ABSTRACT
Objective
The remarkable effect of arsenic trioxide (ATO) was verified, but side effects are generally observed in acute promyelocytic leukemia (APL) patients, especially leukocytosis and hepatotoxicity. Our aims are to study predictors and reduce ATO-induced side effects without inhibiting efficacy.
Methods
Sulfhydryl in ATO-treated APL patients was detected by the Spectra Max M5 microplate reader. And patients were divided into high and low sulfhydryl groups according to median sulfhydryl concentration. The onset time of leukocytosis and the peak value of WBC were compared . Correlations between hepatotoxicity indicators and sulfhydryl concentrations were analysed.
Results
The concentration of sulfhydryl before treatment was significantly higher in the high sulfhydryl group. Leukocytosis ((7.0 ± 5.5) vs. (14.6 ± 8.5) day) and the peak value of WBC occurred earlier in the low sulfhydryl group ((10.8 ± 5.9) vs. (19.3 ± 5.5) day) than in the high group, and the peak value was significantly lower in the low sulfhydryl group ((24.04 ± 15.05) × 109/L) than in the high group ((42.95 ± 25.57) × 109/L). The elevated liver enzymes were smaller in the higher sulfhydryl group between time points before treatment and the treatment one week later (ΔALT 66.57 vs. 9.85 U/L, ΔAST 59.52 vs. 17.76 U/L), as between time points before treatment and peak value. There was a negative correlation between sulfhydryl and elevated liver enzymes.
Conclusions
Higher sulfhydryl compounds contribute to ameliorating ATO-induced leukocytosis and hepatotoxicity in APL patients. The low sulfhydryl before treatment can advance the onset of leukocytosis. For patients with higher sulfhydryl in the early stage, close monitoring of liver enzymes is warranted instead of prophylactic applying any hepatoprotective intervention, to maintain ATO efficacy.
Introduction
Arsenic trioxide (ATO) is a striking medication for patients with acute promyelocytic leukemia (APL). However, ATO has some toxic effects and is accompanied by a series of side effects in a fewer patients [Citation1,Citation2]. ATO-treated patients typically suffer from leukocytosis, hepatotoxicity and cardiac toxicity [Citation3–5]. Concerns have been raised about leukocytosis and hepatotoxicity in APL patients treated with ATO.
Leukocytosis is the most common side effect of ATO treatment, which can induce obviously hypoxemia and hypoglycemia, this can lead to dyspnea, chest tightness, cyanosis of the lips, cold sweating, coma and fatigue [Citation6]. Hepatotoxicity was observed in 65.5% of APL patients treated with single-agent ATO, especially during the induction phase. Increased liver enzymes (ALT, AST and GGT) are indicators of ATO-induced liver toxicity, accounting for 96.6% of the observed hepatotoxicity [Citation7]. ATO is hydrolyzed into trivalent inorganic arsenic (iAsIII), which can be rapidly converted into other metabolites in the liver [Citation8]. The reason for arsenic toxicity is the concentration of trivalent metabolites in the body [Citation9,Citation10]. The main arsenic metabolites can bind to plasma proteins and enter into cells [Citation11,Citation12]. These characteristics are important to evaluate therapeutic effects and side effects [Citation13,Citation14].
The sulfhydryl is the functional group for the total sulfhydryl compounds (R-SH). Sulfhydryl compounds easily bind to ATO, especially trivalent arsenicals which are efficient in promoting APL cell death and likely to damage the liver. The combinations were easily eliminated from the body on the physiological Potential of hydrogen (PH) condition [Citation15]. Researchers demonstrated that ATO is easy to accumulate in liver cells, especially in lower sulfhydryl concentrations, leading to cell membrane damage and liver enzyme leakage [Citation16].
Therefore, the present study aimed to examine the influences of sulfhydryl on ATO-induced leukocytosis and hepatotoxicity in APL patients. To the best of our knowledge, toxicity assessment prediction-outcome studies have not yet been seen and even the basic research on predictors is rarely reported and this study provides a basis for ATO-individualized treatment.
Materials and methods
Ethics approval
This study was approved by the Medical Ethics Committee of our hospital. Written informed consent was obtained from all subjects.
Patients and study design
Patients’ cohort and ATO treatment protocol
The patients’ cohort is a mixed cohort which includes adult and pediatric acute promyelocytic leukemia patients. The ATO solution (10 mg/10 mL) was supplied by Harbin Yida Pharmaceutical Company, dissolved in 500 mL 5% dextrose and administered daily at a dose of 0.20 mg/kg for children > 6 years old and 0.16 mg/kg for children ≤6 years old, with a maximum daily dose of 10 mg. The total ATO dose was infused intravenously for >18 h.
