ABSTRACT
Background
Immune thrombocytopenia (ITP) is the most common acquired bleeding disorder. In both children and adults, the primary goal of any therapeutic approach consists of cessation of bleeding and its prevention. Several options are currently available for first-line therapy in Europe, including corticosteroids and intravenous immunoglobulin (IVIg) infusion, which has a similar efficacy and safety profile in both the pediatric and adult populations. When second-line therapy is needed in the pediatric setting, current guidelines recommend eltrombopag as the drug of choice.
Procedure
The aim of this article is to summarize the available evidence and present real-life experience on eltrombopag as second-line therapy in pediatric patients with ITP, with a focus on dosing and response to therapy as well as its tapering and discontinuation.
Results
In our setting, eltrombopag is associated with good safety profile as well as promising efficacy; dose de-escalation was feasible in 94% of cases and often reached very low pro/kg dosage, with full discontinuation in 15% of cases. In daily practice, a standardized approach for discontinuation of eltrombopag in pediatric patients with ITP is still lacking. Herein, an easy-to-use scheme for tapering and discontinuation in candidate pediatric patients is proposed that proposes 25% dose reduction every four weeks.
Conclusions
In future management of pediatric ITP patients, it will be crucial to assess if thrombopoietin receptor agonists might be more effective in earlier phases of the disease and can modify the course of the disease.
Introduction
Immune thrombocytopenia (ITP) is a common acquired bleeding disorder that occurs in around 5–10 per 100,000 children per year and 3.3 per 100,000 adults per year [Citation1,Citation2]. In children, ITP is usually defined on the basis of a platelet count <100 × 109/L [Citation3]. A platelet count <20 × 109/L at initial presentation appears to be predictive of remission and better outcomes in both children and adults [Citation4]. While the pathophysiology of ITP is not completely clear, it is hypothesized to be linked to the production of autoreactive antibodies leading to destruction and diminished production of platelets [Citation5]. ITP is generally distinguished into pediatric and adult forms [Citation4,Citation6]. Compared to adults, children with ITP have lower bleeding severity, lack of comorbidities, more frequent skin manifestations, and higher rates of spontaneous remission [Citation4,Citation6,Citation7]. ITP in adults tends to evolve more frequently into a chronic disease. Pediatric patients are also treated less often with first-line corticosteroid-based therapy and tend to have a better response to therapy [Citation4,Citation6,Citation7]. Among the pediatric cohort of patients with ITP, boys are more commonly represented than girls, particularly below 5 years of age [Citation8]. In children, ITP can be classified as primary (not clearly connected to a trigger-event) or secondary (connected to a trigger-event such as an infection, immunization, autoimmune-related disorder or associated with aplastic anemia, or immune-deficiency) [Citation9].
Clearly, in both the pediatric and adult settings, the main goal of any therapeutic intervention is the cessation and prevention of bleeding [Citation10]. However, compared to adults, bleeding manifestations may be higher in children at presentation compared to adults, but the risk of serious bleeding such as ICH is lower in children [Citation9]. Thus, close observation and withholding treatment unless clinically warranted is recommended by most guidelines for children. Moreover, children have a higher rate of spontaneous disease remission and fewer co-morbidities. In children, treatment is guided more by bleeding manifestations rather than the platelet count and guided by parent/provider concerns and preferences such as an active child prone to injury. A number of options are available for first-line therapy in Europe, including corticosteroids and intravenous immunoglobulin (IVIg), which have similar efficacy and safety profiles in both children and adults [Citation4]. In a randomized clinical trial in pediatric ITP patients comparing IVIg to observation, no differences were seen in platelet counts at 12 months [Citation11]. If second-line therapy is needed in pediatric patients, all current guidelines recommend eltrombopag or romiplostim [Citation3,Citation12] ().
Figure 1. Algorithm for treatment of pediatric ITP. From Ref. [Citation3].
![Figure 1. Algorithm for treatment of pediatric ITP. From Ref. [Citation3].](/cms/asset/6b1d1de7-e96a-4ae8-bb65-e1f1cf7b91ff/yhem_a_2210906_f0001_ob.jpg)
The aim of the present review is to summarize the available evidence on eltrombopag as second-line therapy in pediatric patients with ITP, focusing on dosing and response to therapy as well as its tapering and discontinuation in the pediatric population.
