ABSTRACT
Objective
To investigate the preventive effect of low-dose porcine anti-thymocyte globulin (P-ATG) on graft versus host disease (GVHD) in patients' donors over 40 years old or female donors undergoing HLA-matched sibling donor hematopoietic stem cell transplantation (MSD-HSCT).
Methods
The clinical data of 30 patients received Low-dose Porcine antithymocyte globulin (P-ATG) as a part of the conditioning regimen (the P-ATG group), while the other 30 patients didn’t receive ATG (the Non-ATG group).
Results
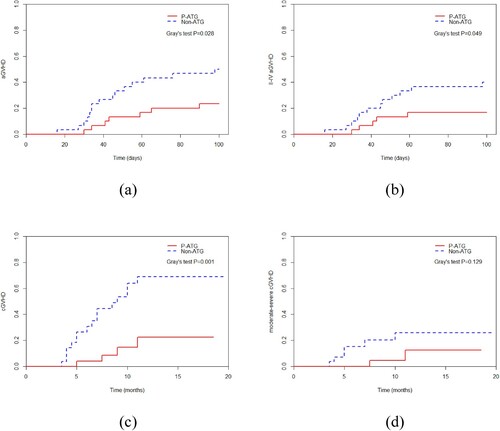
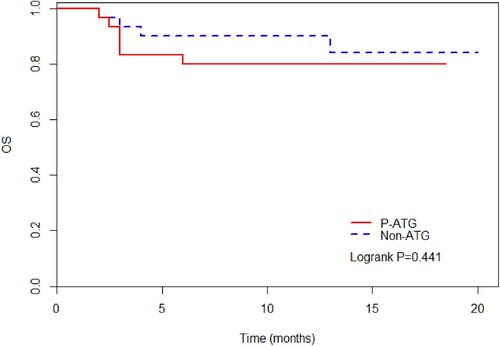
There was a significant difference in the incidence of aGVHD ([23.3 (10.1–39.7) %] vs [50.0 (30.8–66.5) %], P = 0.028), grade II–IV aGVHD ([16.7 (5.94–32.1) %] vs [40.0 (22.4–57.0) %], P = 0.049) and chronic GVHD (cGVHD) ([22.4 (6.03–45.1) %] vs [69.0 (43.4–84.8) %], P = 0.001) between two groups. But there was no significant difference in terms of moderate-severe cGVHD (P = 0.129), 1-year relapse rate (P = 0.742), non-relapse mortality (P = 0.237), or overall survival (P = 0.441).
Conclusion
The application of low-dose P-ATG in patients/donors over 40 years old or female donors undergoing MSD-HSCT for hematological malignancy can significantly reduce the incidence of aGVHD, grade II–IV aGVHD and cGVHD, doesn’t increase the risk of relapse.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is an important therapeutic option and a potentially curative procedure for a variety of hematological malignancies. Graft versus host disease (GVHD) is the most frequent and serious complication following allogeneic HSCT (allo-HSCT), which remarkably impacts a patient’s survival and quality of life [Citation1]. Even among recipients of recipient human leukocyte antigen (HLA)-matched sibling donor HSCT (MSD-HSCT), incidence rates of acute GVHD (aGVHD) and chronic GVHD (cGVHD) reach 40–50% and 30–70%, respectively [Citation2,Citation3]. Especially in patients/donors over 40 years old and female donors with malignant hematological diseases have an increased GVHD risk in MSD-HSCT [Citation4–6]. It is reported that anti-thymocyte globulin (ATG) can effectively decrease the incidence of aGVHD in MSD-HSCT [Citation7]. Porcine ATG (P-ATG) is another ATG preparation that is available in China for clinical use as an immunosuppressive agent. However, there are a few reports that it are used in the conditioning regimen of MSD-HSCT. To address the concern, we investigated the effect of P-ATG on the risk of GVHD in MSD-HSCT.