Detection of sulfhydryl compounds
A total of 20 newly diagnosed APL patients from November 2014 until November 2016 received single-agent ATO (Yida, China) induction treatment without prophylactic hepatoprotective agents in the hematology department of our hospital (The First Affiliated Hospital of Harbin Medical University). The whole blood samples were centrifuged at 2500 rpm for 10 min, and the supernatant was left for the determination of plasma total sulfhydryl group concentration. The total sulfhydryl detection includes free sulfhydryl compounds and sulfhydryl groups on proteins. Plasma sulfhydryl compound concentration was detected by Trace total sulfhydryl determination kit (purchased from Nanjing Jiancheng Bioengineering Institute) and Spectra Max M5 microplate reader (Molecular Devices).
Leukocytosis
For all 20 newly diagnosed APL patients, complete blood cell count monitoring was performed every other day until the end of the treatment and patients achieved CR. Patients with leukocytosis are that with an increase in the number of white blood cells (WBC) greater than 10 × 109/L during ATO treatments. The evaluation of leukocytosis includes the appearance time of leukocytosis, the appearance time of WBC peak value and the peak value of WBC.
Liver function
Liver function was monitored weekly. For patients with impaired liver function, comprehensive monitoring and the necessary supportive therapy were provided until the end of the treatment and patients achieved CR. During ATO treatments, patients with abnormal liver enzymes ALT, AST, or GGT were defined as having impaired liver function (absolute values of ALT > 40 U/L; AST > 40 U/L; GGT > 50 U/L). ΔALT, ΔAST and ΔGGT as changes in liver enzymes between time points between the first occurrence of liver impairment and before ATO treatment.
Statistics
All data were analyzed using SPSS 17.0 software and Graph Pad Prism 6. A Pearson’s correlation coefficient analysis was applied to assess correlations of sulfhydryl with liver enzymes (ΔALT, ΔAST and ΔGGT) between the first occurrence of liver impairment and before ATO treatment; Student t-test was applied to compare the onset time of leukocytosis and the peak value of WBC, as well as the elevated liver enzyme in different sulfhydryl groups. All tests were two-sided, and a p-value of less than 0.05 was considered statistically significant.
Results
Higher sulfhydryl compounds contribute to ameliorating ATO-induced leukocytosis
The initial plasma sulfhydryl concentrations were measured before ATO administration in the enrolled APL patients. There were no relevant studies on the baseline plasma sulfhydryl concentrations of normal or leukemia patients, especially in APL patients; therefore, the dividing line of the grouping criteria is the median value of the detected sulfhydryl concentrations, the ‘high-SH group’ is higher than the median value, and the ‘low-SH group’ is lower than the median value.
Paired t-test was performed on patients grouped by the median of the detected sulfhydryl concentrations, and the sulfhydryl levels were statistically different between the two groups (‘high sulfhydryl group’ and ‘low sulfhydryl group’). The concentration of plasma sulfhydryl before treatment was significantly higher in the high sulfhydryl group than that in the low sulfhydryl group (178.93 vs. 42.15 μmol/L) (Z = 5.34, p = 0.012). We compared the time to appearance of leukocytosis and WBC peak value after the initiation of ATO treatment, and a total of peak values of WBC were recorded in the two groups ().
Table 1. Correlation between sulfhydryl concentration and Leukocytosis (X ± S).
Student t-test was applied to compare both groups. Leukocytosis occurred earlier in the low sulfhydryl group than in the high sulfhydryl group ((7.0 ± 5.5) vs. (14.6 ± 8.5) day, p = 0.029), as well as the appearance time of WBC peak value ((10.8 ± 5.9) vs. (19.3 ± 5.5) day, p = 0.011). The peak value was significantly lower in the low sulfhydryl group ((24.04 ± 15.05) × 109/L) than that in the high sulfhydryl group ((42.95 ± 25.57) × 109/L) (p = 0.021) (). The above results demonstrated statistically significant.
Higher sulfhydryl compounds contribute to ameliorating ATO-induced hepatotoxicity
Correlation of sulfhydryl and elevated enzymes
A total of 20 APL patients were tested for sulfhydryl detection. The above 20 patients were without prophylactic application of hepatoprotective agents during ATO treatment. We divided it into two groups according to sulfhydryl level, higher or lower. We compared change ALT or AST values within the first week (ΔALT(W1) and ΔAST(W1)) for the above groups as well as the change value between the peak value and the initial value (ΔALT(Peak) and ΔAST(Peak)). We also compared time points of the first impaired liver function (defined as ALT, AST or GGT higher than the upper limit of normal).
