ABSTRACT
Invasive pulmonary aspergillosis (IPA) is an infectious disease with a high mortality rate due to diagnostic difficulties associated with the lack of a typical clinical presentation and the inadequacy of the available laboratory testing methods. Nanopore targeted sequencing (NTS) is an alternative method of broad-based pathogen discovery, associated with rapid turnaround and high accuracy. This case report presents a patient with IPA and acute promyelocytic leukemia, diagnosed using the NTS method, which detected Aspergillus flavus in the patient's blood and pleural fluid. The patient was treated effectively with antifungal therapy. Early diagnosis of IPA improved long-term patient prognosis and quality of life.
1. Introduction
Invasive pulmonary aspergillosis (IPA) is an infectious disease caused by the growth of Aspergillus hyphae, invading the lung parenchyma. Prolonged profound neutropenia, hematological malignancies, hematopoietic stem cell or solid organ transplantation, and long-term use of high-dose glucocorticoid or immunosuppressive agents, and inherited or acquired immunodeficiency status are risk factors for IPA infection [Citation1–3]. Early diagnosis and timely treatment are key to good patient outcomes. However, due to the lack of typical clinical manifestations of the disease and various deficiencies in the current main laboratory testing methods, there is an urgent need for a rapid and accurate detection technology to assist in the diagnosis of IPA. This paper describes a case of acute myeloid leukemia with pulmonary infection and large pleural effusion, in which the pathogen Aspergillus flavus was detected by nanopore targeted sequencing (NTS). Based on the NTS results, we performed an effective antifungal therapy and achieved good results.
2. Case description
A 53-year-old female was admitted to our hospital with a 9-day history of fever, and new-onset typical weakness and dizziness. The patient reported poor sleep and noted a decrease in appetite, with weight loss of 4 kilo within 1 month. The patient’s past medical history revealed pulmonary nodules; she developed tinnitus 2 months before admission, which was not examined. At admission, the marrow aspirate indicated signs of acute promyelocytic leukemia, and the patient was diagnosed with neutropenia with fever. Induction therapy (all-trans retinoic acid + arsenite + darubicin) [Citation4] was initiated.
2.1. Treatment
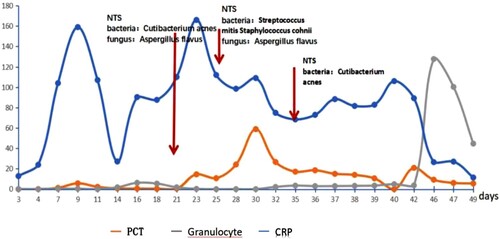
A day after admission, the patient's neutrophil count dropped to 0.07 g/L. Her highest temperature was 38.7°C, and C-reactive protein (CRP) and procalcitonin (PCT) levels were significantly increased, with a maximum CRP level of 159 mg/L (). The sputum culture, blood culture, and (1-3)-b-d-glucan (BDG) + Galactomannan (GM) experiments were negative. After 7 days of anti-infective (voriconazole 200 mg(IV, twice a day) + ceftazidime tazotan sodium 2 g(iv, once every 8 h) + linezolid glucose 600 mg(iv, once every 12 h)) [Citation5] and symptomatic treatment, the patient still experienced fever, diarrhea, and a rapid increase in CRP levels up to 159 mg/L.
At 19 days after admission, a chest computed tomography (CT) scan (A) revealed multiple patchy solid shadows, ground glass shadows, and infectious lesions in the right lung. Blood culture findings were negative. Given all test findings to-date, pulmonary bacterial and fungal co-infection was considered; however, anti-infection therapy (amphotericin B (iv 3.0 4.0 mg/kg/day)+ cefoperazone sodium sulbactam sodium + tetracycline) [Citation6] did not alleviate the patient’s symptoms.
Figure 2. Chest computed tomography findings of a patient (A) 19, (B) 28, (C) 35, (D) 48 days after admission.