Methods
Patients
We conducted a retrospective analysis of the clinical data of 60 patients with hematological malignancies who received MSD-HSCT from July 2020 to October 2021 in our transplantation center. These patients/donors were over 40 years old or the donors were female. Of these, 30 patients received low-dose P-ATG as a part of the conditioning regimen (the P-ATG group), while the other 30 patients didn’t receive ATG (the Non-ATG group). The study was approved by the Ethics Committees of the Institute of Hematology, CAMS & PUMC according to the Guidelines of the Declaration of Helsinki.
In the P-ATG group, 16 patients were male, the rest 14 patients female, with a median age of 47 (33–61) years old. All 30 patients had malignant hematological diseases, 21 patients reached bone marrow complete remission (CR) before transplantation, and the other 9 patients did not reach CR. In the Non-ATG group, 16 patients were male, the rest 14 patients female, with a median age of 35 (18–53) years old. All 30 patients also suffered from malignant hematological diseases, 26 patients reached CR before transplantation, and the other 4 patients did not reach CR.
Some patients experienced infectious events before HSCT. In the P-ATG group, 8, 4 and 4 patients developed severe bacterial infection, invasive fungal disease (IFD) and viral infection, respectively. In the Non-ATG group, 7, 7 and 2 patients developed severe bacterial infection, IFD and viral infection, respectively. The infection was controlled in both groups before HSCT.
Donor
All 60 patients received HLA-A, B, C, DR and DQ-matched MSD-HSCT. In the P-ATG group, 16 donors were male, the rest 14 donors female, with a median age of 47 (34–58) years old. In the Non-ATG group, 11 donors were male, the rest 19 donors female, with a median age of 35 (17–58) years old.
Conditioning regimen
All patients received a myeloablative conditioning regimen. In the P-ATG group, P-ATG 20 mg/kg/day was used as a part of the conditioning regimen in -3, -2, -1 day. In the Non-ATG group, no ATG preparation was used in the conditioning regimen.
Post-transplant immunosuppression
GVHD prophylaxis regimen consisted of a short course of methotrexate (MTX) at a dose of 15 mg/m2 intravenously on day 1 followed by 10 mg/m2 intravenously on days 3 and 6, and CSA 2 mg/kg/day or tacrolimus (FK506) 0.03 mg/kg/day intravenously beginning day -1 in both groups.
Graft source
All patients underwent peripheral blood stem cell transplantation (PBSCT). In the P-ATG group, the median numbers of MNC and CD34+ cells were 11.1 (8.0–21.7) × 108/kg, 2.4 (1.4–4.6) × 106/kg, respectively. In the Non-ATG group, the median numbers of MNC and CD34+ cells were 10.1 (6.4–27.7) × 108/kg, 2.5 (1.7–5.7) × 106/kg, respectively.
Patient routine supportive care
All patients resided in class 100 laminar flow ward, receiving SMZco 1 g twice daily for one week to prevent pneumocystis carinii pneumonia, and ganciclovir 10 mg/kg/day intravenously for one week to prevent cytomegalovirus (CMV) infection before transplantation. Prevention of fungal infections in the patients who hadn’t been diagnosed with IFD before transplantation was applied by voriconazole or posaconazole until 3 months after transplantation. The other patients who had IFD before transplantation received voriconazole, posaconazole or caspofungin according to their individual pre-transplant situations.
Engraftment standard
Neutrophil (ANC) recovery was defined as achieving an absolute ANC count of ≥0.5 × 109/L for 3 consecutive days and platelet (PLT) recovery as achieving a PLT count ≥20 × 109/L, unsupported by transfusion for 7 days. Chimerism analysis was done using STR.
Outcome analysis standard
Diagnosing and grading of aGVHD were based on the Seattle diagnostic criteria. The 2014 NIH consensus of cGVHD [Citation8] was used to diagnose and grade cGVHD. Recurrence after HSCT is defined as the proportion of primitive or immature cells in bone marrow greater than 5%. CMV viremia was defined as positive results of RT-PCR (1 × 103copies/mL) in the blood. We defined IFD according to the revised EORTC/MSG criteria [Citation9]. Severe bacterial infections were defined as bacteremia and severe tissue infections.
