ABSTRACT
Objective
To analyze the effect of granulocyte colony-stimulating factor (G-CSF) on outcomes in patients with acute myeloid leukemia (AML).
Methods
A total of 526 patients with AML in the Haematology Department were enrolled. They were divided into a G-CSF treatment group and a no G-CSF group according to whether G-CSF was administered in the induction chemotherapy period, with 355 cases in the G-CSF group and 171 cases in the no G-CSF group. Cox regression analysis and Kaplan-Meier curve analysis were used to analyze the effect of G-CSF on the first complete remission (CR1) phase and overall survival (OS). In addition, further analysis was performed based on an initial white blood cell count of 50 * 10^9/L.
Results
The application of G-CSF significantly shortened the CR1 phase and OS in patients with high leukocytes.
Conclusions
G/GM-CSF should be used with caution in patients with AML, especially those with high leukocytes.
Introduction
Granulocyte colony-stimulating factor (G-CSF) is a very important cytokine in vivo; it mediates its effects by binding to a special homodimer receptor, G-CSFR, activating the complex signal transduction system, which includes the regulation of the proliferation, differentiation and survival of granulocyte cells, stimulating the secretion of blood vessels in granulocytes, mobilizing bone marrow stem cells to peripheral blood and inducing T cells to undergo immune tolerance in stem cell grafts [Citation1]. The use of G-CSF can promote the recovery of granulocytes after chemotherapy in cancer patients, shorten the duration of granulocytopenia, and reduce the incidence of infection and related mortality. G-CSF can also drive malignant cells into the cell cycle, increasing sensitivity to cell cycle-specific chemotherapy, and it is widely used in chemotherapy among elderly, hypoplastic acute myeloid leukemia (AML) patients [Citation2,Citation3].
However, some myeloid leukemia cells and leukemic cell lines express the G-CSF receptor [Citation4], and they can be induced to proliferate upon G-CSF application [Citation5], which may have an impact on response and prognosis. We retrospectively analyzed the effects of G-CSF on outcomes in 526 anthracycline-based induction chemotherapy-treated patients with non-M3 acute AML.
Materials and methods
Statement of ethics
This study is approved by the Ethics Committee of Fujian Institute of Hematology, Fujian Provincial Key Laboratory on Hematology, Fujian Medical University Union Hospital. Signed informed consent were also obtained from all participants.
Participants
Patients with AML newly diagnosed who were enrolled in the Hematology Department were treated in an alternating regimen, with a main scheme of daunorubicin + cytarabine (DA), homoharringtonine + cytarabine (HA), mitoxantrone and cytarabine (MA) or pirarubicin + cytarabine (TA).
Treatment
For the G/GM-CSF treatment group, when the white blood cell (WBC) count was <1.0 * 109/L or the absolute neutrophil count (ANC) was <0.5 * 109/L, we used G/GM-CSF (1.5–6 µg/kg/d) administered via subcutaneous injection once daily, which was generally discontinued when the WBC count was >3.0 *109/L or the ANC was >1.5*109/L. For the untreated group, when the WBC count was <1.0 * 109/L or the ANC was <0.5 * 109/L in patients after induction chemotherapy, only routine treatment was performed.
Analysis index and reference [Citation6]: The first complete remission (CR1) period, the survival period and so on were analyzed.
Statistical analysis
Statistical analysis was performed using SPSS 17 statistical software. Continuous variables are expressed as means with standard deviations or medians with interquartile ranges, as appropriate. Categorical data are presented as proportions. Cox multivariate regression analysis was used for analysis of CR1 and OS, and Kaplan-Meier analysis was used to determine the difference in the average OS and CR1 for G-CSF and the untreated group. Pearson correlation analysis was calculated for the relation of G-CSF dosage and CR1 stage and OS. Two-tailed P values < .05 were considered statistically significant.
Results
Patient characteristics
A total of 526 hospitalized, non-M3 AML hematology patients in our hospital were enrolled; they were 14–90 years old, with a median age of 40 years, and included 9 M0 cases, 34 M1 cases, 151 M2 cases, 15 M4 cases, 292 M5 cases, 14 M6 cases, 2 M7 cases, and 9 cases with no subgroup. There was no significant difference in age, sex, morphology, number of initial leukocytes, hemoglobin, platelets, bone marrow hyperplasia, and percentage of bone marrow blasts between the G-CSF and untreated groups ().
Table 1. Baseline characteristics of patients.