Dosing with eltrombopag
Compared to rituximab which was widely used off-label until a few years ago in ITP patients [Citation13], eltrombopag has several advantages in terms its safety and tolerability profile and thus represents a valid second-line option [Citation3,Citation12]. Eltrombopag obtained a pediatric extension of the clinical indication relatively quickly, but in practice prescribers still need to observe and consider some precautions. In fact, the therapeutic dose is currently based on age and not weight, with the risk of overexposure of younger children and underexposure of older children and adolescents, in whom the maximum allowed dose of 75 mg/day is often underdosed in relation to weight as shown in the PETIT2 trial [Citation14].
The recommendation of the FDA to reduce the dose in patients with hepatic insufficiency, derived from experience in adults, is not clear in children, in whom thrombocytopenia with liver disease is a rare condition. In addition, the recommendation to reduce the dose in Asian populations is also controversial as shown in a recent real-life Chinese study [Citation15].
The pharmacokinetic data which led to an extension of the indication of eltrombopag to pediatric patients derives mainly from the study by Wire et al., which supported initial doses of 50 mg once daily for non-Asian patients aged ≥6 years and 25 mg daily. For Asian patients, regardless of age, and for all patients aged 1–5 years [Citation16]. The study reported that the time required to reach the Tmax was 4 h at all ages and was comparable to that seen in adults, while the half-life was 46.9–51.9 h in pediatric patients, also similar to the 44.0 h observed in adults, thus allowing daily dosing. However, children aged 1–11 years and female patients showed higher AUC (0 − τ) and Cmax values. These different pharmacokinetic parameters, together with non-weight-proportional dosage, increase the risk of overexposure for younger children.
A more recent study in 36 patients aged 1–17 years found that children aged 1–5 years and female patients showed higher AUC (0 − τ) and Cmax, the time to reach Tmax is 2–4 h at all ages, and that there is no proportionality between dose and AUC (0 − τ) and Cmax, which was higher in patients treated with 50 mg [Citation17]. However, the lack of proportionality between exposure and Cmax should lead to a standard dose increase. The half-life of eltrombopag was highly variable and often very short in patients who do not respond to the drug. It would thus be useful to understand whether, based on more accurate pharmacokinetic studies, it might be useful to split the dose in some patients, even though this approach might be difficult to pursue in the pediatric population, considering the need to administer eltrombopag 4 h after a meal. In the 4 h before and 2 h after taking eltrombopag, patients should not consume dairy foods, antacids, or mineral and vitamin supplements including iron, calcium, magnesium, aluminum, selenium, and zinc [Citation18].
Response to therapy
A real-life retrospective multicenter study conducted in Italy on 386 children with ITP, of whom 71 (20%) were treated with eltrombopag and followed for 6 months, showed a high response rate (80%). Median duration of eltrombopag treatment was 11 months (range 1–32) and the median starting dose was 50 mg/day (range 12.5–75 mg/day). Of those, 32 patients (45%) required one or more concomitant therapies for ITP during the initial 6 months of therapy. Median platelet counts and proportion of patients achieving the target platelet count of at least 30 × 109/L and 100 × 109/L significantly increased during the first 6 months of treatment (p < .0001), confirming the efficacy of eltrombopag. The safety profile was good with headache (7%) and thrombocytosis (6%) as the most common adverse events [Citation19]. Of the 71 patients, 14 (19.7%) discontinued permanently eltrombopag for a constant platelet count >30 × 109/L: 3 patients after 3 months and 11 patients after 6 months of therapy.
A real-life prospective study conducted in China in 116 children treated with eltrombopag also confirmed the efficacy of eltrombopag [Citation15]. Overall response was achieved in 76.7% of patients, and the response was maintained in 67.2% of cases at 12 months (≥50 × 109/L to <150 × 109/L). The median effective dose was 25, 50, and 50 mg/day for children aged ≤5 years, 6–11 years, or ≥12 years, respectively. Median platelet counts were 9 × 109/L before therapy to 26 × 109/L after one week of therapy. The proportion of patients with grade 1–4 bleeding symptoms decreased from 84% at study initiation to 19% at 6 months when only grade 1 was noted. Treatment for 12 months or longer was not associated with any safety concerns.
