Valley, CA, USA), and a two-tailed p value of <0.05 was considered statistically significant. To validate the established RIs, the OOR value of each parameter was calculated.
3 Results
3.1 Study population
We collected 497 (232 males and 265 females) capillary blood specimens from healthy-term newborns aged 5–28 days in Nanjing, China. We excluded 30 specimens that were outliers based on the boxplot method and used the Mann–Whitney U test to compare haematological parameters between males and females. We found significant sex differences in Hct (p < 0.024), PLT (p < 0.042), MCV (p < 0.001), MCH (p < 0.004) and MCHC (p < 0.01). The other parameters showed no significant differences.
3.2 Capillary blood Reference Intervals of 18 CBC parameters for newborns
We calculated the haematological RIs as the nonparametric 2.5th to 97.5th percentiles and 90% CIs for the following 18 CBC parameters: WBC, Mono#, Mono%, Eos#, Eos%, Lymph#, Lymph%, Neut#, Neut%, Baso#, Baso%, RBC, Hb, Hct, MCV, MCH, MCHC and PCT. The RIs are presented in .
Table 1. Reference intervals for 18 hematologic parameters in overall population.
Table 2. Reference intervals for 18 hematologic parameters in males.
Table 3. Reference intervals for 18 hematologic parameters in females.
3.3 Verification of the established RIs
To validate the applicability of the established RIs, we collected another 20 capillary blood samples from healthy newborns who met the same inclusion and exclusion criteria as above. There were four cases in each of the following age groups: 5–10, 11–15, 16–20, 21–25 and 26–28 days. The conformity rate was calculated as the percentage of samples that fell within the established RIs for each CBC parameter. The conformity rate ranged from 90% to 100%, which met the requirements of the CLSI EP28-A3c guideline. The verification details are shown in .
Table 4. Verification of the established RIs (N = 20).
3.4 Correlation between CBC parameters and age
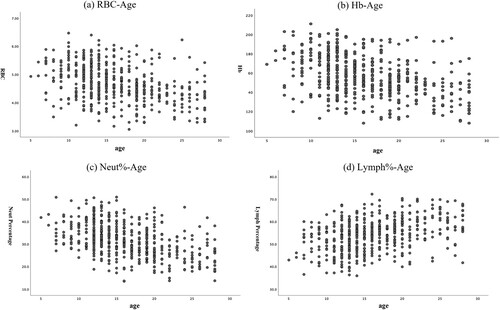
We plotted the scatterplots of Neut%, RBC, Hb and Lymph% against age to illustrate the variation trend of each parameter with age (). The results showed that Neut%, RBC, Hb gradually decreased with increasing age and Lymph% showed a increase with increasing age.
Figure 1. Correlation between CBC parameters and age (in days) in newborns. Scatterplots displayed the correlation of age (in days) versus (A) RBC, (B) Hb, (C) Neu%, and (D) lymph% WBC = white blood cell; RBC = red blood cell; Hb: hemoglobin; Hct = red blood cell specific volume; MCV = mean corpuscular volume; PLT = platelet.
4 Discussion
We compared our results with those from other studies and found some similarities and differences. Our results for the erythrocyte parameters, including RBC, Hb, Hct, MCV, MCH and MCHC, were comparable to those reported by Christensen et al. [Citation22], who defined the erythrocyte indices of neonates using data from over 12,000 patients in a multi-hospital healthcare system. However, our results showed higher values for these parameters than those reported by Wiedmeier et al. [Citation23], who defined the PLT reference ranges for neonates using data from over 47,000 patients in a multi-hospital healthcare system. These differences may be due to variations in the gestational age, birth weight and postnatal age of the neonates as well as the laboratory methods used in the different studies.
Our results for the leukocyte parameters, including WBC, Neut%, Lymph%, Mono%, Eos%, Baso%, Neut#, Lymph#, Mono#, Eos# and Baso#, were similar to those reported by Xanthou [Citation24], who studied the leucocyte blood picture in ill newborn babies with infection.. However, our results revealed lower values for these parameters than those reported by Christensen et al. [Citation25], who defined the reference ranges for blood concentrations of eosinophils and monocytes during the neonatal period using data from over 63,000 records in a multi-hospital healthcare system. These differences may be due to differences in the clinical conditions, infections, medication and inflammatory responses of the neonates as well as the sample collection methods and timings used.
Our results for the PLT parameter were consistent with those reported by Wiedmeier et al. [Citation23], who defined the PLT reference ranges for neonates using data from over 47,000 patients in a multi-hospital healthcare system. However, our results showed lower values for this parameter than those reported by Christensen et al. [Citation22], who defined the erythrocyte indices of neonates using data from over 12,000 patients in a multi-hospital healthcare system. These differences may be due to differences in the thrombopoietin levels, PLT production, PLT consumption and PLT destruction of the neonates as well as the anticoagulant type and sample storage times used.
