ABSTRACT
Objectives
Sickle cell disease (SCD) is characterized by a mutation in the beta-globin gene resulting in abnormal hemoglobin S (HgbS). The significant sequela of SCD include anemia and recurrent vaso-occlusive episodes (VOEs) which may effectuate patients to receive chronic blood transfusions. Current pharmacotherapy options for SCD include hydroxyurea, voxelotor, Lglutamine, and crizanlizumab. Simple and exchange transfusions are often utilized as prophylaxis to prevent emergency department (ED)/urgent care (UC) visits or hospitalizations from VOEs by reducing the level of sickled red blood cells (RBCs). In addition, the treatment of VOEs involves intravenous (IV) hydration and pain management. Studies have demonstrated that sickle cell infusion centers (SCIC) decrease hospital admissions for VOEs, and IV hydration and pain medications are the key components of management employed. Thus, we hypothesized that implementing a structured infusion protocol in the outpatient setting would reduce the incidence of VOEs.
Methods
Here, we discuss two patients with SCD who were trialed on scheduled outpatient IV hydration and opioids with the goal of decreasing the frequency of VOEs in the setting of the current blood product shortage and the patients' refusal to receive exchange transfusions.
Results
Overall, the two patients had opposing outcomes- one demonstrated reduced frequency of VOEs, whereas the other had mixed results due to noncompliance to scheduled outpatient sessions.
Discussion/Conclusion
The use of outpatient SCICs may be an effective intervention for prevention of VOEs in patients with SCD, and further patient-centered research and quality improvement initiatives are needed to further quantify and understand the factors contributing to their efficacy.
Introduction/background
Vaso-occlusive episodes (VOEs) in patients with sickle cell disease (SCD) lead to recurrent presentations for medical attention, whether to the emergency department (ED)/ urgent care (UC) or the hospital for hydration and pain management [Citation1–3]. Endothelial dysfunction and microvascular occlusions arising from adhesions of sickled red blood cells (RBCs) to the endothelial surface are key factors in the pathogenesis of VOEs, which manifest in microvascular inflammation, ischemic injury, and severe pain [Citation1]. As a means to reduce sickled RBC levels and vaso-occlusive complications, patients are often subjected to chronic blood transfusion therapy [Citation1,Citation4].
While prophylactic blood transfusions are commonly implemented in the clinical setting, there remains limited evidence to support their role in preventing VOEs [Citation5]. The complications of chronic transfusions include alloimmunization, iron overload, and infection. Moreover, a worldwide shortage of blood products, especially during the COVID-19 pandemic, has further precluded this strategy from becoming standard-of-care [Citation6,Citation7]. Increased mortality from hyper-hemolytic reactions further limit the use of chronic blood transfusions [Citation2,Citation8]. In addition, patients may refuse blood transfusions due to personal or religious beliefs, and others may decline exchange transfusions due to not being amenable to central line or port placement. Consistently, the American Society of Hematology (ASH) has recommended against the use of chronic monthly blood transfusion therapy as standard, first-line therapy for prevention of acute pain in patients with SCD [Citation5].
In contrast, intravenous (IV) hydration and IV pain medications are first-line treatments used in the acute setting; however, there are no studies on outpatient, scheduled IV hydration for the prevention of VOEs. To improve pain control and quality of life in patients with SCD, especially in the setting of the shortage of blood products available, we developed an outpatient infusion protocol including IV fluid bolus and IV hydromorphone (Dilaudid) in addition to as needed hydromorphone and acetaminophen. Here, we report the clinical response of two patients with SCD who were treated prophylactically with our novel outpatient infusion protocol.