Effect of G/GM-CSF on CR1 stage in AML patients
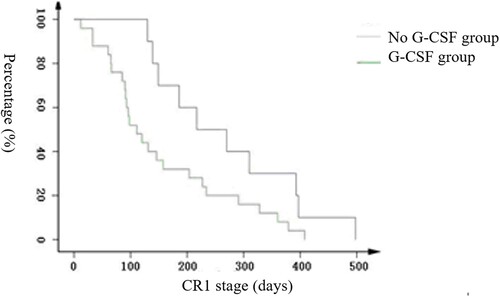
Age, sex, AML subtype, initial bone marrow hyperplasia situation, and different chemotherapy regimens had no effect on CR1 stage (p > 0.05), whereas an initial high WBC count shortened CR1 stage (p < 0.05) as did the use of G-CSF (p < 0.05, ). After application of G-CSF in the AML patient group, the average CR1 stage was 236 days, 95% confidence interval (201, 270), which was lower than the average CR1 stage of the untreated group, which was 292 days, 95% confidence interval (256,328); however, there was no statistically significant difference between the two groups (see Figure S1, p = 0.139).
Table 2. Cox regression analysis of CR1 stage in AML patients.
An initial WBC count of 50 * 109/L was used for layered analysis, which suggested that the CR1 stage was particularly significantly different in the G-CSF-treated group and untreated group for those with a high WBC. The difference was statistically significant (as shown in ), as seen by the Kaplan-Meier graph; CR1 decreased significantly faster in the G-CSF group than the untreated group. The average CR1 period for the high-leukocyte group was as follows: 157 days in the G-CSF group, 95% confidence interval (111, 202), and 264 days in the untreated group, 95% confidence interval (177, 352), log rank: 3.976, p = 0.046.
Effect of G/GM-CSF on survival stage in AML patients
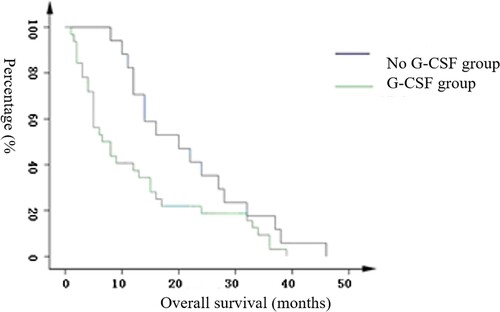
Age, gender, AML subtype, initial bone marrow hyperplasia situation, and different chemotherapy regimens had no effect on the survival stage (p > 0.05), whereas an high initial WBC count shortened the survival stage (p < 0.05) as did the use of G-CSF (p < 0.05, ). The survival time for AML patients with G-CSF was 19.1 months, 95% confidence interval (15.3, 22.8), which was lower than the 22.7 months of the untreated group, 95% confidence interval (19.8, 25.7); however, there was no statistically significant difference between the two groups (see Figure S2), log rank: 1.551, p = 0.213.
Table 3. Cox regression analysis of survival stage in AML patients.
In the layered analysis using an initial WBC count of 50 * 109/L, the survival stage was particularly significantly different in the G-CSF-treated group and untreated group for those with a high WBC (see ). Based on the Kaplan-Meier graph, the survival of the treatment group decreased significantly faster than survival in the untreated group. In the analysis of the overall high-leukocyte group, the average survival period for the treatment group was 12.8 months, 95% confidence interval (8.5,17.0), and for the untreated group, it was 21.9 months, 95% confidence interval (16.4, 27.2), (p = 0.040).
Relationship between G-CSF dosage and CR1 phase and survival stage in AML patients during the induction period
The above results show that the use of G-CSF could shorten the CR1 and survival stages of AML patients. Therefore, Pearson’s test was used to analyze the relationship between G-CSF dosage in AML patients and CR1 and survival stage after induction therapy. The results show that there is no relationship between the dosage of G-CSF and CR1 and survival stage (R −0.149, −0.008; p = 0.252,0.419). In addition, correlation analysis of CR1 and patient survival showed a positive correlation between the two (r = 0.394, p = 0.001).
Discussion
Current standard induction therapy for AML includes a 7-day infusion of cytarabine plus 3 days of daunorubicin or idarubicin [Citation7]. With the application of combination chemotherapy, the remission rate and long-term survival rate of leukemia are greatly improved, but intense chemotherapy-induced myelosuppression and neutropenia, often combined with various infections, especially certain fatal infections, are potential harmful side effects [Citation8]. These side effects are the main cause of early death in patients with leukemia [Citation9].
Based on relevant studies [Citation10], the American Society of Clinical Oncology (ASCO) and the European Organisation for Research and Treatment of Cancer (EORTC) suggest that G-CSF can be used prophylactically in patients with tumors when the risk of severe neutropenia is estimated to be over 20% [Citation11].
During chemotherapy-induced neutropenia, rational use of G-CSF can shorten the duration of neutropenia and reduce the incidence of severe infections and infection-related mortality [Citation12]. The use of G-CSF as a chemosensitizer in AML has been widely recognized and is supported by a large body of laboratory and clinical evidence [Citation13–15].