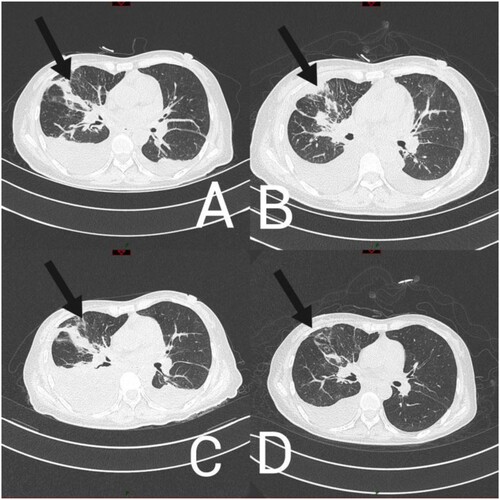
Subsequently, 21 days after admission, a blood sample was sent for testing, using NTS, which detected 1066 sequencing reads of Aspergillus flavus and 24 sequencing reads of Propionibacterium acnes. Based on these findings, the treatment was adjusted to micafungin + amphotericin b + cefoperazone sodium and sulbactam sodium + tigecycline [Citation7]. At 24 days after admission, a sample of pleural effusion was sent for testing using NTS, which detected 17 sequence reads of Aspergillus and 253 sequence reads of Streptococcus mitis. Considering the patient’s clinical symptoms, chest CT imaging findings, failure to respond to antimicrobials, and NTS results,we diagnose probable IPA [Citation8]. After the addition to micafungin + amphotericin B antifungal therapy, which was administered for 4 days, the patient's symptoms were relieved (A and 2B); however, 33 days after admission, the patient presented with fever.
A chest CT scan (C) indicated increased levels of pleural fluid; a sample of pleural effusion was examined with NTS, revealing 271 sequence reads of Micromonas. No sequence reads of Aspergillus were detected, suggesting that the anti-fungal treatment was effective. Micromonas is a gram-positive anaerobic bacterium, which is common in oral and systemic purulent infections [Citation9]. The subsequent antibacterial regimen included amikacin + cefoperazone sodium and sulbactam sodium + tigecycline. At 48 days after admission, the patient had no obvious symptoms of a pulmonary infection, and chest CT (D) scan findings indicated that most of the pleural fluid had been absorbed. Before discharge, the patient’s laboratory test findings were: white blood cell count, 20.89 × 109 g/L; neutrophil count, 15.39 × 109 g/L; CRP levels, 11.4 mg/L; PCT levels, 5.57 g/L; these values were consistent with successful treatment.
Two months post-discharge, the patient received a new round of chemotherapy for leukemia. Physical examination revealed no obvious abnormalities. CT scan findings showed thickening of the texture of the right upper and middle lobes, and thickened interlobular space. A few strand-like high-density shadows were observed. Infectious lesions were considered; the lesions appeared absorbed and significantly improved.
2.2. Sample processing and DNA extraction
The collected clinical samples were sent to the laboratory for DNA extraction within 4 h. The pleural effusion sample was centrifuged at 20,000 g for 10 min; 200 L of sediment was used for DNA extraction. The blood was first centrifuged at 50 g for 10 min, and the supernatant and top sediment were collected and centrifuged at 12,000 rpm for 10 min. The last 200 L of sediment was used for DNA extraction. DNA extraction was performed, using a Sansure DNA Extraction Kit (S1006, Changsha, China).
2.3. Amplification and nanopore targeted sequencing
The universal primer 27F/1492R (for bacterial 16S rRNA gene) [Citation10], ITS1/ITS4 (for fungal ITS1/2) [Citation11] and Genus-specific primers MF/MR (for Mycobacterium spp. rpoB gene) [Citation12] were optimized and used in NTS;the details of primer design and PCR procedure are described elsewhere. The amplification products of 16S rRNA, ITS, and rpoB of the clinical sample and negative controls were pooled for the next sequencing library construction, using the 1D Ligation Kit (SQK-LSK109, Oxford Nanopore Technologies). Sequencing was performed using an R9.4 flow cell on the GridION x5. The whole sequencing process lasted 8 h, and the sequencing data were analyzed, using a real-time bioinformatics analysis pipeline.
2.4. Bioinformatics analysis pipeline and pathogen detection
Base calling and quality control of raw data sequencing were performed in a high accuracy mode, using Guppy (v. 3.1.5). Low-quality reads (Q score of <7 points) and undesired lengths (<200 or >2000 nt) were discarded. The clean reads were then mapped to the 16S rDNA, ITS, and rpoB reference gene databases, which were collected from the NCBI database. The E-value of BLAST was set to 1e-5, and the taxonomy of each read was assigned, according to the taxonomic information of the mapped subject reference with the highest identity and coverage. A consensus sequence of the reads assigned to the same species was generated by Medaka (v. 0.10.1), and the consensus was remapped to the reference database, and the species-level taxonomy of the mapped subject reference was detected as the final result.
