ABSTRACT
Objectives
This Japanese cross-sectional survey evaluated the symptoms, daily living activities, and treatment needs of patients with polycythemia vera (PV), as perceived by patients themselves and their physicians.
Methods
The study was conducted at 112 centers (March to July 2022) and included PV patients aged ≥20 years (n = 265) and their attending physicians (n = 151). The patient and physician questionnaires included 34 and 29 questions, respectively, to assess daily living, PV symptoms, treatment goals, and physician-patient communication.
Results
Concerning daily living (primary endpoint), work (13.2%), leisure activities (11.3%), and family life (9.6%) were most affected by PV symptoms. Patients aged <60 years more frequently reported an impact on daily living than patients aged ≥60 years. Some patients (30%) reported anxiety about their future condition. The most common symptoms were pruritus (13.6%) and fatigue (10.9%). Pruritus was ranked as the first treatment need for patients, while physicians ranked it fourth. Concerning treatment goals, physicians prioritized thrombosis/vascular event prevention, while patients prioritized delaying PV progression. Physicians were less satisfied with physician-patient communication than patients.
Conclusions
Patients’ daily living was largely affected by PV symptoms. There are differences in physician and patient perceptions of symptoms, daily living, and treatment needs in Japan.
Trial registration
UMIN Japan identifier: UMIN000047047.
Introduction
Polycythemia vera (PV) is a neoplastic disease of hematopoietic stem cells that is associated with a marked increase in erythrocyte counts, often accompanied by leukocytosis or thrombocytosis [Citation1]. The main symptoms of PV include fatigue (general malaise); pruritus; night sweats; decreased concentration and motivation; and splenomegaly-related symptoms, such as early satiety and abdominal discomfort and/or pain [Citation2]. PV is generally progressive, and the physical symptoms worsen with disease progression, adversely affecting patients’ daily living and employment.
The impact of PV symptoms on patients’ daily living has been extensively studied [Citation3–9]. For example, in the international myeloproliferative neoplasm (MPN) Landmark Survey, a notable proportion of patients (76%) reported that PV interfered with their daily living [Citation6]. In that study, ‘symptom improvement’ was the most important treatment goal for patients with PV, but ‘prevention of thrombosis’ was the most important treatment goal for physicians [Citation6]. In another study, the living with MPNs survey, 48% of patients experienced a change in employment status, such as leaving a job or taking medical disability leave [Citation7]. Moreover, a study conducted in Korea showed that the most important treatment goal for patients was ‘symptom improvement’, while physicians felt that the most important treatment goal was improving quality of life (QoL). In that study, physicians gave higher scores than patients in terms of the impact of PV on patients’ daily living [Citation8]. These observations suggest differences exist between patients’ and physicians’ perceptions of treatment goals and daily living in PV patients [Citation6,Citation8,Citation10].
Although several studies have examined patients’ perceptions of which PV symptoms have the greatest impact on their daily living and have compared the perceptions between physicians and patients [Citation3–9], especially in Japan, the exact reasons for these differences have not been fully established. Therefore, we conducted a cross-sectional survey of patients with PV and their attending physicians in Japan. The purpose of this study was to clarify what symptoms patients with PV perceive as burdensome, determine how the burden of symptoms affects daily living, and understand the differences between patients and physicians in terms of their perceptions of the treatment goals and impact on daily living.
Materials and methods
Study design and population
This was a cross-sectional survey study conducted in Japan. Participants were recruited from March 2022 to July 2022. The recruitment period was originally planned to continue until August in the study protocol; however, based on the status of questionnaire collection during recruitment, it was determined that it would be difficult to recruit the target sample size (see Supplementary Text 1 for further details of recruitment). Therefore, according to the criteria for study discontinuation, the recruitment period was set until July at the discretion of the principal investigator.
The main patient inclusion criteria were 1) ≥ 20 years old at the time of consent; 2) a diagnosis of PV; and 3) written informed consent for participation. The main physician inclusion criteria were 1) physicians who treated at least one patient diagnosed with PV at the time of consent and 2) physicians who provided written informed consent for participation. All these criteria were self-assessed by the participants. Neither the patients nor the physicians received any remuneration for their participation.