However, the safety of G-CSF in patients with AML during chemotherapy-induced bone marrow suppression remains controversial. Liu et al.’s [Citation16] retrospective analysis of the effect of G-CSF on the prognosis of 171 patients with acute leukemia (AL) suggested that there was no significant difference in the remission rate and CR rate between the G-CSF treatment group and the control group, which is consistent with reports at home and abroad. The use of G-CSF shortens the CR stage and survival time of AML patients but not ALL patients.
There are few reports on the effect of G-CSF on the prognosis of patients with high-leukocyte AML. Therefore, this study expanded the sample size of AML patients and performed a subgroup analysis on WBC count to understand the influence of G-CSF in patients with high-leukocyte AML.
Several trials have investigated the prognostic impact of G-CSF on AML, but the results have varied due to the diversity of AML patients and the differences in test methods. Wheatley et al. [Citation17] prospectively investigated the role of G-CSF as supportive therapy following induction chemotherapy in patients with AML. The use of G-CSF shortened the duration of granulocyte deficiency after chemotherapy and reduced the mean number of hospitalization days. However, it did not affect the duration of hypothermia. There was no difference between the 5-year overall survival time and the disease-free survival time. Both the CR rate and overall survival time were worse with G-CSF in patients aged <40 years. Wolfgang [Citation18] proposed that the use of G-CSF following chemotherapy in AML patients who are older than 60 years can increase the tolerability of chemotherapy and reduce the duration of infection, but there are no relevant data on the impact on overall survival. Ka-Won Kang et al. [Citation19] prospectively investigated the effects of G-CSF on the prognosis in 315 patients with non-M3 AML during the induction period. The results suggest that the recovery time of neutrophils and the duration of iron deficiency fever are shorter in the G-CSF group, which does not affect the incidence of infection, disease-free survival, and overall survival. Minakata et al. [Citation20] suggested that the increase in the number of immature cells in peripheral blood induced by G-CSF predicts a lower response rate in patients with AML. There was a case report [Citation21] on the use of G-CSF during neutropenia in M4 patients after induction of chemotherapy that showed evidence of bone marrow necrosis, which was considered to be associated with microvascular occlusion caused by increased cell proliferation stimulated by G-CSF.
Laboratory evidence suggests that G-CSF stimulates the proliferation of primitive leukemic cells in patients with AML [Citation22,Citation23] and inhibits cell apoptosis by the CXCR4/SDF-1α signaling pathway [Citation23], resulting in adverse effects on the remission rate and recurrence rate of AML.
A prospective, multicentre AML-BFM98 clinical study [Citation24] randomly detected the expression of G-CSFR in 50 out of 154 pediatric patients with standard risk and performed a 5-year survival analysis. The results showed an increased 5-year cumulative incidence of relapse in patients overexpressing G-CSFR isoform IV compared to patients with low-level isoform IV expression.
Our group used quantitative RT–PCR to detect the relative expression levels of G-CSFR IV/G-CSFR I isoforms in healthy and AL patients, and the results suggested that the relative expression level of G-CSFR IV/G-CSFR I is obviously elevated in AML patients compared with ALL patients and controls. Patients with relatively high G-CSFR IV/G-CSFR I expression in AML had lower clinical CR rates than those with low expression [Citation25].
In this study, when patients with AML were divided into a high-leukocyte group and a standard-risk group according to an initial WBC count of 50×10^9/L, the use of G-CSF significantly shortened the remission period and survival period in the high-leukocyte group. It is considered that in patients with high leukocytes, the expression of the G-CSF receptor is higher in immature bone marrow cells, and the relative expression level of G-CSF-R IV/G-CSF-R I is high. Use of G-CSF can stimulate more AML cells to proliferate and inhibit AML cell apoptosis. Therefore, in patients with AML, G-CSF should be used with caution, especially in patients with high leukocytes.
Author contributions
We declare that all the listed authors have participated actively in the study and all meet the requirements of the authorship. Dr. PW designed the study and prepared the paper, Dr. SY managed the literature searches and analyses, Dr. SJX undertook the literature research, Dr. SXZ contributed to the clinical studies, Dr. YW edited the paper, Dr. YZC contributed to the correspondence and paper review. All authors reviewed the manuscript.
Supplemental Material
Download MS Word (222 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Bendall LJ, Bradstock KF. G-CSF: from granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014;25:355–367.
- Law KB, Chang KM, Hamzah NA, et al. Fludarabine, high dose cytarabine and granulocyte colony-stimulating factor (FLAG) as consolidation chemotherapy in older patients with acute myeloid leukemia: a retrospective cohort study. Indian J Hematol Blood Transfus. 2017;33:483–491.
- Saini L, Brandwein J, Turner R, et al. The fludarabine, cytarabine, and granulocyte colony-stimulating factor (FLAG) chemotherapy regimen is an alternative to anthracycline-based therapy for the treatment of acute myeloid leukemia for patients with pre-existing cardiac disease. Eur J Haematol. 2016;97:471–478.