Case description
We describe two female patients with SCD, both with hemoglobin SS (HgbSS), termed ‘Patient 1’ and ‘Patient 2,’ who established care at our comprehensive sickle cell clinic. Patient 1 was 21 years old (baseline Hgb level of 8 g/dL) and had a history of acute chest syndrome with SCD complicated by secondary hemochromatosis from receiving chronic blood transfusions remotely in the pediatric setting. Patient 2 was 19 years old (baseline Hgb of 9 g/dL) with a history of acute chest syndrome and osteonecrosis of the left hip. Patient 1 had been on L-glutamine (Endari), crizanlizumab (Adakveo), and voxelotor (Oxybryta) along with hydroxyurea (average MCV 90–100 fL) prior to the start of the infusions (which were continued during the infusions), along with as needed oral hydromorphone (Dilaudid) for pain. However, Patient 1 was not compliant with these medications. Patient 2’s medication regimen included crizanlizumab and voxelotor along with hydroxyurea (average MCV >110 fL), which the patient had been compliant with prior to starting the infusions, along with as needed hydrocodone/acetaminophen for pain. Both patients were continued on their preventative novel SCD regimens throughout the infusion sessions. Neither patient had prior history of hyperhemolysis syndrome; however, both were affected by the shortage of blood products available for transfusion in the height of the COVID-19 pandemic. In addition, Patient 2 refused prophylactic exchange transfusions due to reluctance about the placement of a central line. The number of VOEs were reported according to the total number of ED/UC visits and hospital admissions specifically for VOEs. Both patients had a history of frequent VOEs in the past with Patient 1 having 8 visits to the ED/UC and 9 admissions in the year prior to Month 0 (the start of as needed infusions) and Patient 2 having 21 ED/UC visits and 3 admissions in the year prior to Month 0.
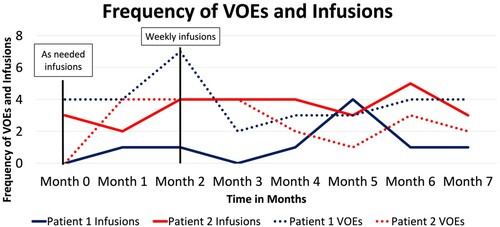
With the goal of decreasing the frequency of VOEs, Patients 1 and 2 were started on as needed IV hydration and IV opioid sessions starting from Month 0, followed by weekly scheduled sessions starting in Month 2. Treatment sessions consisted of 1 L of IV normal saline (NS) with 0.5–1 mg of IV hydromorphone. Patients were able to receive an additional dose of hydromorphone and acetaminophen as needed. Patient 1 had VOEs 2–7 times per month and missed several appointments for the scheduled weekly infusion sessions. She only presented to her weekly scheduled appointments regularly in Month 5. On the other hand, Patient 2 had between 0 and 4 VOEs per month and regularly presented for the scheduled sessions weekly in Months 2, 3, 4, and 6. Overall, Patient 2 received more scheduled weekly infusions compared to Patient 1, and this was associated with reduced frequency of VOEs (see ).
Figure 1. Graph showing the frequency of infusions for Patient 1 (blue solid line), the frequency of VOEs in each month for Patient 1 (blue dotted line), the frequency of infusions for Patient 2 (red solid line), and the frequency of VOEs in each month for Patient 2 (red dotted line). Both Patient 1 and 2 were started on as needed infusions in Month 0 and scheduled for weekly infusions in Month 2. VOE: vaso-occlusive episodes.
Discussion
Recurrent VOEs in patients with SCD are linked to early mortality and financial hardship, which negatively impact quality of life [Citation1,Citation9]. Given a longitudinal medical burden and multifactorial processes underlying VOEs, novel strategies and regimens have been innovated to reduce the occurrence of VOEs. Hydroxyurea was one of the first treatments approved to reduce VOEs in the multicenter MSH study, and prophylactic blood transfusion is commonly used as a preventative measure in addition to hydroxyurea [Citation10]. In the pediatric setting, several landmark randomized control trials have demonstrated the efficacy of chronic blood transfusions and hydroxyurea for secondary stroke prevention [Citation11–15]. However, the use of chronic blood transfusions outside of this indication are limited to observational studies and expert opinion. Recently, the use of L-glutamine was shown to result in reduced VOEs, along with a possible reduction in the frequency of VOEs [Citation16]. Other novel treatment regimens include voxelotor, a hemoglobin S (HgbS) polymerization inhibitor, crizanlizumab, a humanized IgG2 Kappa monoclonal antibody which binds to P-selectin, and fetal hemoglobin inducers [Citation1,Citation17]. While promising, larger randomized control studies are warranted to compare the effectiveness of each treatment options in reducing the frequency of VOEs.
