ABSTRACT
Acute myeloid leukemia (AML) that develops along with prior or concurrent tumors without previous cyto- or radiotherapy (pc-AML) is an essential subset of AML but is often ignored and ambiguous. The biological and genetic characteristics of pc-AML remain largely unknown. Moreover, it is unclear whether pc-AML should be treated as de novo or secondary AML, whereas most clinical trials exclude it due to comorbidities. We performed a retrospective study of 50 patients with multiple neoplasms over five years. We focused on characteristics, treatment regimens, response rate, and prognosis of pc-AML, compared with therapy-related AML (tAML) and AHD-AML (AML discovered following prior hematologic disorders) as controls. We report the first detailed distribution of secondary tumors associated with hematological disorders. The incidence of pc-AML was 30% of all multiple neoplasms, and it was predominantly found in male and older participants. Nearly three-quarters of gene mutations affected epigenetic regulation and signaling pathways, and NPM1, ZRSR2, and GATA2 occurred exclusively in pc-AML. No significant differences were in CR, and pc-AML had an inferior OS similar to that of tAML and AHD-AML. More patients received hypomethylation agents (HMAs) in combination with venetoclax (HMAs + VEN) than intensive chemotherapy (IC) (65.7% vs 31.4%), and there was a trend toward improved OS in HMAs + VEN-based than in IC-based patients, whose 2-year estimated OS times were 53.6% and 35.0%, respectively. In conclusion, our results collectively support pc-AML as a biologically and genetically distinct entity with high-risk and dismal outcomes, and HMAs in combination with venetoclax-based regimens may benefit patients with pc-AML.
Introduction
The incidence of cancer is rapidly increasing with population growth and aging worldwide. At the same time, the appearance of new drugs and technologies has significantly improved prognoses and survival rates, resulting in an increase in secondary tumors [Citation1–3]. The comorbidities of secondary tumors may further aggravate the disease process, bring about more treatment challenges, and seriously affect the quality of life and prognosis of patients. Unfortunately, we still lack comprehensive understanding and in-depth research regarding hematological tumors associated with secondary tumors or hematological tumors as secondary tumors.
Therapy-related acute myeloid leukemia (tAML) is a well-recognized secondary hematological malignancy. The World Health Organization defines tAML as acute myeloid leukemia (AML) occurring as a late complication of cytotoxic chemotherapy, radiotherapy, or both, for neoplastic or non-neoplastic diseases [Citation4]. Similarly, AML discovered following a hematologic disorder, including myelodysplastic syndromes (MDS), chronic myelomonocytic leukemia (CMML), myeloproliferative neoplasms (MPN), or other myeloid neoplasms, is also relatively common and is referred to as AHD-AML [Citation5]. Both tAML and AHD-AML have been associated with dismal outcomes, with response rates of 30-60% and median overall survival (OS) rates of 5–10 months [Citation6–10].
In addition to these two AML subtypes, AML developing with prior or concurrent tumors (pc-AML) without previous cytotoxic or radiotherapy is often ignored. However, in a Danish national population-based study of 3,055 unselected patients with AML diagnosed between 2000 and 2013, nearly 10% of the patients had pc-AML, accounting for a considerable proportion of AML patients [Citation9]. Moreover, it is usually categorized as de novo AML, whereas it is more likely to be treated as t-AML or AHD-AML and has a lower chance of being entered into clinical trials but more opportunity to receive palliative therapy.
However, few studies have focused on these patients. The Danish studies mentioned above only showed the incidence of pc-AML but no information on the response rate or prognosis. Another study from a single center in France, published in 2018, focused on pc-AML in patients with a median age of 73 (70-83) years [Citation11]. The growing incidence and recurrence of cancer worldwide and the advent of new technologies and drugs, such as next-generation sequencing and multiple targeted drugs, may bring about new challenges and opportunities for this type of AML, but we know very little about it. Furthermore, the epidemiology of multiple cancers associated with hematological disorders, other than AML, is unknown.
In this study, we were interested in pc-AML and secondary hematological neoplasms. We focused on the biological and cytogenetic characteristics, treatment strategies, and prognosis of pc-AML, compared with tAML and AHD-AML as controls. We also sought to obtain a detailed and comprehensive description of secondary tumors associated with hematological disorders.
Materials and methods
Patients and design
We enrolled 50 patients with at least two successive or concurrent neoplasms admitted to the Department of Hematology, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, between January 2017 and August 2022. Of these patients, the secondary AML and MDS patients were distributed into three groups according to a history of prior malignancies:1) AHD-AML: AML discovered following a hematologic disorder including MDS, CMML, MPN, or other myeloid neoplasms; 2) therapy-related myeloid neoplasms (tMN): patients presenting with myeloid neoplasms after cytotoxic or radiotherapy, which can be further subdivided into tAML and therapy-related myelodysplastic syndromes (tMDS); 3) prior or concurrent myeloid neoplasms (pcMN): myeloid neoplasms developing with an antecedent or simultaneous solid tumor without prior cytotoxic or radiotherapy, further subdivided into pc-AML and pc-MDS.