These comparisons indicate that there are significant variations in the haematological parameters of neonates among different studies and regions. Therefore, it is important to establish local RIs for these parameters and consider the factors that may affect them when interpreting the results.
We acknowledge that normal values for neonatal haematological parameters depend not only on factors such as region, race and gender but also on factors such as gestational age, birth weight, postnatal time, blood sampling method, blood sampling site, clinical condition and level of care. These factors may account for the differences and heterogeneity in the results of different studies. Therefore, when applying the RIs established in our study, such factors affecting the level of haematological parameters need to be considered and adjusted according to specific situations [Citation26, Citation27].
By utilizing capillary blood, we were able to fill in the information gaps in the published health industry standard WS/T 779–2021 by determining the RIs among babies up to 28 days of age. For the correct interpretation of test findings and the monitoring of dynamic changes during this time, the haematological values of neonates are essential. In the neonatal period, CBC values strongly correlate with gestational age, the location of the blood sample, birth weight, delivery method and many other variables [Citation28]. Our study's revealed that there were statistically significant differences between males and females in the RI haematological parameters Hct, PLT, MCV, MCH and MCHC. These findings are in contrast to those found in Sokoto, Northern Nigeria; Lagos, Nigeria; and Ethiopia, which revealed no statistically significant changes in any haematological parameters between newborn males and females [Citation29, Citation30]. It is debatable if different delivery methods have an impact on haematological variables [Citation31, Citation32]; however, most research on delivery techniques has been conducted on samples of neonatal umbilical cord blood [Citation33]. Although all the neonates chosen were delivered at full term and all capillary blood samples were obtained, numerous other criteria, such as the method of delivery, should also be considered in the future.
Several studies have been conducted in China to determine the paediatric RIs of CBC parameters. To date, children in the neonatal period have not been studied [Citation13, Citation34, Citation35] because the number of neonates (up to 28 days of age) is small and sample collection is challenging; hence, there are no established RIs of CBCs that are typical of newborns. In the current research, we constructed haematologic RIs for neonates aged 5–28 days in Nanjing, which, to the best of our knowledge, is the first of its kind in China. Our results of the 95% RI values of RBC (3.38–5.39), Hct (35.2–61.2), MCH (30.9–36.3), MCHC (310–341) and MCV (94.5–112.2) were similar to those published in Ethiopia (3.69–5.47), (39.4–58.1), (30.5–38.02), (31.7–40.0) and (91.6–113.2); and Iran (3.61–5.29), (39.6–56.9), (31.7–40.0). Our study's lower limit of the WBC (7.17) value was comparable to those reported in studies published in Ethiopia (7.64) and Iraq (7.64). Conversely, the WBC's upper-limit RI (15.69) was lower than that reported in Ethiopia (22.16) but greater than that recorded in Iraq (12.92) [Citation36, Citation37]. This might be related to the different specimen types, which, in our investigation were all capillary blood, while umbilical cord blood was used in the other studies. Geographical location and race may also play a role in the disparity.
Existing studies have verified the importance of age-specific RIs, and most investigations have shown age-dynamic alterations in haematological parameters [Citation34, Citation38]. The present study also found a decline in Neut%, RBC and Hb with age and an increase in Lymph%. Because the number of newborn samples was very limited in the present investigation, age-related dynamic alterations in haematological parameters could not be identified. More research is required to augment the RIs for infants up to four days old and separate the parameters into different ages since they fluctuate with the dynamic growth of babies.
This study has several strengths. First, it is the first research to establish and validate haematological RIs for the umbilical cord blood of healthy-term newborns in the Nanjing region, which can provide a basis for screening and diagnosing haematological disorders in newborns. Second, it used a large sample size and a representative population of newborns from different age groups and genders. Third, it used a non-invasive blood collection method and standardized laboratory instruments to ensure the quality of blood samples and analyses.
This study also has some limitations. First, it only included newborns from one city in China, which may limit the generalisability of the results to other regions and ethnicities. Second, it did not consider other factors that may affect the haematological parameters of newborns, such as maternal health status, gestational complications, delivery mode and feeding patterns. Third, it did not compare the haematological parameters of newborns with those of their mothers or siblings, which may provide more insights into the genetic and environmental influences on haematological parameters.
5 Conclusion
We established and validated haematological RIs for the umbilical cord blood of healthy-term newborns in the Nanjing region and compared them with other studies from different regions and ethnicities. We found some variations in haematological parameters among newborns from different populations, which might be influenced by genetic, environmental, nutritional and methodological factors. These RIs will provide a basis for screening and diagnosing haematological disorders in newborns. Further studies should be conducted to explore the factors affecting the haematological parameters of newborns and evaluate the applicability of these RIs in other populations.