Survival analysis
The last follow-up for all surviving patients was on 28 February 2022. SAS 9.4 software was used to analyze the survival rate and the survival curve by the Kaplan–Meier method. Survival differences between groups were compared by the Log-Rank test. Patient, disease and transplant-related characteristics were compared using the Chi-square test and Rank sum test. The final model of significance attained a P-value of ≤0.05.
Results
Pre-transplant clinical characteristics
The pre-transplant clinical characteristics of the two groups are shown in .
Table 1. Patients’ pre-transplant clinical characteristics.
Treatment regimens
The treatment regimens of the two groups are shown in .
Table 2. Treatment details.
Engraftment
All patients were engrafted in two groups. In the P-ATG group, the median periods for ANC and PLT recovery were 12 (9–16) and 15 (8–38) days, respectively. In the Non-ATG group, the median durations for ANC and PLT recovery were 12 (11–20) and 13 (10–23) days, respectively.
Graft versus host disease
In the P-ATG group, 7 patients developed aGVHD, 2 out of 7 patients had grade I aGVHD, and 5 patients had grade II–IV aGVHD. In the Non-ATG group, 15 patients developed aGVHD, 3 out of 15 patients had grade I aGVHD, and 12 patients had grade II–IV aGVHD. All patients responded to a combination of immunosuppressive agents and steroids, no one died of severe aGVHD. There was significant difference in aGVHD ([23.3 (10.1–39.7)] % vs [50.0 (30.8–66.5)] %, P = 0.028) and grade II–IV aGVHD ([16.7 (5.94–32.1)] % vs [40.0 (22.4–57.0)] %, P = 0.049) between the two groups, respectively.
In the P-ATG group, 4 patients developed cGVHD, and 1 patient had mild-moderate cGVHD. In the Non-ATG group, 16 patients developed cGVHD, and 6 patients had mild-moderate cGVHD. CGVHD was controlled in all patients by immunosuppressive agents. There was significant difference in cGVHD ([22.4 (6.03–45.1)] % vs [69.0 (43.4–84.8)] %, P = 0.001) between the two groups. There was no significant difference in terms of moderate-severe cGVHD between the two groups ([12.5 (1.67–34.8)] % vs [25.9 (9.82–45.6)] %, P = 0.129).
The incidence of GVHD in the two groups is shown in .
Relapse
In the P-ATG group, 3 patients developed relapse, including 2 patients of extramedullary relapse and 1 patient of bone marrow relapse. In the non-ATG group 4 patients developed relapse, including 2 patients of extramedullary relapse and 2 patients of bone marrow relapse. There was no significant difference in relapse rate (RR) between the two groups ([13.6 (2.73–33.2)] % vs [15.2 (4.40–32.1)] %, P = 0.742). Except for 1 patient in the P-ATG group 2 patients in the Non-ATG group gave up treatment and died after relapse, the other patients did not die of relapse directly.
Infection
In the P-ATG group, bacteremia occurred in 3 patients, and pulmonary bacterial infection occurred in 3 patients. These 6 patients recovered after the use of antibacterial therapy. IFD occurred in 7 patients, all of whom had pulmonary infections. The pulmonary IFD was not successfully controlled by antifungal therapy in 3 patients, and they died. The other patients with IFD were successfully treated with antifungal therapy. There were 7 patients with CMV infection. CMV viremia occurred in 5 patients, including 2 patients with CMV enteritis and 1 patient with CMV encephalitis, and the patient with CMV encephalitis died, the other patients with CMV viremia were controlled by antiviral and globulin therapy. Another 2 patients had simple CMV enteritis, 1 died after treatment and 1 survived. Five patients in the P-ATG group developed CMV disease, of which four ultimately died.
In the Non-ATG group, bacteremia occurred in 1 patient, 1 patient soft tissue bacterial infection, and 1 patient intestinal bacterial infection. The patient’s intestinal bacterial infection was not successfully treated with antibacterial therapy and finally died. IFD occurred in 6 patients, all of whom had pulmonary infections. All patients with IFD were successfully treated with antifungal therapy. CMV viremia occurred in 4 patients, no patients with CMV disease, and all patients were successfully treated with antiviral and globulin therapy.