Ethics
This study was conducted in accordance with the principles of the Declaration of Helsinki and the Clinical Trials Act in Japan. The study protocol was approved by the Ethics Committee of Juntendo University School of Medicine (approval No. E21-0300-H01). The study was prospectively registered with UMIN-CTR with the identifier number UMIN000047047. All patients and physicians provided written informed consent before enrollment.
Survey instrument
All original surveys were written in Japanese, and the English translations of the questionnaires are shown in Supplementary Text 2. Questions regarding PV symptoms were adapted from the MPN Landmark Survey [Citation4,Citation6] and the MPN Symptom Assessment Form [Citation11].
The patient and physician questionnaires included 34 and 29 questions (partially multiple-choice), respectively. The physician and patient questionnaires each consisted of four main domains: demographic characteristics, PV symptoms (27 different symptoms) and their impact on daily living, treatment-related items (treatment goals, phlebotomy and drug therapy implementation, treatment efficacy and satisfaction), and physician-patient communication. The primary endpoint was the impact of PV symptoms on patients’ daily living or employment status. The level of impact was assessed on a 5-point scale ranging from 1 (no impact) to 5 (large impact). The secondary endpoint was the perceptions of the patients and physicians on the survey items. In addition, patients were asked how they obtained information about PV.
Statistical analyses
The sample size was set to 400 for patients and 200 for physicians, based on a previous study [Citation6]. In this study, no formal hypothesis was tested; therefore, statistically significant differences were not calculated. The data were analyzed using descriptive statistics. Statistical analyses were performed using SAS v9.4 (SAS Institute Inc., Cary, NC, USA).
Comparisons of physician-patient perceptions were made across three domains: PV symptoms and their impact on daily living (family life, social life, work, leisure life, and sex life), treatment-related items (treatment goals, phlebotomy/drug therapy, and treatment assessment), and physician-patient communication. Subgroup analyses of patients stratified by age were also conducted when assessing the impact of PV on daily living and phlebotomy and drug therapy implementation. Invalid answers for each question were excluded from the analysis.
Results
Patient and physician demographics
Of the 566 facilities invited to participate in the study, responses were received from 112 facilities, with a total of 265 patients and 151 physicians included in the analysis set.
A total of 55.8% of the patients were male, and the mean age of the patients was 67.3 years: 205 patients (77.4%) were aged ≥60 years, while 58 patients (21.9%) were aged <60 years. The mean duration of PV was 6.5 ± 6.9 years, and 43.4% of the patients were employed (). The main reason for a patient’s first visit to the hospital for diagnosis was abnormal blood laboratory values (90%).
Table 1. Baseline characteristics of patients.
A total of 76.8% of the physicians were male, and the mean age of the physicians was 48.5 years. The physicians were most frequently (57.6%) affiliated with a university hospital ().
Table 2. Baseline characteristics of physicians.
The impact of PV on daily living and QoL
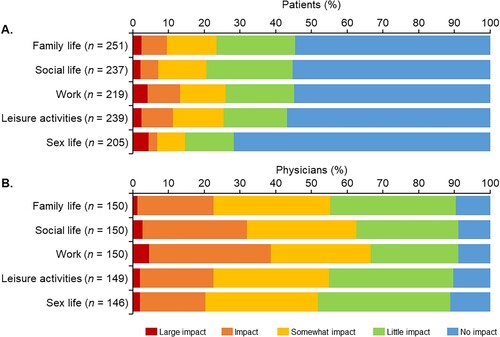
The impact of PV on daily living based on five items, namely family life, social life, work, leisure activities, and sex life (primary endpoint), is shown in . More than half of the patients answered ‘no impact’ for each of the items. Work had the highest percentage of respondents (13.2%) who answered ‘large impact’ or ‘impact’, followed by leisure activities (11.3%) and family life (9.6%) (A). The percentage of physicians who reported ‘no impact’ on any of the items was around 10%, which was notably lower than for patients (B). Similar to the patients, of the five areas of daily living, work had the highest proportion of physicians who answered ‘large impact’ or ‘impact’ (38.7%), and the proportion of physicians who gave this response was higher than the proportion of patients.
Figure 1. Impact of polycythemia vera symptoms on five areas of daily living (family life, social life, work, leisure activities, and sex life) ranked on a 5-point scale (large impact; impact; somewhat impact; little impact; no impact) as perceived by (A) patients and (B) physicians.