Targeted gene sequencing
The 55 genes related to myeloid neoplasms (Supplemental Table 1) were identified by analyzing deoxyribonucleic acid (DNA) from mononuclear cells of the bone marrow. Each DNA sample was quantified using agarose gel electrophoresis and a NanoDrop™ (Thermo Fisher Scientific Inc., Waltham, MA, USA). Databases were prepared using the Illumina standard protocol. The amplified DNA was captured with a 55 Gene Panel using biotinylated oligoprobes (MyGenosticsGenCap Enrichment Technologies, MyGenostics, Baltimore, MD, USA). Capture experiments were conducted according to the manufacturer’s protocol. Notably, samples with FLT3-ITD positivity were further verified by capillary electrophoresis. The median depth of sequencing was 2000x. All putative mutations were compared against multiple databases (e.g. 1000 genomes, COSMIC, PolyPhen, and SIFT).
Treatment and prognosis
Patients received induction therapy with either standard intensive chemotherapy (IC) with cytarabine and anthracycline-based regimens, regimens containing aclarubicin or idarubicin, or with a hypomethylation agent (HMA) in combination with venetoclax (VEN) (HMAs + VEN-based) regimens. The latter regimen is based on HMAs (azacitidine, 75 mg/m2 d1-7 or decitabine, 20 mg/m2 d1-5) and venetoclax (100 mg d1, 200 mg d2, 400 mg d3-28; when combined with a potent CYP3A inhibitor, the amount should be reduced to 100 mg/d). No patients were treated with CPX 3-5-1 because this drug is only registered for clinical trials in China.
Complete remission (CR) was defined as morphological remission after two cycles of induction therapy, according to international consensus criteria. Incomplete remission (Cri) was defined as remission with incomplete blood count recovery. Partial remission (PR) was defined as a decrease of at least 50% of the blast percentage in the bone marrow aspirate to 5-25%. The objective response rate (ORR) included CR and PR rates. Latency time was defined as the time from the first hospital contact for the preceding disease of interest to the date of AML diagnosis. OS was calculated as the time from the date of diagnosis to the date of death or last follow-up visit.
Statistical analyses
Statistical analyses were performed using SPSS version 23.0 (IBM Corp., Armonk, NY, USA) and GraphPad PRISM version 8.0(GraphPad Software, San Diego, CA, USA). The normality of the data was evaluated using the Kolmogorov–Smirnov test. Differences were assessed using the chi-squared test, t-test, or Mann–Whitney U test when appropriate. Survival analysis was performed using the Kaplan–Meier method, and survival curves were compared using the log-rank test. Statistical significance was set at P < 0.05.
Results
Detailed distribution of secondary tumors associated with hematological disorders
As outlined in , 50 patients with at least two successive or concurrent neoplasms were enrolled. Overall, about one-third of patients had pc-MN, including 15 (30%) with pc-AML and 3 (6%) with pc-MDS. One-tenth of patients had t-AML (10%, n = 5) with one diagnosed with t-MDS (2%), and 30% were diagnosed with AHD-AML. In addition, nearly one-quarter of the patients (n = 11) had a relatively rare condition.
Figure 1. Distribution of different types of multiple neoplasms. A. Incidence of tAML, AHD-AML, pc-AML, pc-MDS, and rare cases. B. Descriptive data on the relationship between prior exposure and secondary disorders. C. The types of secondary hematological malignancies after previous solid tumors in rare cases. Abbreviations: HM, hematological malignancy; ST, solid tumor; tAML, therapy-related acute myeloid leukemia; AHD-AML, AML discovered following a prior hematologic disorder; pc-AML and pc-MDS, AML and MDS developing with an antecedent or simultaneous tumors without prior cytotoxic or radio-therapy.
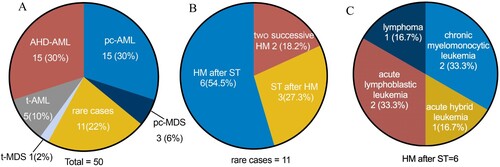
Among these 11 patients with rare conditions, more than half presented with a hematological malignancy (HM) after a solid tumor (ST), whereas two had successive HMs, and patients with an HM prior to an ST accounted for about one-fifth. Of the six patients diagnosed with HMs after STs, three had acute leukemia (acute lymphoblastic leukemia = 2, hybrid acute leukemia = 1); two had CMML after lung or thyroid cancer, respectively; and one was diagnosed with B-cell non-Hodgkin lymphoma following renal cancer.
We also noted that two non-acute promyelocytic leukemia (non-APL) AML patients were diagnosed with T-lymphoblastic leukemia or diffuse large B-cell lymphoma. Interestingly, three patients with lung cancer had previous hematological diseases, including APL, MDS, and mantle cell lymphoma. A more detailed description is provided in .