Recently, the use of SCICs has been shown to improve patient outcomes and reduce hospital admission rates [Citation18]. Lanzkron et al demonstrated a statistically significant decrease in the rate of hospital admissions for VOEs per month after the use of an SCIC. The infusion clinic utilized IV pain medications along with IV hydration to prevent VOEs in patients with SCD. Interestingly, the ESCAPED trial analyzed the clinical outcomes between VOEs treated in a specialty infusion clinic versus the ED and reported significant reduction in VOEs managed at specialty infusion clinics (NCT02411396). Specifically, patients who were closely followed in the infusion clinics had a relative risk reduction of 75% for admission compared to those who received initial care in the ED (P < 0.001) [Citation18].
Similar findings were seen in our patients, especially in Patient 2. Prior to the initiation of the outpatient infusion protocol, both patients initially had similar frequencies of VOEs. There was a significant decrease in the frequency of VOEs in the months associated with compliance to weekly infusions. For example, Patient 2 had four episodes in Month 2 down to one VOE in Month 5 after being compliant with weekly infusions in Months 2–4. Conversely, Patient 1 had an unchanged frequency of VOEs. Notably, Patient 2 reported subjective improvement of symptoms related to VOEs and recognized this improvement in the frequency of VOEs after the outpatient protocol was initiated. Thus, both objective and patient-reported data are encouraging.
While our data shows the outpatient infusion clinic sessions were key in reducing the frequency of VOEs, the specific factors contributing to its efficacy need to be further validated. Both Patient 1 and 2 were on medications currently approved for preventative purposes in SCD; however, compliance was an issue. Clinical trials have demonstrated improvement in the frequency of VOEs with L-glutamine and crizanlizumab, and though not correlated with reduced VOEs, voxelotor showed improved Hgb levels and decreased markers of hemolysis [Citation19–21]. Thus, the individual and combinatorial role of the medications and their combination with IV infusions are areas requiring further study. Unfortunately, patients may not be amenable to taking hydroxyurea and other approved regimens, and in such patients, IV infusions may be the only available option. Thus, the IV infusion regimen is a promising prophylactic measure in patients who are noncompliant to medications such as hydroxyurea, crizanlizumab, and voxelotor.
One major contributor to the findings may be IV hydration. In general, patients with SCD are at risk for dehydration due to defects in the concentrating mechanism of the renal system and decreased oral intake during severe VOEs, which ultimately worsens RBC dehydration, further exacerbating RBC sickling [Citation4]. As a result, the current standard for acute management of VOEs in patients with SCD includes IV hydration therapy [Citation4]. This decreases the rate of HgbS polymerization by decreasing plasma osmolality, allowing free water and electrolytes to remain intracellularly, thus maintaining cellular integrity [Citation22]. There are benefits to utilizing hypotonic solutions compared to the more commonly used NS, but there is need for further studies to guide clinical practice [Citation22,Citation23]. Yet, there is no evidence-based guideline on the rate or quantity of fluid, such as bolus versus continuous fluid [Citation1]. Current ASH guidelines do not recommend for or against IV fluids as a standard preventative component for patients with SCD; however, in cases of clinical dehydration leading to or contributing to VOEs, IV fluids are warranted [Citation4]. Still, it is within reason to assume that IV hydration is an integral part of the infusion protocol of SCICs in treating and preventing VOEs, especially in light of the effect of dehydration in the development of sickled RBCs and the data presented in the ESCAPED trial [Citation24]. In addition, the co-administration of IV pain medications with hydration may also play a role in treating and preventing VOEs, although likely to a lesser extent.