In the subgroup analysis by patient age, the percentage of patients who reported a greater impact (level of impact, ‘large impact’ or ‘impact’) for all five items was higher in the subgroup aged <60 years than in the subgroup aged ≥60 years (Supplementary Fig. 1A–E). The percentage of patients who reported ‘no impact’ was lower in patients aged <60 years than those aged ≥60 years. Compared with patients aged ≥60 years, patients aged <60 years more frequently answered ‘reduced hours at work,’ ‘change of workplace,’ and ‘job change’ when asked about the reasons for the impact of PV on their work (Supplementary Fig. 2). The employment rate was 80.7% for patients aged <60 years and 33.3% for patients aged ≥60 years (data not shown).
Treatment needs for PV symptoms
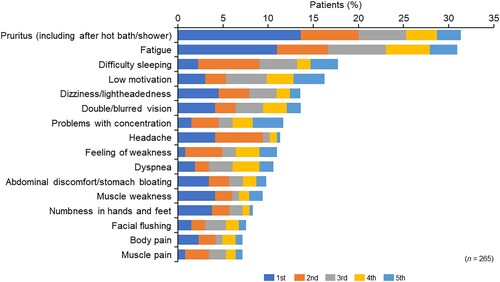
Of the 27 symptoms listed in the questionnaires (Q2-5), all symptoms were selected as symptoms perceived to be experienced by the patients ( and Supplementary Fig. 3A). The PV symptoms that patients most wanted to resolve were pruritus (including after a hot bath/shower) (13.6%), and fatigue (10.9%) (). Dizziness/lightheadedness, double/blurred vision, headache, and muscle weakness also ranked highly as symptoms that patients most wanted to resolve (). Pruritus (including after a hot bath/shower) (31.3%) and fatigue (30.9%) were the most selected symptoms among the top five most common symptoms that patients wanted to resolve. Meanwhile, the four most common symptoms that physicians perceived as most important to resolve were fatigue (33.8%), headache (19.9%), dizziness/lightheadedness (9.3%), and pruritus (including after a hot bath/shower) (6.0%) (Supplementary Fig. 3B).
Figure 2. Polycythemia vera symptoms and the proportion of patients that ranked each symptom within the top five symptoms to be resolved.
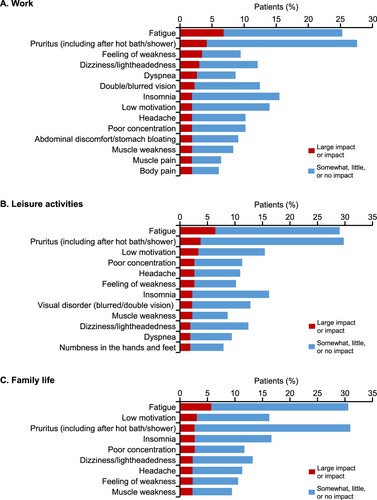
For patients who specified a ‘large impact’ or ‘impact’ on daily living (A), the impact of each symptom on three items of daily living (work, leisure activities, and family life) was assessed (A–C). Pruritus (including after a hot bath/shower) and fatigue were selected as the main symptoms that impacted work, leisure activities, and family life. Additionally, a feeling of weakness had a strong impact on work, and low motivation had an impact on family life and leisure activities.
Treatment goals and satisfaction
Regarding the treatment goals other than treating PV (first choice), patients placed the highest value on ‘slow disease progression’, followed by ‘prevention of thrombotic event’, ‘symptom improvement’, and ‘hematocrit level <45%’ (A). Physicians placed the highest value on ‘prevention of thrombotic event,’ followed by ‘symptom improvement’ and ‘hematocrit level <45%’ (A). With the exceptions of ‘slow disease progression’ and ‘prevention of thrombotic event,’ the most important physician – patient goals were generally consistent. ‘Slow disease progression’ was selected by only 2.6% of physicians versus 24.5% of patients, and ‘prevention of thrombotic event’ was selected by 51.7% of physicians versus 23.0% of patients.
Figure 4. (A) Most important polycythemia vera treatment goals and (B) target hematocrit level as determined by patients and their physicians.