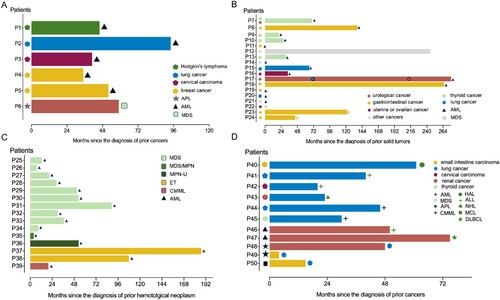
Figure 2. Latency time from first hospital contact for the preceding disease to the date of the secondary neoplasms in tAML (A), pc-AML(B), AHD-AML(C), and rare cases(D). Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasms; ET, primary thrombocytosis; CMML, chronic myelomonocytic leukemia; APL, acute promyelocytic leukemia; HAL, hybrid acute leukemia; ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin’s lymphoma; MCL, mantle cell lymphoma; DLBCL, diffuse large B-cell lymphoma.
Clinical characteristics of pc-AML compared with t-AML and AHD-AML
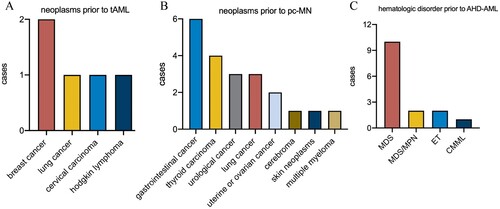
The distributions of prior neoplasms in t-AML and pc-MN were quite different (). The STs prior to t-AML mainly consisted of breast, lung, and cervical carcinomas, which are well recognized. However, gastrointestinal cancer and thyroid carcinoma accounted for the majority of previous pc-MN disorders. The distribution of hematologic disorders prior to AHD-AML is shown in detail in C (prior MDS: 10 [66.7%]; prior MDS/MPN: 2 [13.3%]; prior ET(primary thrombocytosis): 2(13.3%); prior CMML: 1[6.7%]).
Figure 3. Distribution of previous disease in 5 patients with tAML(A), 15 patients with pc-AML(B), and 16 patients with AHD-AML(C).
To further understand the biology and prognostic features of pc-AML, we retrospectively analyzed the sex, age, latency time, routine blood indices, karyotype, and gene mutation of next-generation sequencing in 15 pc-AML patients. Five tAML patients and 15 AHD-AML patients were used as controls.
Female predominance was noted in tAML, while pc-AML and AHD-AML both showed male predominance. Patients with pc-AML and AHD-AML were also similar in age (pc-AML vs AHD-AML: 66 [28-76] vs 63 [49-88] years, P = 0.169), whereas tAML patients were younger (43 [34-76] years). The latency time was 34 (0-276) months in pc-AML, shorter than that in tAML (46 [35-94] months) and longer than that in AHD-AML (30 [4-187] months), although there was no significant difference between the three groups ().
Table 1. Patient Characteristics.
Abnormal karyotypes were more frequent in tAML patients (n = 4; 80%), and pc-AML had the largest proportion of normal karyotypes of the two others (pc-AML vs t-AML: 60% vs 0%, P = 0.023; pc-AML vs AHD-AML: 60% vs 26%, P = 0.065). Similarly, intermediate cytogenetics were more frequent in pc-AML (53%), while more than 60% of t-AML and over 80% of AHD-AML cases had adverse cytogenetics. The differences between pc-AML and AHD-AML were statistically significant.(P = 0.002).
Targeted mutational analysis of pc-AML in the era of next-generation sequencing
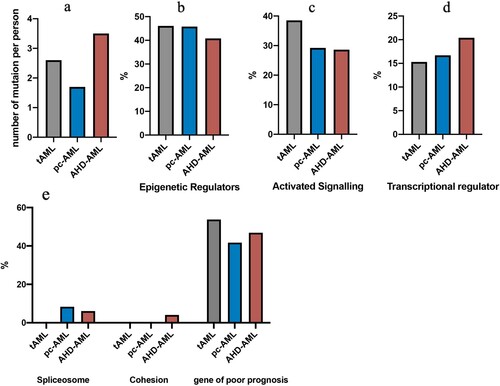
Targeted mutational analysis may be crucial to gain a deeper insight into the relationship between pc-AML, tAML, and AHD-AML; therefore, we focused our investigation on a gene set of 55 genes commonly associated with myeloid neoplasms. We identified 31 types and a total number of 91 mutations (). Mutations in NPM1, ZRSR2, and GATA2 were specific for pc-AML patients, whereas t-AML had unique mutations in NF1 and KDM6A. In contrast, 10 mutations, including JAK2, JAK3, SETBP1, U2AF1, KRAS, STAG2, CBL, KMT2A, and KMT2D occurred exclusively in AHD-AML patients. Among the 18 shared mutations in the three subsets, a high frequency of mutations affected RUNX1 (n = 9), DNMT3A (n = 7), TET2 (n = 6), ASXL1 (n = 6), TP53 (n = 5), BCOR (n = 4), and IDH2 (n = 4). The mutational sites and frequencies of the high-frequency mutations are listed in Supplemental Table 2.