Though the findings are promising, this study has limitations. Though the IV fluid infusions and scheduled opioids were well-tolerated without immediate side-effects, there is need for further study on the potential for developing opioid tolerance and the safe use of IV fluids. For example, IV hydration should be utilized judiciously to minimize adverse effects associated with fluid overload, and as such, there is need for developing protocols for the amount and frequency of IV fluids tailored to each patient [Citation24]. In addition, both patients had previous exposure to as needed opioids for pain control before the infusions; however, for patients who are opioid-naïve, care must be taken to monitor for side effects. On the other hand, patients may develop analgesic tolerance to opioids with long-term use [Citation25]. At this time, the use of pharmacotherapy to prevent and treat VOEs is not standardized [Citation9]. Thus, protocols for safe use of opioids in infusion clinics and the expansion of infusions to include alternative analgesic pharmacotherapies are needed. Lastly, the sample size for this current study is limited. Given the encouraging findings, further studies involving larger samples are warranted to study the effect of IV infusion protocols and the association with frequency of VOEs.
Conclusion
The findings of this study are promising and suggest that SCICs may reduce VOEs in patients with SCD. Our infusion protocol, consisting of weekly IV fluids and IV opioid administration, led to reduction in the frequency of these episodes. The use of infusion clinics can be beneficial for the prevention of VOEs and has the potential to contribute to the improvement of quality of life in patients with SCD in addition to decreasing the cost of health care related to VOEs and complications from chronic blood transfusions. In the future, related quality improvement projects should continue to focus on further development of SCICs and identification of factors helpful in preventing VOEs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Won Jin Jeon
Dr. Won Jin Jeon is a physician in the Internal Medicine program at Loma Linda University with plans to pursue the subspecialty of Hematology/ Oncology.
Bowon Joung
Dr. Bowon Joung is a physician in the Internal Medicine program at Loma Linda University with plans to pursue the subspecialty of Hematology/ Oncology.
Jin Hyun Moon
Dr. Jin Hyun Moon is a physician in the Internal Medicine program at Loma Linda University with plans to pursue the subspecialty of Hematology/ Oncology.
Christopher Hino
Dr. Christopher Hino is a physician in the Internal Medicine program at Loma Linda University with plans to pursue the subspecialty of Hematology/ Oncology.
Daniel Park
Dr. Daniel Park is a physician in the Internal Medicine program at the University of California San Franciso-Fresno who plans to pursue Hematology/ Oncology.
Bryan Pham
Dr. Bryan Pham is a physician in the Internal Medicine program at Loma Linda University with plans to pursue the subspecialty of Hematology/ Oncology.
Dan Ran Castillo
Dr. Dan Ran Castillo is a physician in the Hematology/ Oncology program at Loma Linda University with passion for research and resident mentorship.
Esther Chong
Dr. Esther Chong is a physician in the Hematology/ Oncology program at Loma Linda University with passion for research and resident mentorship.
Simmer Kaur
Dr. Simmer Kaur is a physician in the Hematology/ Oncology program at Loma Linda University with passion for research and resident mentorship.
Huynh Cao
Dr. Huynh Cao is a core faculty of Hematology/ Oncology at Loma Linda University who is active in student/ resident mentorship, clinical and pre-clinical research, and numerous publications on various topics in the field of Hematology/ Oncology.
References
- Okomo U, Meremikwu MM. Fluid replacement therapy for acute episodes of pain in people with sickle cell disease. Cochrane Database Syst Rev. 2021;2021(7):CD005406. doi:10.1002/14651858.CD005406.pub5
- Tanhehco YC, Shi PA, Schwartz J. Transfusion therapy in sickle cell disease. Ann Blood. 2022;7(0):9. doi:10.21037/aob-21-67
- Peslak SA, Akins AB, Foxwell AM, et al. A novel, effective, and efficient strategy for treating sickle cell vaso-occlusive events in the infusion center setting. Blood Adv. 2023;410:410–413. doi:10.1182/bloodadvances.2022007307
- Gaartman AE, Sayedi AK, Gerritsma JJ, et al. Fluid overload due to intravenous fluid therapy for vaso-occlusive crisis in sickle cell disease: incidence and risk factors. Br J Haematol. 2021;194(5):899–907. doi:10.1111/bjh.17696
- Howard J. Sickle cell disease: when and how to transfuse. Hematology Am Soc Hematol Educ Program. 2016;2016(1):625–631. doi:10.1182/asheducation-2016.1.625
- Brandow AM, Carroll CP, Creary S, et al. American Society of Hematology 2020 guidelines for sickle cell disease: management of acute and chronic pain. Blood Adv. 2020;4(12):2656–2701. doi:10.1182/bloodadvances.2020001851.