There was no significant difference in terms of incidence of severe bacterial infections (P = 0.472), IFD (P = 1.000) or CMV infection (P = 0.298) between the two groups.
Deaths
In all, 6 patients died in the P-ATG group, 3 with pulmonary IFD, the others account for giving up treatment after relapse, CMV encephalitis, and gastrointestinal bleeding caused by CMV enteritis. A total of 4 patients died in the Non-ATG group, 2 with relapse, and the other 2 patients with intestinal infection perforation, and intracranial hemorrhage, respectively. 80% (4/5) of patients in the P-ATG group died of a secondary infection after grade II–IV aGVHD, and 16.7% (2/12) in the Non-ATG group.
There was no significant difference in terms of the 1-year non-relapse mortality (NRM) between the two groups ([16.7 (5.95–32.1) %] vs [6.67 (1.14–19.5) %], P = 0.237).
Survival
In the P-ATG group, at a median follow-up of 10 (2–18.5) months, 24 patients were alive and the 1-year overall survival (OS) was 79.9 (60.5–90.4) %. In the Non-ATG group, at a median follow-up of 12.5 (2–20) months, 26 patients were alive and the 1-year OS was 84.0 (61.2–94.0) %. There was no significant difference in terms of the 1-year OS between the two groups (P = 0.441).
The outcomes of the two groups patients are shown in and .
Table 3. The outcomes of the two groups’ patients.
Discussion
AGVHD and cGVHD are the leading causes of non-relapse mortality following allo-HSCT. The most consistently reported factors significantly associated with an increased risk of aGVHD were recipient HLA mismatching with the donor, alloimmunization of the donor, the use of a female donor for male recipients, and older patient age. For cGVHD, the most consistently reported risk factors include prior aGVHD, grafting with growth factor–mobilized blood cells, the use of a female donor for male recipients, older patient age, and mismatched and unrelated donors [Citation10–12]. In vivo T-cell depletion using ATG-Fresenius (ATG-F), and rabbit ATG (R-ATG) has been shown to reduce the risks of aGVHD and cGVHD in unrelated HSCT [Citation13,Citation14]. Some researchers tried to add ATG preparations to a conditioning regimen in MSD-HSCT to reduce the incidence of GVHD: D-Y Shin et al. [Citation15] demonstrated that a high dose (⩾4.5 mg/kg) of R-ATG reduces the risk of aGVHD following MSD-HSCT using busulfan (Bu) Fludarabine (Flu) conditioning with no significant effect on relapse-free survival (RFS) and OS. Kroger et al. [Citation16] investigated the use of ATG-F at a dose of 10 mg/kg on 3, 2, and 1 days before the transplantation of MSD-HSCT in patients with acute leukemia (AML). The rate of grades II–IV aGVHD was 10.8%, the 2-year cumulative incidence of cGVHD was 32.2%, and the 2-year cumulative incidence of clinical extensive cGVHD was 7.6%. Liping Dou et al. [Citation17] used low-dose R-ATG for GVHD prophylaxis in patients or donors aged ≥40 years with hematological malignancies receiving MSD-HSCT. R-ATG was administered to 40 patients at an intravenous dose of 5 mg/kg divided over day 5 and day 4 before graft infusion. The cumulative incidence of grades II–IV and grades III–IV aGVHD at day +100 was 30.0% and 2.6%, respectively. The 2-year cumulative incidence of extensive cGVHD and severe cGVHD was 11.4% and 14.7%. The 2-year cumulative incidence of transplant-related mortality (TRM) and relapse was 14.0% and 22.6%, respectively. The cumulative incidence of CMV reactivation, Epstein–Barr virus reactivation, and fungal infection was 22.3%, 12.9%, and 12.5%, respectively. Kaplan–Meier estimates for OS, disease-free survival (DFS), and GVHD-free and relapse-free survival 3 years after transplantation were 68.9%, 68.9%, and 54.0%, respectively. Thus, consensus-based recommendations by an international expert panel agreed that ATG should be recommended before MSD-HSCT to reduce the occurrence of GVHD [Citation18].