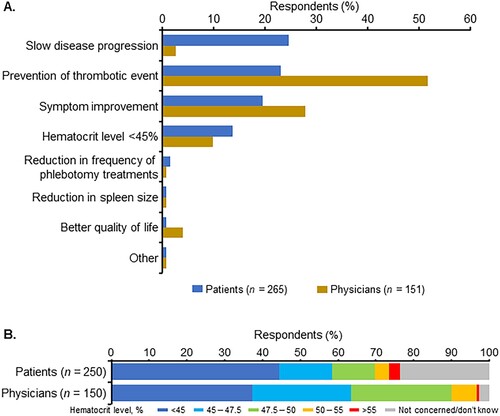
Of the therapeutic target hematocrit levels, a hematocrit level of <45% which is recommended in the Japanese Society of Hematology guidelines [Citation12] received the highest proportion of responses from both physicians and patients (approximately 40% in each group), while 32.0% of patients and 60.0% of physicians specified a hematocrit level of >45% (B). Just over 20% of patients reported that they were not concerned about or did not know their hematocrit level (B).
In terms of the psychological impact of PV, both patients and physicians reported anxiety about their symptoms, although the percentage of physicians who reported patient anxiety about symptoms as a perceived psychological impact was greater than the percentage of patients (Supplementary Fig. 4A, B).
Approximately 50% of patients and 60% of physicians responded ‘very satisfied’ or ‘somewhat satisfied’ with phlebotomy treatment (A). A total of 27.1% of patients responded with ‘don’t know.’ Approximately 20% and 30% of patients and physicians, respectively, responded ‘very dissatisfied’ or ‘somewhat dissatisfied’ with phlebotomy treatment (A), with the most frequent reason being ‘treatment was painful’ (Supplementary Fig. 5A).
Figure 5. Satisfaction of patients and their physicians with (A) phlebotomy treatment, (B) drug therapy, and (C) overall treatment situation.
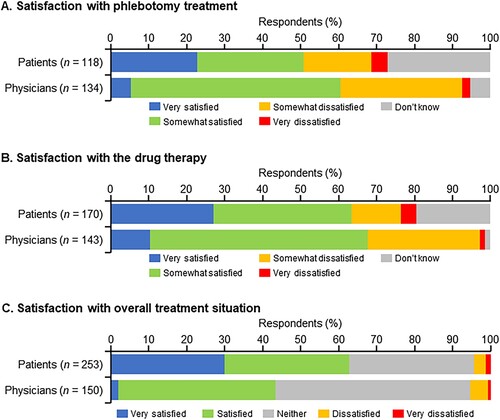
More than 60% of patients and physicians answered that they were ‘very satisfied’ or ‘somewhat satisfied’ with drug therapy (B). Approximately 20% of patients and 30% of physicians were ‘somewhat dissatisfied’ or ‘very dissatisfied.’ The most frequent reason for dissatisfaction with drug therapy (70%) as reported by patients was ‘high medical costs,’ while approximately half of the physicians reported ‘side effects are horrible’ as being the reason for patient dissatisfaction (Supplementary Fig. 5B).
Additionally, 30.0% of patients were ‘very satisfied’ and 62.8% were ‘very satisfied’ or ‘satisfied’ with the overall treatment situation, indicating a high level of treatment satisfaction. A lower percentage of physicians than patients answered ‘very satisfied’ (2.0%), and ‘very satisfied’ or ‘satisfied’ (43.3%) (C).
In the patient subgroup analysis by age (<60 years and ≥60 years), the satisfaction with phlebotomy treatment (‘very satisfied’ or ‘somewhat satisfied’) was similar between the subgroups, but the proportion of patients who answered ‘very dissatisfied’ or ‘somewhat dissatisfied’ was different between age subgroups (Supplementary Fig. 6A). In patients aged ≥60 years, satisfaction with drug therapy (‘very satisfied’ or ‘somewhat satisfied’) was higher than in patients aged <60 years (Supplementary Fig. 6B).
Physician-patient communication and satisfaction
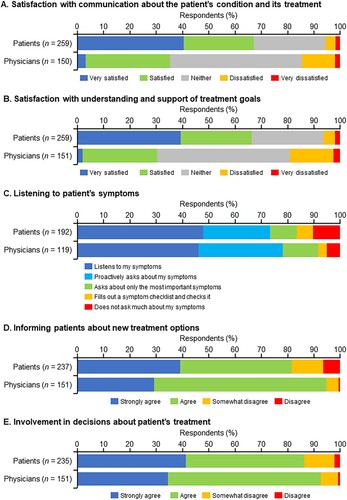
In terms of satisfaction with communication about the patient’s condition and its treatment, the patients were more satisfied than the physicians, with approximately 40% of patients responding ‘very satisfied’ compared with 3.3% of physicians (A). A similar tendency was observed in ‘understanding and support of treatment goals’ (B). Regarding the response of ‘listening to patient’s symptoms,’ the distribution of responses was roughly consistent between the patients and physicians (C). Regarding the response ‘informing patients about new treatment options’ and ‘involvement in decisions about patient’s treatment,’ more than 80% of patients and physicians answered ‘strongly agree’ or ‘agree’ (D and E).