Figure 4. The mutational profiles among pc-AML, tAML, and AHD-AML of 55 genes associated with myeloid neoplasms. A Total of 31 types and a total number of 91 gene mutations are shown.
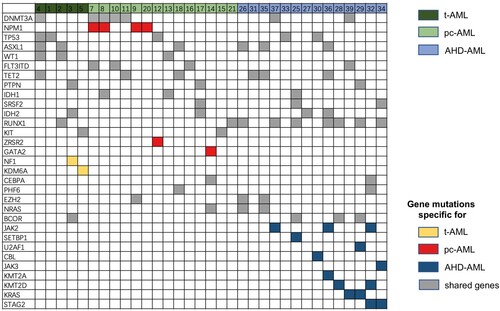
Next, we categorized these mutations by molecular function for AML progression (). In pc-AML, 45.8% of the mutations affected epigenetic regulation, followed by activated signaling pathways (29.2%), transcriptional regulators (16.7%), and spliceosomes (8.3%). The same switch also occurred in AHD-AML and t-AML, and AHD-AML had a higher proportion of epigenetic or transcriptional regulators. Moreover, we defined a group of gene mutations associated with poor prognosis, including TP53, ASXL1, RUNX1, DNMT3A, and spliceosome chromatin modification (SF3B1, U2AF1, SRSF2, ZRSR2, EZH2, BCOR, and STAG2). It was observed that the mutations with poor prognoses accounted for about half of t-AML and AHD-AML and over 40% of pc-AML patients. In summary, these three types of AML, especially pc-AML, and AHD-AML, share similar molecular mutation profiles.
Dismal outcomes in pc-AML but improved OS with HMAs + VEN-based regimen
A total of 34 patients received chemotherapy, and one pc-AML patient rejected chemotherapy and only accepted supportive treatment for personal reasons. Compared to t-AML and AHD-AML, pc-AML had superior response rates (pc-AML, 78.6%; t-AML, 40%; AHD-AML, 53.3%), which could be attributed to the higher percentage of patients with intermediate-risk cytogenetics (53%). However, the difference was not statistically significant. AHD-AML patients more frequently received HMAs + Ven therapy (n = 13, 86.7%). Conversely, among the 14 pc-AML patients who underwent chemotherapy, 7 (50%) received IC, while the other half received HMAs + Ven. Of the tAML patients, 2 received IC-based, and 3 received HMAs + VEN-based regimens (A).
Figure 6. The response rate and overall survival rate. The response rate (CR/CRi rates) among pc-AML, tAML, and AHD-AML patients (A) and the response rate in three types of secondary AML patients with HMAs + VEN-based and IC-based regimens (B). Probabilities of overall survival (OS) in pc-AML compared with tAML and AHD-AML patients (C), and OS in three types of secondary AML patients with HMAs + VEN-based and IC-based regimens (D). Abbreviations: CR, complete remission; CRi, CR with incomplete blood count recovery; HMAs + VEN, hypomethylating agents in combination with venetoclax; IC, intensive chemotherapy.
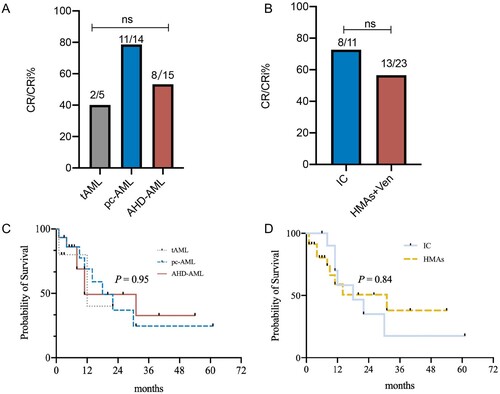
No significant difference was observed between IC-based and HMAs + VEN-regimens in all patients (B), but patients who received IC seemed to have a higher CR rate, at 72.7%, than those with HMAs + VEN, at 56.5%. Five patients subsequently underwent allogeneic haploidentical hematopoietic stem cell transplantation (tAML, 1 [20.0%]; pc-AML, 3 [20.0%]; and AHD-AML, 1 [6.7%]). The tAML patient died three months after transplantation due to acute graft-versus-host disease of the intestinal tract. Two of the three pc-AML patients relapsed at 11 months and four months after transplantation, respectively, and the former died of pneumonia during the relapse. The AHD-AML patient relapsed at one and a half year after transplantation, and died 7 months later for uncontrolled primary disease with severe pneumonia.
Finally, crude survival rates according to AML type and treatment strategy are shown in C and D. The median OS was 12, 18, and 11 months for tAML, pc-AML, and AHD-AML, respectively. Pc-AML had comparable survival rates to the other two groups (P = 0.95). The 2-year estimated OS rates were 40% and 36.9% in tAML and pc-AML patients, respectively, and 49.2% in AHD-AML patients.
Although there was no significant difference between patients who received IC and HMAs + VEN (P = 0.84), patients with HMAs showed a trend toward improved OS. The median OS was 18 months for the IC group and 31 months for the HMAs + VEN group. The 2-year estimated OS rates were 35.0% for IC-based and 53.6% for HMAs + VEN-based regimens.