In the lower sulfhydryl group, ΔALT (W1) and ΔAST (W1) were 66.57 U/L and 59.52 U/L, respectively, whereas 9.85 U/L and 17.76 U/L in the higher sulfhydryl group (). A Pearson’s correlation coefficient analysis was applied to assess sulfhydryl levels and variations in liver enzymes after a week of treatment for a total of 20 patients. The results showed that there was a negative correlation between sulfhydryl levels and variations in liver enzymes after one-week treatment ( and ), the correlation in ΔALT(W1) is R = −0.797 p < 0.01 and ΔAST(W1) is R = −0.684 p < 0.01, the above correlations were all considered statistically significant.
Figure 1. The correlations between sulfhydryl and elevated liver enzymes. – SH, sulfhydryl; ΔALT(W1) and ΔAST(W1), changes in ALT and AST after one-week ATO treatment; ΔALT(peak) and ΔAST(peak), change values of ALT and AST between the peak value and initial value; (** p ≤ 0.01;* p ≤ 0.05).
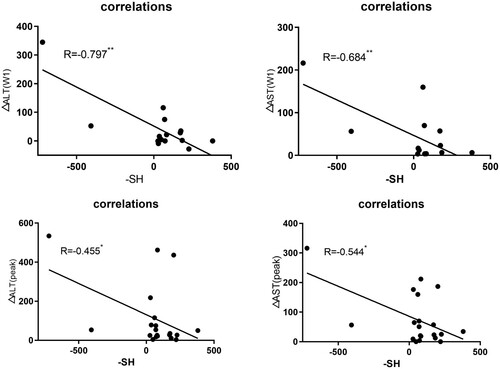
Figure 2. The mean elevated liver enzymes of lower sulfhydryl and higher sulfhydryl groups.△ALT(W1) and △AST(W1), changes in ALT and AST after one-week ATO treatment; △ALT(peak) and △AST(peak), change values of ALT and AST between the peak value and initial value.
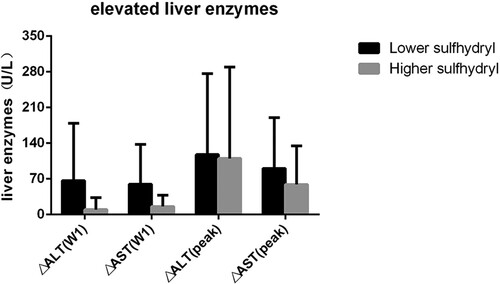
The elevated peak values of the higher sulfhydryl group were less than the lower sulfhydryl group (ΔALT (Peak) and ΔAST (Peak), 110.21 U/L and 59 U/L vs. 117.8 U/L and 90.7 U/L). Comparisons of sulfhydryl levels and elevated peak values of a total of 20 patients were performed using Pearson’s test. A negative correlation was found between sulfhydryl levels and the elevated values in ALT peak values (ΔALT (Peak)) (R = −0.455 p < 0.05), and also a negative correlation was found between sulfhydryl levels and changes in AST peak values (ΔAST (Peak)) (R = −0.544 p < 0.05). The results (p < 0.05) were all considered statistical significance, while a weaker correlation of elevated peak values than changes a week treatment later, in other words, the correlation of changes a week treatment later is more noticeable.
In the lower sulfhydryl group, the average time point of the first impaired liver function appeared on the 4.7th day. In the higher sulfhydryl group, the average time point of the first impaired liver function appeared was on the 9.7th day, in terms of, the appeared time of elevated liver enzymes is later than the lower sulfhydryl group.
Discussion
Arsenic trioxide is a striking medication for patients with acute promyelocytic leukemia. However, ATO side effects affected therapeutic effects. How to reduce side effects without reducing the efficacy of ATO is an urgent problem. In our study, we suggest that it is necessary to detect sulfhydryl compound concentration before ATO application in the early stage.
Sulfhydryl can bind to toxic substances, thereby ensuring chemical reactions normally involving thiol-containing enzymes such as pyruvate dehydrogenase and glutathione peroxidase [Citation17,Citation18]. The major mechanism of iAsIII-mediated APL treatment is targeting and destabilizing PML-RARα, which contains cysteine residues and sulfhydryl functional groups [Citation19]. In contrast to previous studies, the total sulfhydryl compounds, which include free sulfhydryl compounds and sulfhydryl groups on proteins, were detected in our study. And the maximum detection of sulfhydryl groups bound to iAsIII can better illustrate the role of the sulfhydryl group in ATO-induced side effects of acute promyelocytic leukemia patients.