A limitation of these studies is that the response is measured only considering the platelet count, while other parameters should also be evaluated, to better reflect the clinical situation, such as bleeding score and overall quality of life. As mentioned, another important aspect that emerges from the study from China is that there is no difference between Asian and Caucasian populations, which does not substantiate the different treatment indications reported in its product characteristics. There is also a definite lack of studies in other ethnic groups.
Tapering and discontinuation of eltrombopag
In case of persistent response, defined as a platelet count above 50,000 for more than eight weeks, the question of discontinuation of therapy then arises, and currently there is no data available on the proposed duration of therapy or tapering regimen and discontinuation of eltrombopag in pediatric patients. Even if eltrombopag is associated with good tolerability, the opportunity of its tapering and discontinuation should be considered in patients with a persistent response. One reason for this is that there is limited data on possible long-term side effects, which are important to understand particularly if the therapy is started in a pediatric age and continued over a long period of time. In addition, the risk of ITP-related mortality is very low [Citation20], and the treatment is aimed at improving overall quality of life [Citation3,Citation12]. It is therefore important to answer the question of whether eltrombopag should be administered on a chronic basis, also considering that remission is observed in around 70% of children [Citation4] and its impact on the quality of life is still unclear. The costs related to long-term therapy are also worthy of consideration. Recent evidence suggests that in patients achieving a stable response in terms of platelet count and symptoms with eltrombopag, therapy can be tapered and/or discontinued [Citation21]. Therefore, it makes sense to try and reduce the dose or discontinue the treatment, based on the information available in adults.
In adults, the EXTEND study, conducted on 302 patients (median age 50 years, 115 splenectomized), with a fairly long follow-up (2.37 years), showed that the ITP is very different in adults compared to children [Citation22]. At the start of therapy with eltrombopag, 52 patients had platelet levels between 30 and 50 × 109/L, while 39 patients had levels >50 × 109/L. The study identified low platelet count and the number of previous therapies as predictors of lower efficacy, information not translatable to pediatric patients considering that in children the platelet count is always very low, and this is predictive of more common spontaneous recovery.
A prospective study on a limited number of adult patients provides some indications about the predictors of response to treatment [Citation23]. In particular, patients with high levels of IL-10, IL-4, TNF-α and osteopontin at baseline had a lower probability of response to eltrombopag, while there was no association between thrombopoietin levels and response. Regarding tapering and discontinuation, patients were eligible if they achieved stable corticosteroid independence – with or without IVIg – and achieved a mean platelet count ≥30 × 109/L and at least a two-fold increase of baseline platelet count confirmed by at least two subsequent sampling (every other week) and absence of bleeding events. The starting dose of 75 mg/day was reduced to 50 mg/day for 14 days, followed by 25 mg/day for 14 days, 25 mg/day every other day for 14 days, and finally 25 mg/day every 4 days before full discontinuation. Sustained remission for six months after the discontinuation of treatment was achieved in 13 out of 51 (25%) treated patients and in 13 of 34 (38%) patients who started the tapering.
Another study evaluated lymphocyte phenotypes in 56 treated adult patients (38 with complete response, of whom 28 discontinued treatment), as a possible predictor of response to treatment [Citation24]. It was found that the number of B and NK cells at discontinuation of eltrombopag was higher in patients who maintained response compared to those who relapsed.
A recent expert consensus from the US considered TPO-RA tapering inappropriate in the following conditions in both adult and pediatric patients [Citation25]:
low platelet count (30–50 × 109/L)
lower than normal platelet count (50–150 × 109/L) with a history of significant bleeding
platelet count below normal (50–150 × 109/L) with the need of intensification of treatment in the previous three to six months and on anticoagulants or antiplatelet drugs
high risk of trauma on anticoagulant or anti-platelet therapy, regardless of platelet count
In a recent paper by UK hematologists, participants estimated that around 30% of patients were candidates for tapering or discontinuation of thrombopoietin receptor agonist (TPO-RA) therapy [Citation21]. Moreover, among those discontinuing the therapy, about one-third required re-initiation of therapy within three months. A time of 6–12 months with adequate treatment response (platelet count >50 × 109/L at ≥75% of assessments in the preceding six months) could be considered a sufficient criterion to consider tapering or discontinuation. Treatment should be reinitiated if the platelet count decreases to <30 × 109/L or in the presence of symptoms. While the participants did not identify factors that could be predictive of suitability or response to tapering, several factors were considered to be associated with poor response to tapering, including history of major bleeding events, ineffective rescue therapy, and large variability in platelet counts, as well as concomitant use of anticoagulant or antiplatelet medications. Altogether, the consensus highlighted the following predictors of remission vs. relapse: age, duration of ITP, current dose of TPO-RA, duration of therapy, response to TPO-RA, platelet count, and use of concomitant therapy. Patients eligible for tapering should have satisfied the following criteria during the last six months.