Further noteworthy findings
Notably, a patient was diagnosed with diffuse large B-cell lymphoma (DLBCL) six years after receiving chemotherapy for AML. The incidence of tMN following radio- or chemotherapy for lymphoma has been reported as 0.8-6.3% at 20 years, but secondary non-Hodgkin lymphoma with prior AML is quite rare and has only been reported in a few cases [Citation12,Citation13]. Therefore, we performed whole exome sequencing in tumor tissues and non-carcinoma adjacent tissues. We identified several germline mutations and selected cancer-predisposing genes via the Cancer Gene Census database (http://cancer.sanger.ac.uk/cancergenome/projects/census/). Mutations in CBLB, HOXC13, PTPRC, ERCC4, TSC1, SETBP1, and AHCTF1 were included: mutations in CBLB, HOXC13, and SETBP1 were more predisposed to AML; mutation of PTPRC was predisposed to T-ALL; and mutations of ERCC4 and TSC1 were predisposed to cancer of skin cells or renal and bladder cells, respectively.
We were also surprised to note that a patient with APL developed lung cancer four years later with an associated APL. APL accounts for approximately 10% of AML, developing from a fatal disease to a highly curable one with the administration of arsenic trioxide and all-trans-retinoic acid [Citation14,Citation15]. The patient achieved CR through induction therapy of retinoic acid and arsenic trioxide and then received DA (daunorubicin: 40mg/m−2*d−1, d1-3; cytarabine: 100mg/m−2*d−1, d1-5) and HA (homoharringtonine: 2mg/m−2*d−1, d1-7; cytarabine: 100mg/m−2*d−1, d1-5) once each followed with 2-years retinoic acid and arsenic trioxide maintenance therapy. Although arsenic and low-dose chemotherapeutants are known to be therapeutic factors for lung cancer, the pathogenesis of secondary malignancies may consist of multiple potential factors, including genetic abnormalities, daily habits, regional theories, and tumor immunity. More clinical trial participation, whole genome and exome sequencing, and germline mutation analysis should be encouraged in these patients.
Discussion
In recent years, there has been a growing cancer burden of an estimated 18.1 million new cancer cases worldwide and 4.82 million in China annually. Accordingly, the co-occurrence of multiple primary cancers with hematological neoplasms is increasing [Citation1–3]. However, few studies have focused on the true epidemiology and related conditions of these patients, except for sporadic case reports. To our knowledge, this is the first report of a detailed description of hematological neoplasms concurrently or successively with other malignant diseases in a single center over five years.
We found some interesting cases that were not commonly seen, and they accounted for nearly one-quarter of the patients with multiple cancers. We also performed whole exome sequencing in an AML and DLBCL patient and identified several germline cancer-predisposing gene mutations. More importantly, our results showed that pc-AML is biologically and prognostically different and should be regarded as a distinct subset of AML.
The results showed that about one-third of the patients had pc-MN, one-third had AHD-AML, and nearly one-tenth had tAML. Registry-based studies reported that tAML and AHD-AML comprised approximately 25% of all AML patients, with 18.7-19.8% of AHD-AML and 6.6-7.7% of t-AML. Our ratio between AHD-AML and t-AML was similar, and we noted that pc-AML also accounted for a considerable proportion, compared to previous studies, which could be interpreted as the increasing incidence of whole cancers and suggested that more attention should be paid to this group of patients [Citation11,Citation16].
Furthermore, recent studies have shown that specific mutation patterns play a potential role in response and survival outcomes [Citation17,Citation18] and that t-AML and AHD-AML have completely different mutation profiles [Citation19,Citation20]. However, studies on mutation analysis of pc-AML are scarce, and only a few studies of pc-AML have been reported in the pre-mutation era. In this study, we performed a targeted mutational analysis of pc-AML compared to t-AML and AHD-AML to determine the biological characteristics of pc-AML. Previous studies reported that mutations in eight genes (SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2) were >95% specific for AHD-AML, while NPM1, MLL/11q23 rearrangements, and core-binding factor rearrangements were >95% specific for de novo AML. In contrast, t-AML had a higher frequency of DNMT3A, FLT3, NPM1, and NRAS and a far lower frequency of ASXL1, BCOR, RUNX1, and SRSF2 than AHD-AML [Citation21].
In our results, mutations in NPM1, ZRSR2, and GATA2 were specific to patients with pc-AML. It shares 18 mutations with t-AML and AHD-AML, mainly comprising RUNX1, DNMT3A, TET2, ASXL1, BCOR, TP53, and IDH2, the majority of which belong to signaling pathways and transcriptional regulators, including some genes that are either specific for AHD-AML or t-AML and are associated with poor prognoses. Our results suggest that pc-AML is genetically distinct but partly similar to t-AML and AHD-AML, with some gene mutations associated with inferior outcomes.