Figure 6. Satisfaction of patients and their physicians with (A) physician-patient communication about the patient’s condition and its treatment, (B) the support provided to the patient to understand the treatment goal, (C) the ability of the physician to listen to the patient’s symptoms, (D) ability of the physician to inform the patient about new treatment options, and (E) ability of the physician to involve patients in the decisions about treatment.
More than half of the patients obtained helpful information about PV from materials provided by the physician (55.1%), followed by the internet (18.5%) (Supplementary Fig. 7A). The most frequent websites visited for information about PV by patients were health-related websites (36.6%) and hospital websites (15.5%) (Supplementary Fig. 7B).
Discussion
In this cross-sectional survey study, we disseminated questionnaires to patients with PV and their attending physicians to investigate the impact of PV symptoms on patients’ daily living in Japan. We also examined differences in patients’ and physicians’ perceptions of treatment goals and physician-patient communication during treatment. Additionally, we performed a subgroup analysis according to the cut-off age of 60 years, which was selected as it is a prognostic factor for PV [Citation13], and it is also generally accepted as the mandatory retirement age in Japan. This is the first and largest physician-patient survey study in this disease area in Japan, involving 265 patients with PV and 151 treating physicians.
Qol and daily living
In the present study, for the patients who did report an impact of PV on daily living, the largest proportion of patients reported an impact on work (13.2%), followed by leisure activities (11.3%) and family life (9.6%). Moreover, a higher percentage of patients aged <60 years reported a greater impact on all three items than patients aged ≥60 years. Work was particularly affected in terms of a reduction in working hours, change of workplace, and job change. Although this study did not examine changes in the actual number of hours worked and the rates of turnover and layoff, previous US studies have reported that 37% of patients decreased their working hours, 21% of patients voluntarily terminated their jobs, and 5% of patients involuntarily terminated their jobs [Citation4]. In another study, 48.0% of patients changed employment status after the PV diagnosis, with leaving their job being the most frequent reason (26.6%) [Citation7]. These findings suggest that the impact of PV on work may be substantial. It should also be noted that more than half of patients answered ‘no impact’ with respect to the impact of PV on daily living (family life, social life, work, leisure activities, and sex life). This result differs from the international Landmark Survey [Citation4,Citation6,Citation10], which included 38 Japanese patients with PV. In the Landmark Survey, 72% of patients responded ‘strongly agree’ or ‘somewhat agree’ when asked about the impact of PV symptoms on their QoL. The differences between the present study and the Landmark Survey may be because the majority of patients in the present study were not employed (55.8% vs. approximately 33%). Moreover, patients were more frequently older and had a longer disease duration than in the Landmark Survey [Citation4,Citation6,Citation10], which could have led to age-related differences in the results. Nevertheless, the results suggest that the impact of PV on those who work cannot be ignored.
In the present study, despite less of an impact on patients’ daily living, in terms of the psychological impact of PV, approximately 30% of patients responded that they felt anxious about their condition. These results indicate that although patients with PV may feel that PV does not greatly impact their daily living in Japan, they have anxiety about the future of their condition. Interestingly, physicians were more likely than patients to report that the impact of PV on patients’ daily living was great. Although this study did not calculate the physician-patient agreement, the results suggest that there may be a low agreement rate between physicians and patients in terms of their understanding of the impact of PV on patients’ daily living.