3. Discussion
IPA is associated with a variety of bacterial infections. In such cases, despite the use of antibiotics, fever persists and may be the only clinical symptom of a fungal infection [Citation13]. The timely diagnosis of IPA is important for effective treatment, reducing the risk of antibiotic resistance and mortality. However, common pathogen detection methods have limitations. Galactomannan (GM) is a specific component of the cell wall of Aspergillus, relevant to the early diagnosis and treatment of IPA. Fluid-based GM detection (in particular, that of BAL) is associated with IPA diagnostic sensitivity higher than that associated with culture. However, the sensitivity of serum GM testing is significantly lower in non-neutropenic than in neutropenic patients, and serial screening is not recommended in patients on mold-active prophylaxis. In addition, (1-3)-b-d-glucan (BDG) is a component of the cell wall of many species and genera of fungi, released into body fluids when the fungus is infected. GM testing cannot distinguish between fungal species, so its use in IPA diagnosis is limited; however, when used in combination with GM or PCR, its specificity improves [Citation14,Citation15].
Blood culture can only identify some microorganisms. As the conditions under which Aspergillus grows differ greatly from those associated with bacteria, the capacity of blood culture techniques to identify fungi is limited, in particular, in the case of Aspergillus [Citation8].
Most PCR-based diagnostic methods require the design of specific primers for specific pathogens. Alternatively, specific probes with panfungal PCR can be used to identify fungal species in the tested samples, which limits the detection scope of a single test. Panfungal PCR combined with Sanger sequencing of PCR products may help identify fungal species. Clinical samples, in particular, those obtained from the respiratory tract, tend to be colonized by more than one fungus. Pathogenic fungi mixed with colonized fungi or real mixed fungal infections generate mixed PCR products, when using panfungal PCR, which cannot be resolved by Sanger sequencing. Consequently, we used the nanopore sequencer, which is a high-throughput sequencer, to analyze targeted fragments of all fungal marker genes in the sample, regardless of whether the fragment was a mixture or a single band [Citation1].
The library preparation process of mNGS requires a long sequencing cycle and adherence to strict requirements in the laboratory environment. Meanwhile, data analysis must be performed after the completion of sequencing. Bioinformatics analysis of NGS data is associated with challenges [Citation16,Citation17]. The NTS technology used in this case is extremely time-sensitive. First, it can reach the level of broad-spectrum pathogen identification consistent with that of mNGS technology; second, it can deliver preliminary and final results within 2 and 8 h, respectively [Citation18].
In the present case, we first suspected bacterial and fungal coinfection; however, the corresponding treatment was not effective. The NTS of blood samples detected Aspergillus flavus and several other pathogens, and that of pleural effusion samples confirmed this infection. The patient's symptoms improved after adjusting the antifungal regimen. Ten days thereafter, the patient presented with new-onset fever and dyspnea, and CT scan findings showed increased levels of pleural fluid. However, the second pleural effusion NTS presented no evidence of Aspergillus flavus. Adjustments to the antibacterial regimen improved the patient’s symptoms. Clinical practice guidelines suggest voriconazole as the primary treatment for most IPA cases [Citation1]. However, voriconazole showed poor efficacy in the present case. Finally, we combined micafungin and amphotericin B to achieve a better therapeutic effect [Citation13,Citation19].
4. Conclusion
This case report presented an acutely ill patient with a severe infection, precluding some types of diagnostic examinations (e.g.biopsy.) to confirm the pathogen. The patient did not respond well to the initial treatment, resulting in the use of NTS, which has helped in the diagnosis and treatment, achieving a good outcome. This approach may be used in other patients that present with similar characteristics. Voriconazole is the first-line treatment for IPA; however, in patients that show resistance to this treatment and in those that are prone to mixed infections, combined antifungal therapy can be used.
This case report has some strengths and limitations. In this study, the patient was treated with antibiotics prior to sampling, and the isolation and culture findings of other microorganisms detected by sequencing were negative, which may be related to the use of antibiotics. Given the patient’s presentation and her relatively poor overall condition, there were restrictions on the types of pathological examinations that could be performed. Consequently, the diagnosis remains unclear; however, the NTS findings were strongly indicative of IPA. The treatment based on these findings was effective and have saved the patient's life.
Ethics statement
The study involving human participants was reviewed and approved by the appropriate institutional department of the Department of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Written informed consent was obtained from the individual(s) for publication of potentially identifiable images or data included in this article.
Author contributions
QXH and YHW took the lead in drafting the manuscript and provided CT images. LHX and XL provided supervision and participated in the literature review and in drafting the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The author would like to acknowledge all who contributed in this case diagnosis, therapy, and decision-making. Acknowledgment is given to the Union Hospital of Huazhong University of Science and Technology, especially for the Department of Hematology.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All datasets generated for this study are included in the article/Supplementary Material/NCBI DRA database (https://www.ncbi.nlm.nih.gov/sra/PRJNA692057).