Both t-AML and AHD-AML have been associated with inferior outcomes of lower response rates and shorter survival times compared to de novo AML [Citation6–10]. Several large population-based studies demonstrated CR rates for t-AML of 54-61%, while AHD-AML had a relatively lower CR rate of 33-59%. Furthermore, the median OS of t-AML and AHD-AML patients was 6–12 months. In our study, the CR/CRi rates of t-AML and AHD-AML were 40% and 53.3%, respectively, similar to previous studies.
Our results showed that pc-AML seemed to have a better response rate of 78.6%, with a higher proportion of normal karyotypes, and NPM1 was a pc-AML-specific mutation. Our study had several limitations. Firstly, we could not avoid the relatively small number of these types of AML in our study population and the limitations of a single-center retrospective analysis resulting in a possible bias. Moreover, similar to the entire AML group, there may also be heterogeneity within the so-called ‘pc-AML’ subgroup. Part of the clinical and biological features of some pc-AML patients could overlap with de novo AML, and we cannot distinguish them perfectly. That is to say, in some cases, pc-AML likely represents a de novo disease with a coincidental previous history of a concomitant or prior tumor; but in other cases, the occurrence of an earlier tumor and subsequent pc-AML may be due to innate genetic susceptibility. Could it even be considered that adverse environments and customs have created a similar genetic susceptibility in another sense, leading to multiple tumors and, ultimately, poor prognoses? Considering its interesting background of multi-tumor and inferior outcomes similar to AHD-AML and t-AML shown in our results, pc-AML deserves more attention and research. In future studies, large-scale, long-term, multi-dimensional data and further detection techniques will help us better identify the ‘true’ pc-AMLs, which can be used for personalized treatment, and improved effectiveness of treatments and even prevention.
At the same time, we should not ignore the vital role of immune dysregulation in solid and hematological tumors. Turk S et al showed that downregulation of some critical genes related to the RAS gene family and neurotransmitter-associated pathways play an important role in NK-cell dysfunction, resulting leukemia immunity evasion [Citation22]. In KRAS-driven lung cancer, IL-6/STAT3 signaling promotes inflammatory microenvironment and thereby enhances tumor progression. Hyperactivation of the KEAP1/NRF2 pathway is found in multiple solid tumors including lung, colorectal, liver, gastric, ovarian, and breast cancer, leading to metabolic liabilities. In the microenvironment of KEAP1/NRF2 mutant tumors, massive accumulation of lipid peroxidation products and bilirubin, as well as increased glycolysis ultimately inhibit dendritic cell function, induce CD8+ T cell apoptosis, and promote T regulatory cell Proliferation and infiltration [Citation23,Citation24]. It is also reported that DNMT3A mutation promotes acute leukemic cell survival by regulating glycolysis through the NRF2/NQO1 axis [Citation25]. Incorporating immune factors into future research will help us better understand and individualize the treatment of pc-AML.
However, we also noted that more patients received HMAs + VEN than IC (65.7% vs 31.4%). Patients receiving HMAs + VEN compared with IC-based regimens had slightly lower CR rates (56.5% vs 72.7%) but experienced a trend toward improved OS. Similar results were confirmed in several other studies, and the potential reason was that HMAs + VEN may be associated with lower early mortality, higher quality of life, and more time to undergo stem cell transplantation [Citation10,Citation26–29]. The recent approval of venetoclax in combination with HMAs is a welcome addition to the treatment approach for AML and has improved response rates [Citation30,Citation31]. Venetoclax is a selective small-molecule inhibitor of B-cell lymphoma 2 protein, which is overexpressed in leukemia stem cells. Its overexpression is positively correlated with drug resistance and poor prognosis in AML [Citation32]. Studies have shown that leukemic cells are sensitive to venetoclax, both in vitro and in vivo. Additionally, azacitidine could inhibit another anti-apoptotic protein, MCL-1, which is vital for the survival of leukemic cells and may play a crucial role in the resistance to venetoclax [Citation33,Citation34]. Both clinical trials and real-world clinical practice demonstrated that venetoclax with HMAs had a high response rate (60-83.3%) in older patients and newly diagnosed unfit AML patients [Citation31,Citation35,Citation36]. Even for relapsed or refractory AML patients, the regimen can achieve CR/CRi rates of 32-51%. A retrospective study reported that 60% of patients with t-AML achieved CR/CRi.
Recent studies also confirmed that patients with mutations in FLT3, IDH1/IDH2, or TP53 could benefit from venetoclax [Citation37–40]. Besides our abovementioned findings, there are still many prognostic biomarkers regarding pc-AML that can offer a deeper understanding of the genetic landscape. For example, Ivosidenib and Enasidenib can be used to treat pc-AML with IDH mutation, raising myeloid differentiation and decreasing blast counts by repressing IDH1 and IDH2 mutants, respectively. In Renin-angiotensin system genes, ATP6AP2 was associated with resistance, and IGF2R and CTSA were associated with sensitivity for Doxorubicin. Moreover, midostaurin, a small-molecule inhibitor of FMS-like tyrosine kinase 3 (FLT3), was administered in combination with conventional chemotherapy in AML with FLT3 mutation, and had an extended OS and significantly higher EFS [Citation41–44].