PV symptoms
The main clinical symptoms of PV are reported to be fatigue (general malaise), pruritus, night sweats, poor concentration and motivation, and splenomegaly and splenomegaly-related symptoms (abdominal pain, early satiety) [Citation2]. In the present study, the most common symptoms experienced by patients were pruritus and fatigue (13.6% and 10.9%, respectively), which may be symptoms that correlate well with effects on work, leisure activities, and family life. Additionally, pruritus was ranked as the greatest treatment need by patients, but it was ranked as the fourth greatest treatment need by physicians, indicating a disagreement in perception. Although this study did not calculate the rate of agreement between the physicians and patients, this difference may indicate a discrepancy between patients and their physicians in terms of the symptoms of PV that are perceived to be burdensome. Nevertheless, the results suggest that active treatment to improve or alleviate symptoms, such as fatigue and pruritus, may improve patients’ daily living [Citation14,Citation15].
Treatment goals and satisfaction
In terms of treatment goals, physicians emphasized the prevention of thrombosis/vascular events, while patients focused on delaying PV progression. These results are similar to those of the Landmark Survey [Citation4,Citation6,Citation10]. The goal of physicians’ daily practice is likely to be thrombosis prevention, which can improve patient prognosis, rather than control of PV progression. Physicians are aware that there is currently no curative treatment for PV, and treatments to prevent thrombotic events and improve symptoms are the focus. However, this study did not survey whether patients fully understood that there is no curative treatment or treatment to delay PV progression. Thus, we cannot tell from these results whether there is a gap in the treatment needs between patients and physicians. Unmet needs, such as delaying PV progression, still exist in the treatment of PV, and it is hoped that newly developed therapeutic agents will help to alleviate this [Citation16].
Physician – patient communication and satisfaction
In this study, patient satisfaction with overall treatment and with the provision of general information, and patient understanding of the purpose of treatment were relatively high. Additionally, the main method by which patients obtained information about PV was from educational materials from their attending physician. These findings suggest that patients feel that their physicians provide adequate explanations of the treatment plan. However, physicians were less satisfied than patients in terms of physician-patient communication. Although a clear cause is unknown from the present study, physicians may have a better understanding of PV and its available treatment option and thus may not underestimate the impact of PV on patients’ daily living. Physicians may also try to help their patients understand the disease using educational materials, resulting in patients having a better comprehension of the disease and perhaps feeling satisfied with the communication. However, physicians may not have enough time to listen to feedback on how the patient perceives the explanation, and thus they may not know the patient’s level of understanding. As a result, physicians may feel that communication is inadequate, causing their satisfaction with communication to be lower than that of patients. This assumption is consistent with the result that ‘neither’ was the most common response among physicians regarding physician-patient communication. Patient satisfaction with communication was actually higher than the physicians thought; thus, physicians might benefit from listening to feedback from their patients, which could help to improve the physicians’ satisfaction with physician-patient communication. Furthermore, PV symptoms and anxiety about disease progression affected employed patients’ daily living. For these patients, it would be helpful to deepen their understanding of PV by explaining the risks of complications and disease progression and helping them to understand the symptoms that physicians may find difficult to identify through interviews.
Limitations
This study has some limitations that should be noted. First, when recruiting participants to complete this survey, the patients were administered the survey by their treating physicians. Therefore, it is possible that patients with relatively good physician-patient relationships were more likely to be selected, which may have resulted in patient selection bias. Second, the analysis did not link patients to their primary physicians; therefore, individual physician-patient differences could not be identified. Third, the results of the physician-patient responses were self-reported. For this reason, accuracy in the responses cannot be ensured. Fourth, information on PV diagnosis was based on physician-patient self-reporting. Moreover, some of the symptoms investigated in this study are not known to be related to PV. Fifth, 112 of the 566 facilities invited to participate responded to the survey, with a total of 265 patients and 151 physicians included in the analysis set, giving a high response dispersion rate. Sixth, the 151 physicians who responded were mostly hematologists or oncologists and likely included both MPN experts and non-MPN experts. However, the ratio of the two groups participating in this study was not assessed, so the results may have been influenced by the ratio of MPN experts to non-MPN experts. Finally, this study was conducted during the COVID-19 pandemic, and limitations in social activities during the pandemic may have affected the results.
Conclusions
Patients’ daily living was largely affected by PV symptoms. Although satisfaction with treatment and physician-patient communication was high among patients in Japan with PV, patients were concerned about the future impact of their PV symptoms on their work, leisure activities, and family life. It should be noted that discrepancies exist between patients and physicians in the symptoms most requiring treatment, the impact of PV on patients’ daily living, satisfaction with communication, and treatment goals.
Geolocation information
Tokyo, Japan.