- Liongue C, Wright C, Russell AP, et al. Granulocyte colony-stimulating factor receptor: stimulating granulopoiesis and much more. Int J Biochem Cell Biol. 2009;41:2372–2375.
- Curtis DJ, Metcalf D, Alexander B, et al. Leukemic cells from murine myeloid leukemia display an intrinsic ability for autonomous proliferation. Exp Hematol. 2000;28:36–45.
- Zhang ZN, editor. Blood disease diagnosis and efficacy standard. 3rd ed. Beijing: Science Press; 2007, p. 131–134.
- Teuffel O, Leibundgut K, Lehrnbecher T, et al. Anthracyclines during induction therapy in acute myeloid leukaemia: a systematic review and meta-analysis. Br J Haematol. 2013;161:192–203.
- Valcarcel D, Montesinos P, Sanchez-Ortega I, et al. A scoring system to predict the risk of death during induction with anthracycline plus cytarabine-based chemotherapy in patients with de novo acute myeloid leukemia. Cancer. 2012;118:410–417.
- Rubnitz JE, Lensing S, Zhou Y, et al. Death during induction therapy and first remission of acute leukemia in childhood: the St. Jude experience. Cancer. 2004;101:1677–1684.
- Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33:3199–3212.
- Holmes FA, O'Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002;20:727–731.
- Beksac M, Ali R, Ozcelik T, et al. Short and long term effects of granulocyte colony-stimulating factor during induction therapy in acute myeloid leukemia patients younger than 65: results of a randomized multicenter phase III trial. Leuk Res. 2011;35:340–345.
- Nomdedeu M, Lara-Castillo MC, Etxabe A, et al. Treatment with G-CSF reduces acute myeloid leukemia blast viability in the presence of bone marrow stroma. Cancer Cell Int. 2015;15:122.
- Martinez-Cuadron D, Boluda B, Martinez P, et al. A phase I-II study of plerixafor in combination with fludarabine, idarubicin, cytarabine, and G-CSF (PLERIFLAG regimen) for the treatment of patients with the first early-relapsed or refractory acute myeloid leukemia. Ann Hematol. 2018;97(5).
- Uy GL, Rettig MP, Stone RM, et al. A phase 1/2 study of chemosensitization with plerixafor plus G-CSF in relapsed or refractory acute myeloid leukemia. Blood Cancer J. 2017;7:e542.
- Liu XM, Chen YZ, et al. The potential prognostic influence of granulocyte-colony stimulating factor in acute leukemia. Chin J Intern Med. 2005;44(7):518–521.
- Wheatley K, Goldstone AH, Littlewood T, et al. Randomized placebo-controlled trial of granulocyte colony stimulating factor (G-CSF) as supportive care after induction chemotherapy in adult patients with acute myeloid leukaemia: a study of the United Kingdom Medical Research Council Adult Leukaemia Working Party. Br J Haematol. 2009;146:54–63.
- Sperr WR, Herndlhofer S, Gleixner K, et al. Intensive consolidation with G-CSF support: tolerability, safety, reduced hospitalization, and efficacy in acute myeloid leukemia patients >/=60 years. Am J Hematol. 2017;92:E567–E574.
- Kang KW, Kim DS, Lee SR, et al. Effect of granulocyte colony-stimulating factor on outcomes in patients with non-M3 acute myelogenous leukemia treated with anthracycline-based induction (7+3 regimen) chemotherapies. Leuk Res. 2017;57:1–8.
- Minakata D, Fujiwara SI, Ikeda T, et al. Relationship between white blood cell count elevation and clinical response after G-CSF priming chemotherapy for acute myeloid leukemia. Int J Hematol. 2017;106:411–417.
- Osuorji I, Goldman L. G-CSF-associated bone marrow necrosis in AML after induction chemotherapy. Case Rep Hematol. 2012;2012:314278.
- Dale DC, Cottle TE, Fier CJ, et al. Severe chronic neutropenia: treatment and follow-up of patients in the Severe Chronic Neutropenia International Registry. Am J Hematol. 2003;72:82–93.
- Kutlay S, Beksac M, Dalva K, et al. The detection of flow cytometric G-CSF receptor expression and it's effect on therapy in acute myeloid leukemia. Leuk Lymphoma. 2003;44:791–795.
- Ehlers S, Herbst C, Zimmermann M, et al. Granulocyte colony-stimulating factor (G-CSF) treatment of childhood acute myeloid leukemias that overexpress the differentiation-defective G-CSF receptor isoform IV is associated with a higher incidence of relapse. J Clin Oncol. 2010;28:2591–2597.
- Wu Y, Chen YY, Chen YZ. Expression of G-CSFRIV isoform in adult acute myeloid leukemia and its clinical significance. J Exp Hematol. 2014;22(4):899–902.