In conclusion, our results collectively support pc-AML as a biologically and genetically distinct entity with high risk and dismal outcomes, and HMAs + VEN-based regimens may benefit patients with pc-AML. Large-scale, multicenter, randomized, prospective clinical trials are warranted to better understand the disease and obtain more optimal treatment regimens.
Authorship contributions
J.W., and Z.G. conceived of the study. Y.L. and J.T. collected and analyzed the clinical data. L.M. contributed to data collection, diagnosis, and treatment of the diseases. Y.L., Z.G., and J.W. wrote the manuscript with the help of all the authors.
Ethics statement
Clinical data were obtained from the medical records after approval by the Ethics Committee of Shanxi Bethune Hospital. This research was conducted in accordance with the guidelines of the Declaration of Helsinki.
Acknowledgments
The authors acknowledge the team of researchers at the Clinical Laboratory Center in Shanxi Bethune Hospital for their assistance.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394–424. doi:10.3322/caac.21492.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. Ca A cancer J clin. 2020;70(1):7–30. doi:10.3322/caac.21590.
- Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022 Feb 9;135(5):584–590. doi:10.1097/CM9.0000000000002108.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544.
- Björkholm M, Hultcrantz M, Derolf ÅR. Leukemic transformation in myeloproliferative neoplasms: therapy-related or unrelated?. Best Pract Res Clin Haematol. 2014;27:141–153. doi:10.1016/j.beha.2014.07.003.
- Kayser S, Döhner K, Krauter J, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117:2137–2145. doi:10.1182/blood-2010-08-301713.
- Dhakal P, Pyakuryal B, Pudasainee P, et al. Treatment strategies for therapy-related acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2020 Mar;20(3):147–155. doi:10.1016/j.clml.2019.12.007.
- Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36:2684–2692. doi:10.1200/JCO.2017.77.6112.
- Collinge E, Loron S, Larcher MV, et al. Elderly patients (Age 70 years or older) with secondary acute myeloid leukemia or acute myeloid leukemia developed concurrently to another malignant disease. Clin Lymphoma Myeloma Leuk. 2018 May;18(5):e211–e218. doi:10.1016/j.clml.2018.02.018.
- Boddu PC, Kantarjian HM, Ravandi F, et al. Characteristics and outcomes of older patients with secondary acute myeloid leukemia according to treatment approach. Cancer. 2017 Aug 15;123(16):3050–3060. doi:10.1002/cncr.30704.
- GranfeldtØstgård LS, Medeiros BC, Sengeløv H, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: A national population-based cohort study. J Clin Oncol. 2015 Nov 1;33(31):3641–3649. doi:10.1200/JCO.2014.60.0890.
- Chakraborty S, Sun CL, Francisco L, et al. Accelerated telomere shortening precedes development of therapy-related myelodysplasia or acute myelogenous leukemia after autologous transplantation for lymphoma. J Clin Oncol. 2009;27:791–798. doi:10.1200/JCO.2008.17.1033.
- Higuchi M, Sasaki S, Kawadoko S, et al. Epstein-Barr virus-positive diffuse large B-cell lymphoma following acute myeloid leukemia: a common clonal origin indicated by chromosomal translocation t(3;4)(p25;q21). Int J Hematol. 2015;102(4):482–487. doi:10.1007/s12185-015-1802-4.
- Powell BL. Arsenic trioxide in acute promyelocytic leukemia: potion not poison. Expert Rev Anticancer Ther. 2011;11:1317–1319. doi:10.1586/era.11.128.
- Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–121. doi:10.1056/NEJMoa1300874.
- Hulegårdh E, Nilsson C, Lazarevic V, et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry. Am J Hematol. 2015 Mar;90(3):208–214. doi:10.1002/ajh.23908.
- Ohgami RS, Ma L, Merker JD, et al. Next-generation sequencing of acute myeloid leukemia identifies the significance of TP53, U2AF1, ASXL1, and TET2 mutations. Mod Pathol. 2015;28:706–714. doi:10.1038/modpathol.2014.160.
- Devillier R, Gelsi-Boyer V, Brecqueville M, et al. Acute myeloid leukemia with myelodysplasia-related changes are characterized by a specific molecular pattern with high frequency of ASXL1 mutations. Am J Hematol. 2012;87:659–662. doi:10.1002/ajh.23211.
- Lindsley RC, Mar BG, Mazzola E, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015 Feb 26;125(9):1367–1376. doi:10.1182/blood-2014-11-610543.
- Higgins A, Shah MV. Genetic and genomic landscape of secondary and therapy-related acute myeloid leukemia. Genes (Basel). 2020 Jul 6;11(7):749, doi:10.3390/genes11070749.
- Awada H, Kuzmanovic T, Kishtagari A, et al. Mutational patterns and clonal architecture of therapy-related acute myeloid Leukemia. Blood. 2019;134:1405, doi:10.1182/blood-2019-131953.