Supplemental Material
Download PDF (687 KB)Acknowledgments
The authors would like to thank the patients and physicians who participated in this study. The authors would also like to thank Emily Woodhouse, PhD, of Edanz (www.edanz.com) for providing medical writing services, which were funded by PharmaEssentia Japan K.K. The authors would like to thank PharmaEssentia Japan K.K., which supported them in the study design, planning of the data analysis, data interpretation, and development of the manuscript, but was not involved in the data management or statistical analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on reasonable request from the corresponding author, Y.E. or the sponsor, PharmaEssentia Japan K.K.
Additional information
Funding
Notes on contributors
Yoko Edahiro
Yoko Edahiro is the associate professor of Department of Hematology, Juntendo University School of Medicine in Tokyo, Japan. She received her education at Juntendo University School of Medicine (M.D.), and Juntendo University Graduate School of Medicine (Ph.D.). She continued teaching, researching and holding appointments centered on myeloproliferative neoplasms in Juntendo University School of Medicine and in Juntendo Hospital.
Keita Kirito
Keita Kirito is the professor of department of Hematology and Oncology in University of Yamanashi. He received his education at Jichi Medical School (M.D and Ph.D.). He worked as an assistant professor at Jichi Medical School and moved to University of Washington (Seattle) at 2001 as visiting scientist. In 2002, he moved to University of California, San Diego as visiting assistant professor. He became associate professor at University of Yamanashi in 2005 and became professor in 2010. His publications include Blood, Haematologica, Lancet Hematology, Nature communications and Annals of Oncology.
Akihiko Gotoh
Akihiko Gotoh is the Professor & Chairman of the Department of Hematology at Tokyo Medical University, Tokyo. He received his education at Tokyo Medical University (M.D. and PhD.). He was a postdoctoral fellow at Walther Oncology Center of Indiana University School of Medicine, Indiana, under the supervision of Professor Hal E. Broxmeyer. His practice, education, and research focused on general hematology, especially malignant and non-malignant hematopoietic stem cell diseases. He has served in his current position since 2019, concurrently serving as a visiting professor at Juntendo University and Tokyo University of Pharmacy and Life Sciences. He is currently a member of the board of directors of the Japanese Society of Hematology and serves as associate editor of the Japanese Journal of Clinical Hematology and editor-in-chief of the Journal of Tokyo Medical University.
Katsuto Takenaka
Katsuto Takenaka is a professor of Department of Hematology, Clinical Immunology and Infectious Disease at Ehime University Graduate School of Medicine in Japan. He received his medical degree from Kyushu University in Japan in 1991. His first academic post was the faculty of Kyushu University, and he studied as a post-doctoral fellow at Hospital for Sick Children and University of Health Network in Canada in 2002-2005. His research focuses are in the areas of myeloproliferative neoplasm, hemophagocytic syndrome and allogeneic hematopoietic stem cell transplantation.
Yuka Sugimoto
Yuka Sugimoto (M.D., PhD.) works as the Associate professor of Department of Hematology and Oncology in Mie University Graduate School of Medicine. She received her education at Faculty of Medicine and Graduate School of Medicine in Mie University. She holds appointments in Mie University Hospital. She is also the medical adviser of MPN-JAPAN, and the committee member of Public Relations Committee, Research Committee for MPN, and Genomic Medicine Committee in Japanese Society of Hematology. Her research interests focus on the clinical characteristics and genetic abnormalities in myeloproliferative neoplasms.
Norio Komatsu
Norio Komatsu is the project professor at Juntendo University Graduate School of Medicine and chairperson of PharmaEssentia Japan. He graduated from Niigata University in 1981 and acquired his Ph.D. at Jichi Medical University in 1988. In the years 1991-1997, he succeeded in establishing a human leukemic cell line with megakaryocytic features (UT-7) and its sublines (Cancer Res 1991; Blood 1993; Blood 1996; Blood 1997), an accomplishment which had and still has a major impact on the world of science. He was professor and chairman of the Department of Hematology and Oncology at Yamanashi University from 2004 to 2009. In the years 2009–2021, He was professor and chairman of the Department of Hematology at Juntendo University Graduate School of Medicine. His major interests and expertise lie in the area of myeloproliferative neoplasms (MPN). He was chairman of the MPN Study Group of the Japanese Society of Hematology in the years 2014-2018 and now leads the multi-center prospective observational study of Japanese MPN patients (JSH-MPN-15). He also worked as editor-in-chief of The Japanese Journal of Clinical Hematology during 2012-2020. He won the 56th Erwin von Bälz Prize (Second Prize) in 2019 and the 9th JSH Award in 2020.