Ethics approval and consent to participate
The present study was conducted in accordance with the Declaration of Helsinki. This study has been approved by the Ethics Committee of Nanjing Maternity and Child Health Care Hospital. Written informed consent was obtained from LARs/ parents of the new born children.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Competing interest
All of the authors had no any personal, financial, commercial, or academic conflicts of interest separately.
Author contributions
Conception and design: Li CL
Administrative support: Tan C and Gao L
Provision of study materials or patients: Chang Y
Collection and assembly of data: Fan WM
Data analysis and interpretation: Chang Y
Manuscript writing: All authors
Final approval of manuscript: All authors
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ringoringo HP, Sari LP, Yunanto A, et al. Reticulocyte hemoglobin equivalent as a diagnostic parameter of iron deficiency anemia in infants age 1-4 months. Arch Med Sci. 2022. doi:10.5114/aoms/152280.
- Ringoringo HP. Prevalence of Iron deficiency anemia and reference range of complete blood count, reticulocyte parameters in infants aged 9-11 months. Int J Gen Med. 2022;15:8017–8024. doi:10.2147/IJGM.S383055.
- Ringoringo HP. Reference range of complete blood count in healthy term newborns 1 week after birth. Open Access Maced J Med Sci. 2021;9(B):1565–1569. https://doi.org/10.3889/oamjms.2021.7781.
- Castellone DD. Establishing reference intervals in the coagulation laboratory. Int J Lab Hematol. 2017;39(Suppl 1):121–127. doi: 10.1111/ijlh.12661.
- Horowitz G, Altaie S, Boyd J, et al. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline, CLSI document C28-A3. Clinical and Laboratory Standards Institute, Wayne, PA. 2008.
- Jones G, Barker A. Reference intervals. Clin Biochem Rev. 2008;29(Suppl 1):S93–S97.
- Katayev A, Balciza C, Seccombe DW. Establishing reference intervals for clinical laboratory test results: is there a better way? Am J Clin Pathol. 2010;133(2):180–186. doi: 10.1309/AJCPN5BMTSF1CDYP.
- CLSI. Defining, establishing, and verifying reference intervals in the clinical laboratory; Approved Guideline – Third Edition. CLSI EPC28-A3c. Wayne (PA): Clinical and Laboratory Standards Institute, 2010.
- International Organization for Standardization. ISO 15189:2012 (E) Medical laboratories – Requirements for quality and competence, Third edition, 2012-11-01.
- Song WQ, Yan RH, Peng MT, et al. Age and sex specific reference intervals of 13 hematological analytes in Chinese children and adolescents aged from 28 days up to 20 years: the PRINCE study. Clin Chem Lab Med. 2022;60(8):1250–1260. doi:10.1515/cclm-2022-0304.
- Ozarda Y, Higgins V, Adeli K. Verification of reference intervals in routine clinical laboratories: practical challenges and recommendations. Clin Chem Lab Med. 2019;57(1):30–37. doi: 10.1515/cclm-2018-0059.
- Wu XZ, Zhao M, Pan BS, et al. Complete blood count reference intervals for healthy Han Chinese adults. PLoS One. 2015;10:e0119669), doi: 10.1371/journal.pone.0119669.
- Noguera NI, Detarsio G, Pérez SM, et al. Hematologic study of newborn umbilical cord blood. Medicina (B Aires). 1999;59(5 Pt 1):446–448. PMID: 10684163.
- Strecker T, Palyi B, Ellerbrok H, et al. Field evaluation of capillary blood samples as a collection specimen for the rapid diagnosis of ebola virus infection during an outbreak emergency. Clin Infect Dis. 2015;61(5):669–675. doi: 10.1093/cid/civ397.
- Krleza JL, Dorotic A, Grzunov A, et al. Capillary blood sampling: national recommendations on behalf of the croatian society of medical biochemistry and laboratory medicine. Biochem Med (Zagreb). 2015;25(3):335–358. doi: 10.11613/BM.2015.034.
- Sasse Edward A, Doumas Basil T, D’Orazio Paul EJH, et al. Stanton Noel v: How to Define and Determine Reference Intervals in the Clinical Laboratory Approved Guideline. NCCLS Document C28-A2 2nd edn National Committee for Clinical Laboratory Standards.
- Approved Standard – Sixth Edition. Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute (CLSI) document GP42-A6: Procedures and Devices for the Collection of Diagnostic Capillary Blood Specimens. 2008.