being treated with a stable dose of TPO-RA
platelet count consistently >50 × 109/L
no immunosuppressive therapy (except for comorbidities)
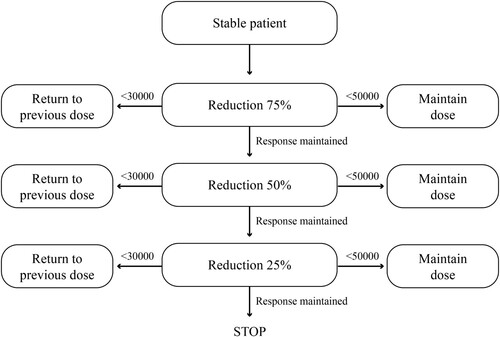
Based on a previous scheme in adult patients [Citation23] and real-world recommendations [Citation26], the following modality of tapering and discontinuation can be suggested in suitable pediatric patients (). The scheme considers a 25% dose reduction for successive four-week intervals (dose reduced by half every other day = 25% reduction; dose reduced by half every day = 50% reduction; dose suspended every other day = 25% reduction; then discontinued completely). The scheme is not modified for platelet counts <50 × 109/L, but is increased to the previous dose in case of a platelet count <30 × 109/L. The scheme preserves the daily dose as much as possible and is easy to understand for parents.
Conclusions
Eltrombopag can be considered to be safe and effective in pediatric patients with ITP. However, there is currently very limited data about the effects of the drug, especially toxicity, when administered to pediatric patients in the long term. This, together with other considerations such as the high rate of spontaneous remission and costs, makes it worth attempting to taper/discontinue the TPO-RA. Further studies are thus needed to understand in which patient tapering and discontinuation is likely to be more successful in terms of sustained response.
In the future, it will be necessary to develop a standardized approach for management of pediatric patients with ITP receiving eltrombopag. Such an approach must consider the amount of loading dose prior to reducing it to the minimal effective dose, as well as age-related dose adjustments. The criteria and modality of the tapering and discontinuation must also be standardized. On this note, we propose an easy-to-use scheme, even if not evidence based. In the management of pediatric ITP patients, it will also be important to understand if a TPO-RA might be more effective in earlier phases of the disease to possibly modify its course.
Acknowledgements
The authors thank Dr Ceglie for her contribution in revising the final manuscript. Content Ed Net provided the editorial support, with the helpful assistance of Patrick Moore.
Disclosure statement
UR received support for attending meetings by Novartis Farma Italy; CD attended to advisory boards sponsored by Novartis Farma Italy; GP, PF, GR, AB, MS, FG, SL, MZ, SP, AP, PG have no conflicts to declare.
Additional information
Funding
References
- Reid MM. Chronic idiopathic thrombocytopenic purpura: incidence, treatment, and outcome. Arch Dis Child. 1995;72(2):125–128. DOI: 10.1136/adc.72.2.125
- Terrell DR, Beebe LA, Vesely SK, et al. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85(3):174–180.
- AIEOP. Available at: https://www.aieop.org/web/
- Despotovic JM, Grimes AB. Pediatric ITP: is it different from adult ITP? Hematology Am Soc Hematol Educ Program. 2018;2018(1):405–411.
- Matzdorff A, Meyer O, Ostermann H, et al. Immune thrombocytopenia – current diagnostics and therapy: recommendations of a joint working group of DGHO, OGHO, SGH, GPOH, and DGTI. Oncol Res Treat. 2018;41:51–30.
- Kuhne T. Diagnosis and management of immune thrombocytopenia in childhood. Hamostaseologie. 2017;37(1):36–44. DOI: 10.5482/HAMO-16-06-0017
- Parodi E, Russo G, Farruggia P, et al. Management strategies for newly diagnosed immune thrombocytopenia in Italian AIEOP Centres: do we overtreat? Data from a multicentre, prospective cohort study. Blood Transfus. 2020;18(5):396–405.