P-ATG is another ATG preparation that is a new product developed in China. It is first applied to immunosuppressive therapy (IST) of severe aplastic anemia (SAA). Several studies have shown that P-ATG exhibited good therapeutic effects in SAA for IST [Citation19]. Zhang FK et al. (data not yet published) showed that the half-life of P-ATG was about 15 days and effective serum concentration of P-ATG was maintained for at least 60 days in vivo. The drug metabolism curve of P-ATG had an advantage over R-ATG in IST, P-ATG therapy combined with cyclosporine A had significant long-term efficacy and high OS in SAA [Citation20,Citation21]. Subsequently, P-ATG was gradually applied in the conditioning regimens for SAA. The clinical data of 113 SAA patients who received MSD-HSCT from January 2005 to November 2016 in our center showed [Citation22]: 58 patients received R-ATG as a part of the conditioning regimen (R-ATG group), whereas the other 55 patients received P-ATG (the P-ATG group). There was a significant difference in the incidence of aGVHD (20.7% ± 5.3% versus 43.4% ± 7.0%, P = 0.015) and cGVHD (20.1% ± 5.8% versus 46.0% ± 7.9%, P = 0.003) between the R-ATG and P-ATG groups. However, there was no significant difference in terms of 3-year OS (93.1% ± 3.3% versus 84.4% ± 5.7%, P = 0.235), grades III–IV aGVHD (3.4% ± 2.4% versus 12.3% ± 4.7%, P = 0 .098), moderate-severe cGVHD (12.6% ± 4.9% versus 11.5% ± 4.9%, P = 0.905), or graft rejection (GR) (7.4% ± 3.6% versus 5.5% ± 3.1%, P = 0.852). There was also no significant difference with regard to the incidence of severe bacterial infection (P = 0.075), IFD (P = 0.701), or CMV viremia (P = 0.770). P-ATG showed satisfactory efficacy and safety compared with R-ATG in the setting of MSD-HSCT for SAA patients. A total of 91 patients with SAA who received haploid HSCT (haplo-HSCT) in our center between January 2014 and December 2020 were retrospectively reviewed [Citation23]. 28 patients were in the P-ALG group while 63 patients were in the R-ATG group. There was no significant difference in 5-year OS (74.83% ± 8.24% vs 72.29% ± 6.26%, P = 0.830), GVHD-free, failure-free survival (71.05% ± 8.65% vs 62.71% ± 6.22%, P = 0.662), failure-free survival (74.83% ± 8.24% vs 66.09% ± 5.84%, P = 0.647) and TRM (25.17% ± 8.24% vs 26.29% ± 6.22%, P = 0.708) between the two groups. The incidence of aGVHD (65.39% ± 9.33% vs 62.71% ± 6.30%, P = 0.653), II–IV aGVHD (38.46% ± 9.54% vs 35.64% ± 6.24%, P = 0.695), III–IV aGVHD (19.23% ± 7.73% vs 10.53% ± 4.07%, P = 0.291), cGVHD (chronic graft versus host disease) (22.22% ± 12.25% vs 22.31% ± 6.30%, P = 0.915), and moderate-severe cGVHD (5.56% ± 5.40% vs 9.28% ± 4.46%, P = 0.993) was not significantly different. Similar outcomes were observed between the P-ALG and R-ATG groups for severe bacterial infection (17.9% vs 25.4%, P = 0.431), IFD (3.6% vs 9.5%, P = 0.577) and GR (0% vs 9.5%, P = 0.218). However, the incidence of CMV infection and Epstein–Barr virus infection was significantly lower in the P-ALG group (46.4% vs 71.4%, P = 0.022; 3.6% vs 25.4%, P = 0.014).