- World Health Organization. WHO guidelines on drawing blood: best practices in phlebotomy[M]. World Health Organization; 2010.
- Box G, Cox D. An analysis of transformations. J Roy Stat Soc. 1964;B26:2111252.
- Dixon WJ. Processing data for outliers. Biometrics. 1953;9:74–89.
- Daly CH, Higgins V, Adeli K, et al. Reference interval estimation: methodological comparison using extensive simulations and empirical data. Clin Biochem. 2017;50:1145–1158. doi: 10.1016/j.clinbiochem.2017.07.005.
- Christensen RD, Jopling J, Henry E, et al. The erythrocyte indices of neonates, defined using data from over 12,000 patients in a multihospital health care system. J Perinatol. 2008;28(1):24–28. doi: 10.1038/sj.jp.7211852.
- Wiedmeier SE, Henry E, Sola-Visner MC, et al. Platelet reference ranges for neonates, defined using data from over 47,000 patients in a multihospital healthcare system. J Perinatol. 2009;29(2):130–136. doi: 10.1038/jp.2008.141.
- Xanthou M. Leucocyte blood picture in ill newborn babies. Arch Dis Child. 1972;47(255):741–746. doi: 10.1136/adc.47.255.741.
- Christensen RD, Jensen J, Maheshwari A, et al. Reference ranges for blood concentrations of eosinophils and monocytes during the neonatal period defined from over 63 000 records in a multihospital health-care system. J Perinatol. 2010;30(8):540–545. doi: 10.1038/jp.2009.196.
- Maconi M, Rolfo A, Cardaropoli S, et al. Hematologic values in healthy and small for gestational age newborns. Lab Hematol. 2005;11(2):152–156. doi: 10.1532/LH96.04076.
- da Silva Brito R, de Lima Barros LM, Moreira LW, et al. Basic biochemical and hematological parameters of structural hemoglobin variants in the postpartum women and their respective newborn from Manaus, Amazonas, Brazil. BMC Pregnancy Childbirth. 2022;22(1):936. doi: 10.1186/s12884-022-05143-7.
- Proytcheva MA. Issues in neonatal cellular analysis. Am J Clin Pathol. 2009 Apr;131(4):560–573. doi: 10.1309/AJCPTHBJ4I4YGZQC.
- Imoru M, Momodu I, Buhari H, et al. Haematological reference values for full term healthy neonates delivered within 24 hours in Sokoto, Northern Nigeria. Int J Hematol Ther. 2016;2(2):1–4.
- Adewumi A, Titilope AA, Akinsegun AA, et al. Cord blood full blood count parameters in Lagos, Nigeria. Pan Afr Med J. 2014;17(1):192), doi: 10.11604/pamj.2014.17.192.3680.
- Tiruneh T, Kiros T, Getu S. Hematological reference intervals among full-term newborns in Ethiopia: a cross-sectional study. BMC Pediatr. 2020 Sep 2;20(1):417. doi: 10.1186/s12887-020-02320-5.
- El Gendy FM, Allam AA, Allam MM, et al. Haematological parameters of newborns delivered vaginally versus caesarean section. Menoufia Med J. 2016;29(2):259), DOI:10.4103/1110-2098.192429.
- Glasser L, Sutton N, Schmeling M, et al. A comprehensive study of umbilical cord blood cell developmental changes and reference ranges by gestation, gender and mode of delivery. J Perinatol. 2015;35(7):469–475. doi: 10.1038/jp.2014.241.
- Li J, Zhang H, Huang X, et al. Establishment of reference intervals for complete blood count parameters in venous blood for children in the Xiamen area, China. Int J Lab Hematol. 2019;41(5):691–696. doi: 10.1111/ijlh.13095.
- Song M, Dai S, Li J, et al. Establishment of pediatric reference intervals for complete blood count parameters in capillary blood in Beijing. Int J Lab Hematol. 2021;43(6):1363–1372. doi: 10.1111/ijlh.13631.
- Keramati MR, Mohammadzadeh A, Farhat AS, et al. Determination of hematologic reference values of neonates in Mashhad Iran. UHOD - Uluslar Hematol-Onkol Derg. 2011;21(2):101–105. DOI:10.4999/uhod.10059.
- Al-Marzoki JM, Al-Maaroof ZW, Kadhum AH. Determination of reference ranges for full blood count parameters in neonatal cord plasma in Hilla, Babil, Iraq. J Blood Med. 2012;3:113–118. doi: 10.2147/JBM.S35895.
- Zierk J, Arzideh F, Rechenauer T, et al. Age- and sex-specific dynamics in 22 hematologic and biochemical analytes from birth to adolescence. Clin Chem. 2015;61(7):964–973. doi: 10.1373/clinchem.2015.239731.