- Turk S, Baesmat AS, Yılmaz A, et al. NK-cell dysfunction of acute myeloid leukemia in relation to the renin-angiotensin system and neurotransmitter genes. Open Med (Wars). 2022 Sep 20;17(1):1495–1506. doi:10.1515/med-2022-0551.
- Kerins MJ, Ooi A. A catalogue of somatic NRF2 gain-of-function mutations in cancer. Sci Rep. 2018 Aug 27;8(1):12846, doi:10.1038/s41598-018-31281-0.
- Pillai R, Hayashi M, Zavitsanou AM, et al. Nrf2: KEAPing tumors protected. Cancer Discov. 2022 Mar 1;12(3):625–643. doi:10.1158/2159-8290.CD-21-0922.
- Chu X, Zhong L, Dan W, et al. DNMT3A R882H mutation promotes acute leukemic cell survival by regulating glycolysis through the NRF2/NQO1 axis. Cell Signal. 2023 May;105:110626, doi:10.1016/j.cellsig.2023.110626.
- Vachhani P, Al Yacoub R, Miller A, et al. Intensive chemotherapy vs. hypomethylating agents in older adults with newly diagnosed high-risk acute myeloid leukemia: A single center experience. Leuk Res. 2018 Dec;75:29–35. doi:10.1016/j.leukres.2018.10.011.
- Maurillo L, Buccisano F, Spagnoli A, et al. Comparative analysis of azacitidine and intensive chemotherapy as front-line treatment of elderly patients with acute myeloid leukemia. Ann Hematol. 2018 Oct;97(10):1767–1774. doi:10.1007/s00277-018-3374-x.
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17. doi:10.1182/blood-2018-08-868752.
- Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33:379–389. doi:10.1038/s41375-018-0312-9.
- Guerra VA, DiNardo C, Konopleva M. Venetoclax-based therapies for acute myeloid leukemia. Best Pract Res Clin Haematol. 2019;32(2):145–153. doi:10.1016/j.beha.2019.05.008.
- Richard-Carpentier G, DiNardo CD. Venetoclax for the treatment of newly diagnosed acute myeloid leukemia in patients who are ineligible for intensive chemotherapy. Ther Adv Hematol. 2019;10:204062071988282, doi:10.1177/2040620719882822.
- Pan R, Ruvolo VR, Wei J, et al. Inhibition of Mcl-1 with the pan-Bcl-2 family inhibitor (-)BI97D6 overcomes ABT-737 resistance in acute myeloid leukemia. Blood. 2015;126:363–372. doi:10.1182/blood-2014-10-604975.
- Pan R, Hogdal LJ, Benito JM, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4:362–375. doi:10.1158/2159-8290.CD-13-0609.
- Bose P, Gandhi V, Konopleva M. Pathways and mechanisms of venetoclax resistance. Leuk Lymphoma. 2017;58:2026–2039. doi:10.1080/10428194.2017.1283032.
- Yu WJ, Jia JS, Wang J, et al. Short-term efficacy of venetoclax combined with azacitidine in acute myeloid leukemia: a single-institution experience. ZhonghuaXue Ye Xue Za Zhi. 2022 Feb 14;43(2):134–140. doi:10.3760/cma.j.issn.0253-2727.2022.02.008.
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137–2145. doi:10.1182/blood.2020004856.
- Aldoss I, Yang D, Aribi A, et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica. 2018;103(9):e404–e407. doi:10.3324/haematol.2018.188094.
- Tenold ME, Moskoff BN, Benjamin DJ, et al. Outcomes of adults With relapsed/refractory acute myeloid leukemia treated With venetoclax plus hypomethylating agents at a comprehensive cancer center. Front Oncol. 2021;11:649209, doi:10.3389/fonc.2021.649209.
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629. doi:10.1056/NEJMoa2012971.
- DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(2):216–228. doi:10.1016/S1470-2045(18)30010-X.
- Largeaud L, Berard E, Bertoli S, et al. Outcome of AML patients with IDH2 mutations in real world before the era of IDH2 inhibitors. Leuk Res. 2019;81:82–87. doi:10.1016/j.leukres.2019.04.010.
- Popovici-Muller J, Lemieux RM, Artin E, et al. Discovery of AG-120 (ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of idh1 mutant cancers. ACS Med Chem Lett. 2018;9:300–305. doi:10.1021/acsmedchemlett.7b00421.
- Turk S, Turk C, Akbar MW, et al. Renin angiotensin system genes are biomarkers for personalized treatment of acute myeloid leukemia with Doxorubicin as well as etoposide. PLoS One. 2020 Nov 25;15(11):e0242497, doi:10.1371/journal.pone.0242497.
- Stone RM, Manley PW, Larson RA, et al. Midostaurin: its odyssey from discovery to approval for treating acute myeloid leukemia and advanced systemic mastocytosis. Blood Adv. 2018;2:444–453. doi:10.1182/bloodadvances.2017011080.