Additional information
Funding
References
- Patterson TF, Thompson III GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1–e60. doi:10.1093/cid/ciw326
- Baddley JW. Clinical risk factors for invasive aspergillosis. Med Mycol. 2011 Apr;49(Suppl 1):S7–S12. doi:10.3109/13693786.2010.505204
- Vandewoude K, Blot S, Benoit D, et al. Invasive aspergillosis in critically ill patients: analysis of risk factors for acquisition and mortality. Acta Clin Belg. 2004 Sep-Oct;59(5):251–257. doi:10.1179/acb.2004.037
- Iland HJ, Bradstock K, Supple SG, et al. Australasian Leukaemia and Lymphoma group. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012 Aug 23;120(8):1570–1580. doi:10.1182/blood-2012-02-410746
- Pizzo PA. Management of patients with fever and neutropenia through the Arc of time: a narrative review. Ann Intern Med. 2019 Mar 19;170(6):389–397. doi:10.7326/M18-3192
- Yoshida I, Saito AM, Tanaka S, et al. Intravenous itraconazole compared with liposomal amphotericin B as empirical antifungal therapy in patients with neutropaenia and persistent fever. Mycoses. 2020 Aug;63(8):794–801. doi:10.1111/myc.13100
- Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015 Jan 20;162(2):81–89. doi:10.7326/M13-2508
- Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2020 Sep 12;71(6):1367–1376. PMID: 31802125; PMCID: PMC7486838. doi:10.1093/cid/ciz1008
- Chamilos G, Luna M, Lewis RE, et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003). Haematologica. 2006 Jul;91(7):986–989. Epub 2006 Jun 1. PMID: 16757415.
- Calus ST, Ijaz UZ, Pinto AJ. NanoAmpli-Seq: a workflow for amplicon sequencing for mixed microbial communities on the nanopore sequencing platform. Gigascience. 2018 Dec 1;7(12):giy140. PMID: 30476081. doi:10.1093/gigascience/giy140
- Fujita SI, Senda Y, Nakaguchi S, et al. Multiplex PCR using internal transcribed spacer 1 and 2 regions for rapid detection and identification of yeast strains. J Clin Microbiol. 2001 Oct;39(10):3617–3622. PMID: 11574582. doi:10.1128/JCM.39.10.3617-3622.2001
- Kim BJ, Lee SH, Lyu MA, et al. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB). J Clin Microbiol. 1999 Jun;37(6):1714–1720. PMID: 10325313. doi:10.1128/JCM.37.6.1714-1720.1999
- Walsh TJ, Teppler H, Donowitz GR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med. 2004 Sep 30;351(14):1391–1402. doi:10.1056/NEJMoa040446
- Duarte RF, Sánchez-Ortega I, Cuesta I, et al. Serum galactomannan-based early detection of invasive aspergillosis in hematology patients receiving effective antimold prophylaxis. Clin Infect Dis. 2014 Dec 15;59(12):1696–1702. Epub 2014 Aug 27. PMID: 25165088. doi:10.1093/cid/ciu673
- Teering S, Verreth A, Peeters A, et al. Prognostic value of serum galactomannan in mixed ICU patients: a retrospective observational study. Anaesthesiol Intensive Ther. 2014 Jul-Aug;46(3):145–154. PMID: 25078766. doi:10.5603/AIT.2014.0027
- Russell JA, Campos B, Stone J, et al. Unbiased Strain-Typing of Arbovirus Directly from mosquitoes using nanopore sequencing: a field-forward biosurveillance protocol. Sci Rep. 2018 Apr 3;8(1):5417. PMID: 29615665. doi:10.1038/s41598-018-23641-7
- Petersen LM, Martin IW, Moschetti WE, et al. Third-Generation sequencing in the clinical laboratory: exploring the advantages and challenges of nanopore sequencing. J Clin Microbiol. 2019 Dec 23;58(1):e01315–19. doi:10.1128/JCM.01315-19
- van Dijk EL, Jaszczyszyn Y, Naquin D, et al. The third revolution in sequencing technology. Trends Genet. 2018 Sep;34(9):666–681. doi:10.1016/j.tig.2018.05.008
- Russo A, Tiseo G, Falcone M, et al. Pulmonary aspergillosis: an evolving challenge for diagnosis and treatment. Infect Dis Ther. 2020 Sep;9(3):511–524. doi:10.1007/s40121-020-00315-4