However, a few studies about P-ATG had been done on conditioning regimens for hematological malignancy. We conducted this retrospective analysis to compare the incidence of GVHD in the P-ATG group and the Non-ATG group in patients/donors over 40 years old or female donors undergoing MSD-HSCT for hematological malignancy. In our study, in addition to the fact that the ages of the patients and donors were older in the P-ATG group, all patients’ other baseline characteristics and donor conditions of the two groups were similar. The results showed that all patients were engrafted in two groups. There was significant difference in aGVHD ([23.3 (10.1–39.7)] % vs [50.0 (30.8–66.5)] %, P = 0.028), grade II–IV aGVHD ([16.7 (5.94–32.1)] % vs [40.0 (22.4–57.0)] %, P = 0.049), cGVHD ([22.4 (6.03–45.1)] % vs [69.0 (43.4–84.8)] %, P = 0.001). There was no significant difference in terms of moderate-severe cGVHD ([12.5 (1.67–34.8)] % vs [25.9 (9.82–45.6)] %, P = 0.129), 1-year RR ([13.6 (2.73–33.2)] % vs [15.2 (4.40–32.1)] %, P = 0.742), NRM ([16.7 (5.95–32.1)] % vs [6.67 (1.14–19.5)] %, P = 0.237), or OS ([79.9 (60.5–90.4)] % vs [84.0 (61.2–94.0)] %, P = 0.441). There was also no significant difference with regard to the incidence of severe bacterial infections (P = 0.472), IFD (P = 1.000) or CMV infection (P = 0.298) between the two groups. In our study, the ages of the patients were older (P < 0.001) in the P-ATG group. 80% (4/5) of patients in the P-ATG group died of a secondary infection after grade II–IV aGVHD, and 16.7% (2/12) in the Non-ATG group. The risk of death was significantly higher caused by a secondary infection after grade II–IV aGVHD. In addition, Jhong-Lin Wu et al. As reported while P-ATG reduced the incidence of GVHD, its long half-life may cause delayed immune reconstitution, particularly delayed cluster of differentiation (CD)4+ T-cell reconstitution, which increases the risk of CMV infection [Citation24]. Although there was no significant difference in terms of incidence of CMV infection between the two groups (P = 0.298) in our research, the incidence of CMV disease with poor prognosis was significantly increased in the P-ATG group. Five patients in the P-ATG group developed CMV disease, of which four ultimately died, while no one in the Non-ATG group developed CMV disease. So it may be the reason for a little high NRM and slightly inferior OS in the P-ATG group. It also indicates that older patients with II–IV aGVHD after MSD-HSCT need strict monitoring of infection and early prevention of CMV to reduce the mortality of subsequent secondary infection and occurrence of severe CMV disease. Subsequently, it is also necessary to expand the sample size to clarify whether there are differences in NRM and OS between the two groups.
In summary, the application of low-dose P-ATG in patients/donors over 40 years old or female donors undergoing MSD-HSCT for hematological malignancy can significantly reduce the incidence of aGVHD, grade II–IV aGVHD and cGVHD, doesn’t increase the risk of relapse. However, this report was a retrospective analysis and the sample size of each group was relatively small, our results should be considered preliminary. Further studies with larger cohorts and longer follow-up periods are needed to derive more conclusive findings.
Author contributions
Xin Chen performed the data analyses and wrote the manuscript; Erlie Jiang guided article ideas and writing; Rongli Zhang, Weihua Zhai, Qiaoling Ma, Aiming Pang, Donglin Yang, Jialin Wei, Yi He collected data;Yueshen Ma, Zhen Song carried out statistical analysis; Sizhou Feng, Mingzhe Han helped perform the analysis with constructive discussions.
Acknowledgements
The authors would like to thank all of the doctors and nurses in the Therapeutic Centre of Hematopoietic Stem Cell Transplantation, Center for information and resources for their professional assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data are available on request from the authors.
Additional information
Funding
References
- Phan M, Chavan R, Beuttler R, et al. Evaluating risk factors for acute graft versus host disease in pediatric hematopoietic stem cell transplant patients receiving tacrolimus. Clin Transl Sci. 2021;14:1303–1313.
- Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307.
- Stem Cell Trialists’ Collaborative G. Allogeneic peripheral blood stem cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23:5074–5087.
- Zhang H. Impact of donor and recipient characteristics on graft-versus-host disease and survival in HLA-matched sibling hematopoietic stem cell transplantation. Transfus Apher Sci. 2020;59:102743.
- Loren AW, Bunin GR, Boudreau C, et al. Impact of donor and recipient sex and parity on outcomes of HLA-identical sibling allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:758–769.
- Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043–2051.
- Chang Y-J, Wu D-P, Lai Y-R, et al. Antithymocyte globulin for matched sibling donor transplantation in patients with hematologic malignancies: a multicenter, open-label, randomized controlled study. J Clin Oncol. 2020;38:3367–3376.
- Moon JH, Sohn SK, Lambie A, et al. Validation of National Institutes of Health global scoring system for chronic graft-versus-host disease (GVHD) according to overall and GVHD-specific survival. Biol Blood Marrow Transplant. 2014;20:556–563.
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821.
- Koch C, Li L, Figueroa P, et al. Transfusion and pulmonary morbidity after cardiac surgery. Ann Thorac Surg. 2009;88:1410–1418.
- Nassereddine S, Rafei H, Elbahesh E, et al. Acute Graft versus host disease: a comprehensive review. Anticancer Res. 2017;37:1547–1555.
- Oran B, Champlin RE, Wang F, et al. Donor clonal hematopoiesis increases risk of acute graft versus host disease after matched sibling transplantation. Leukemia. 2022;36:257–262.
- Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–864.
- Walker I, Panzarella T, Couban S, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17:164–173.
- Shin D-Y, Lee J-H, Park S, et al. Role of thymoglobulin in matched sibling allogeneic hematopoietic stem cell transplantation with busulfan and fludarabine conditioning in myeloid malignanicies. Bone Marrow Transplant. 2018;53:207–212.
- Kroger N, Solano C, Wolschke C, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med. 2016;374:43–53.
- Dou L, Hou C, Ma C, et al. Reduced risk of chronic GVHD by low-dose rATG in adult matched sibling donor peripheral blood stem cell transplantation for hematologic malignancies. Ann Hematol. 2020;99:167–179.
- Bonifazi F, Rubio M-T, Bacigalupo A, et al. Rabbit ATG/ATLG in preventing graft-versus-host disease after allogeneic stem cell transplantation: consensus- based recommendations by an international expert panel. Bone Marrow Transplant. 2020;55:1093–1102.
- Lv Q, Huiqin Z, Na X, et al. Treatment of severe aplastic anemia with porcine anti-human lymphocyte globulin. Curr Pharm Des. 2020;26:2661–2667.
- Chen M, Liu C, Zhuang J, et al. Long-term follow-up study of porcine anti-human thymocyte immunoglobulin therapy combined with cyclosporine for severe aplastic anemia. Eur J Haematol. 2016;96:291–296.
- Ma X, Wang J, Zhang W, et al. Comparison of porcine anti-human lymphocyte globulin and rabbit anti-human thymocyte globulin in the treatment of severe aplastic anemia: a retrospective single-center study. Eur J Haematol. 2016;96:260–268.
- Chen X, Wei J, Huang Y, et al. Effect of antithymocyte globulin source on outcomes of HLA-matched sibling allogeneic hematopoietic stem cell transplantation for patients with severe aplastic anemia. Biol Blood Marrow Transplant. 2018;24:86–90.
- Chen J, Zhang Y, Chen X, et al. Comparison of porcine ALG and rabbit ATG on outcomes of HLA-haploidentical hematopoietic stem cell transplantation for patients with acquired aplastic anemia. Cancer Cell Int. 2022;22:89.
- Wu J-L, Ma H-Y, Lu C-Y, et al. Risk factors and outcomes of cytomegalovirus viremia in pediatric hematopoietic stem cell transplantation patients. J Microbiol Immunol Infect. 2017 Jun;50(3):307–313.