- Yong M, Schoonen WM, Li L, et al. Epidemiology of paediatric immune thrombocytopenia in the general practice research database. Br J Haematol. 2010;149(6):855–864. DOI: 10.1111/j.1365-2141.2010.08176.x
- Kim TO, Despotovic JM. Primary and secondary immune cytopenias: evaluation and treatment approach in children. Hematol Oncol Clin North Am. 2019;33(3):489–506. DOI: 10.1016/j.hoc.2019.01.005
- Neunert CE, Cooper N. Evidence-based management of immune thrombocytopenia: ASH guideline update. Hematology Am Soc Hematol Educ Program. 2018;2018(1):568–575.
- Heitink-Polle KMJ, Uiterwaal C, Porcelijn L, et al. Intravenous immunoglobulin vs observation in childhood immune thrombocytopenia: a randomized controlled trial. Blood. 2018;132(9):883–891. DOI: 10.1182/blood-2018-02-830844
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–3866. DOI: 10.1182/bloodadvances.2019000966
- Liang Y, Zhang L, Gao J, et al. Rituximab for children with immune thrombocytopenia: a systematic review. PLoS One. 2012;7(5):e36698. DOI: 10.1371/journal.pone.0036698
- Grainger JD, Locatelli F, Chotsampancharoen T, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;386(10004):1649–1658. DOI: 10.1016/S0140-6736(15)61107-2
- Cheng X, Fu L, Ma J, et al. Spotlight on eltrombopag in pediatric ITP in China: a long-term observational study in real-world practice. Blood Adv. 2021;5(19):3799–3806. DOI: 10.1182/bloodadvances.2020004110
- Wire MB, Li X, Zhang J, et al. Modeling and simulation support eltrombopag dosing in pediatric patients with immune thrombocytopenia. Clin Pharmacol Ther. 2018;104(6):1199–1207. DOI: 10.1002/cpt.1066
- Dionisi M, Cairoli S, Simeoli R, et al. Pharmacokinetic evaluation of eltrombopag in ITP pediatric patients. Front Pharmacol. 2021:6;12:772873.
- Eltrombag. (n.d.). Summary of product characteristics.
- Giordano P, Lassandro G, Barone A, et al. Use of eltrombopag in children with chronic immune thrombocytopenia (ITP): a real life retrospective multicenter experience of the Italian Association of Pediatric Hematology and Oncology (AIEOP). Front Med (Lausanne). 2020;28;7:66.
- Frederiksen H, Maegbaek ML, Norgaard M. Twenty-year mortality of adult patients with primary immune thrombocytopenia: a Danish population-based cohort study. Br J Haematol. 2014;166(2):260–267. DOI: 10.1111/bjh.12869
- Cooper N, Hill QA, Grainger J, et al. Tapering and discontinuation of thrombopoietin receptor agonist therapy in patients with immune thrombocytopenia: results from a modified Delphi panel. Acta Haematol. 2021;144:418-426..
- Wong RSM, Saleh MN, Khelif A, et al. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017;130(23):2527–2536. Blood. 2018;131(6):709.
- Lucchini E, Palandri F, Volpetti S, et al. Eltrombopag second-line therapy in adult patients with primary immune thrombocytopenia in an attempt to achieve sustained remission off-treatment: results of a phase II, multicentre, prospective study. Br J Haematol. 2021;193:386–396.
- Oka S, Ono K, Nohgawa M. The association of lymphocyte phenotypes and outcomes after discontinuing eltrombopag in immune thrombocytopenia. Int J Clin Pract. 2021;75(5):e14057.
- Cuker A, Despotovic JM, Grace RF, et al. Tapering thrombopoietin receptor agonists in primary immune thrombocytopenia: expert consensus based on the RAND/UCLA modified Delphi panel method. Res Pract Thromb Haemost. 2021;5(1):69–80. DOI: 10.1002/rth2.12457
- Zaja F, Carpenedo M, Barate C, et al. Tapering and discontinuation of thrombopoietin receptor agonists in immune thrombocytopenia: real-world recommendations. Blood Rev. 2020:41:100647.