- McGann PT, Weyand AC. Lessons learned from the COVID-19 pandemic blood supply crisis. J Hosp Med. 2022;17(7):574–576. doi:10.1002/jhm.12843
- Banks M, Shikle J. Hyperhemolysis syndrome in patients with sickle cell disease. Arch Pathol Lab Med. 2018;142(11):1425–1427. doi:10.5858/arpa.2017-0251-RS
- Arzoun H, Srinivasan M, Sahib I, et al. Opioid use in patients with sickle cell disease during a vaso-occlusive crisis: a systematic review. Cureus. 2022;14(1):e21473. doi:10.7759/cureus.21473
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the multicenter study of hydroxyurea in sickle cell anemia. N Engl J Med. 1995;332(20):1317–1322. doi:10.1056/NEJM199505183322001
- Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11. doi:10.1056/NEJMoa050460.
- Adams RJ, Brambilla D. Optimizing primary stroke prevention in sickle cell anemia (STOP 2) trial investigators. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353(26):2769–2778.
- Ware RE, Helms RW. Stroke With transfusions changing to hydroxyurea (SWiTCH). Blood. 2012;119(17):3925–3932. doi:10.1182/blood-2011-11-392340
- DeBaun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med. 2014;371(8):699–710. doi:10.1056/NEJMoa1401731
- Ware RE, Davis BR, Schultz WH, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial Doppler flow velocities in children with sickle cell anaemia—TCD with transfusions changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet. 2016;387(10019):661–670. doi:10.1016/S0140-6736(15)01041-7
- Lanzkron S, Carroll CP, Hill P, et al. Impact of a dedicated infusion clinic for acute management of adults with sickle cell pain crisis. Am J Hematol. 2015;90(5):376–380. doi:10.1002/ajh.23961v
- Dick MH, Abdelgadir A, Kulkarni VV, et al. Comparing the safety and efficacy of L-glutamine, voxelotor, and crizanlizumab for reducing the frequency of vaso-occlusive crisis in sickle cell disease: a systematic review. Cureus. 2022;14(5):e24920. doi:10.7759/cureus.24920
- Lanzkron S, Little J, Wang H, et al. Treatment of acute pain in adults with sickle cell disease in an infusion center versus the emergency department: a multicenter prospective cohort study. Ann Intern Med. 2021;174(9):1207–1213. doi:10.7326/M20-7171
- Salinas Cisneros G, Thein SL. Recent advances in the treatment of sickle cell disease. Front Physiol. 2020;11:435. Published 2020 May 20. doi:10.3389/fphys.2020.00435
- Ali MA, Ahmad A, Chaudry H, et al. Efficacy and safety of recently approved drugs for sickle cell disease: a review of clinical trials. Exp Hematol. 2020;92:11–18.e1. doi:10.1016/j.exphem.2020.08.008
- Howard J, Ataga KI, Brown RC, et al. Voxelotor in adolescents and adults with sickle cell disease (HOPE): long-term follow-up results of an international, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Haematol. 2021;8(5):e323–e333. doi:10.1016/S2352-3026(21)00059-4.
- Purnell MC, Rayborn M. Novel hydration and nutritional strategies for sickle cell disease. EJHaem. 2020;1(1):230–234. doi:10.1002/jha2.9
- Carden MA, Fay ME, Lu X, et al. Extracellular fluid tonicity impacts sickle red blood cell deformability and adhesion. Blood. 2017;130(24):2654–2663. doi:10.1182/blood-2017-04-780635
- Gaut D, Jones J, Chen C, et al. Outcomes related to intravenous fluid administration in sickle cell patients during vaso-occlusive crisis. Ann Hematol. 2020;99(6):1217–1223. doi:10.1007/s00277-020-04050-1
- Mercadante S, Arcuri E, Santoni A. Opioid-induced tolerance and hyperalgesia. CNS Drugs. 2019;33(10):943–955. doi:10.1007/s40263-019-00660-0