Kazuya Shimoda
Kazuya Shimoda is a professor of Internal Medicine at the University of Miyazaki, Miyazaki, Japan. Dr. Shimoda received his medical degree from Kyushu University in Fukuoka, Japan, in 1987. His first academic post was the faculty of Kyushu University in 1999, and he moved to Miyazaki University in 2006. His main research field is the cytokine signal transduction in normal and abnormal hematopoiesis, focusing on both basic sciences to understand the molecular mechanism of myeloproliferative neoplasms and translational approaches directed at improving prognosis.
References
- Tefferi A, Vannucchi AM, Barbui T. Polycythemia vera: historical oversights, diagnostic details, and therapeutic views. Leukemia. 2021;35:3339–3351. doi:10.1038/s41375-021-01401-3
- Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109:68–76. doi:10.1002/cncr.22365
- Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30:4098–4103. doi:10.1200/JCO.2012.42.3863
- Mesa R, Miller CB, Thyne M, et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: the MPN landmark survey. BMC Cancer. 2016;16:167. doi:10.1186/s12885-016-2208-2
- Mesa R, Boccia RV, Grunwald MR, et al. Patient-reported outcomes data from REVEAL at the time of enrollment (baseline): a prospective observational study of patients with polycythemia vera in the United States. Clin Lymphoma Myeloma Leuk. 2018;18:590–596. doi:10.1016/j.clml.2018.05.020
- Harrison CN, Koschmieder S, Foltz L, et al. The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN landmark survey. Ann Hematol. 2017;96:1653–1665. doi:10.1007/s00277-017-3082-y
- Yu J, Parasuraman S, Paranagama D, et al. Impact of myeloproliferative neoplasms on patients’ employment status and work productivity in the United States: results from the living with MPNs survey. BMC Cancer. 2018;18:420. doi:10.1186/s12885-018-4322-9
- Byun JM, Bang SM, Choi EJ, et al. How myeloproliferative neoplasms patients’ experience and expectations differ from physicians’: the international MPN landmark survey. Korean J Intern Med. 2022;37:444–454. doi:10.3904/kjim.2021.475
- Brochmann N, Flachs EM, Christensen AI, et al. Health-related quality of life in patients with Philadelphia-negative myeloproliferative neoplasms: a nationwide population-based survey in Denmark. Cancers (Basel). 2020;12:3565. doi:10.3390/cancers12123565
- Mesa RA, Miller CB, Thyne M, et al. Differences in treatment goals and perception of symptom burden between patients with myeloproliferative neoplasms (MPNs) and hematologists/oncologists in the United States: findings from the MPN landmark survey. Cancer. 2017;123:449–458. doi:10.1002/cncr.30325
- Scherber R, Dueck AC, Johansson P, et al. The myeloproliferative neoplasm symptom assessment form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118:401–408. doi:10.1182/blood-2011-01-328955
- Shimoda K, Takahashi N, Kirito K, et al. JSH Practical guidelines for hematological malignancies, 2018: I. Leukemia-4. chronic myeloid leukemia (CML)/myeloproliferative neoplasms (MPN). Int J Hematol. 2020;112:268–291. doi:10.1007/s12185-020-02964-0
- Passamonti F. New and old prognostic factors in polycythemia vera. Curr Hematol Malig Rep. 2009;4:19–24. doi:10.1007/s11899-009-0003-8
- Scherber RM, Kosiorek HE, Senyak Z, et al. Comprehensively understanding fatigue in patients with myeloproliferative neoplasms. Cancer. 2016;122:477–485. doi:10.1002/cncr.29753
- Siegel FP, Tauscher J, Petrides PE. Aquagenic pruritus in polycythemia vera: characteristics and influence on quality of life in 441 patients. Am J Hematol. 2013;88:665–669. doi:10.1002/ajh.23474
- Duek A, Berla M, Ellis MH. Recent advances in the treatment of polycythemia vera. Leuk Lymphoma. 2022;63:1801–1809. doi:10.1080/10428194.2022.2057491