Higher sulfhydryl may weaken iAsIII role in promoting differentiation and reduce/delay leukocytosis. In our study, the occurrence time of leukocytosis and the peak value of WBC count were relatively late may be caused by the iAsIII effect of promoting differentiation, which affected the ATO therapeutic effect on APL. The first week of ATO treatment is a critical period for APL patients, we can predict the time of occurrence of leukocytosis and take intervention measures to maximize the ATO therapeutic effect, reduce the adverse reactions and realize individualized treatment, according to the initial plasma sulfhydryl level. And our result suggested higher sulfhydryl compound levels may reduce and delay liver impairment. The main causes may be the quicker binding of sulfhydryl groups to the active products of arsenic and the easier excretion of sulfhydryl groups from the body which inhibit the arsenic therapeutic effect [Citation20]. The initial values of ALT and AST are different; therefore; we couldn’t evaluate liver impairment and just rely on absolute values of ALT and AST to evaluate liver damage for APL patients’ undergoing ATO treatment. In our study, we carried out dynamic data analysis: changes in ALT and AST a week of treatment later and elevated peak values. The first week of ATO treatment is a critical period, so we focus on the first week of liver function changes to rule out any interference in the ATO effect. Although iAsIII may lead to liver injury, the free iAsIII proportion is small in plasma arsenic metabolites [Citation21].
However, previous studies have confirmed that too many sulfhydryl compounds reduce the curative effect of ATO [Citation10,Citation15,Citation20,Citation22]. Simone Kann demonstrated that sensitivity to ATO was altered in APL cells when regulating the cell glutathione (GSH) level [Citation20]. Akao et al demonstrated that NB4 cells (an acute promyelocytic leukemia cell line) failed to apoptosis when the intracellular GSH level was more than 40 mmol/l and the therapeutic effects were attenuated [Citation13]. The As2O3-resistant cell line (NB4/As) has a higher cellular GSH level than the As2O3-sensitive cell line (NB4). The GSH synthesis inhibitor can completely restore the sensitivity of NB4/As to As2O3. Akao revealed that ATO resistance is stronger in higher intracellular GSH-level animals [Citation23].
The above results lay the foundation for future research about arsenic metabolites; efficacy and side effects in APL patients. Consideration of regulated arsenic metabolism is important to improve appropriate ATO therapy. Arsenic methylations were mainly affected by Arsenic (+3) methyltransferase (AS3MT) and methylation materials (e.g. methionine) [Citation24,Citation25]. These are also our further research studies. All the above, to get better efficacy and lower toxicity is our final goal. This study was conducted in newly diagnosed and first treated APL patients. The effect of plasma sulfhydryl level in recurrent and maintenance treatment of APL needs to be confirmed by further study.
Conclusions
Taken together, our works suggest that it is necessary to detect sulfhydryl component concentration before ATO application in APL patients. The low sulfhydryl concentration can advance the onset of leukocytosis. For patients with lower sulfhydryl concentrations, we should closely monitor liver function and apply hepatoprotective agents if necessary; for patients with higher sulfhydryl concentrations, close monitoring of liver function is warranted instead of prophylactic applying any hepatoprotective intervention in APL patients.
Authors’ contributions
All authors contributed to the study’s conception and design. Material preparation was performed by QZ and KG. Data collection was performed by HW and XS. Data analysis was performed by MS and XY Z. The first draft of the manuscript was written by MS and ZZ. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors gratefully acknowledge all teachers in the Central Laboratory of The First Affiliated Hospital of Harbin Medical University for the continued generous support of this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Kuo YJ, Liu YJ, Way TD, et al. Synergistic inhibition of leukemia WEHI-3 cell growth by arsenic trioxide and Hedyotis diffusa Willd extract in vitro and in vivo. Exp Ther Med. 2017;13(6):3388–3396. doi:10.3892/etm.2017.4392
- Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–121. doi:10.1056/NEJMoa1300874
- Shinjo K, Takeshita A, Sahara N, et al. Delayed recovery of normal hematopoiesis in arsenic trioxide treatment of acute promyelocytic leukemia: a comparison to all-trans retinoic acid treatment. Intern Med. 2005;44(8):818–824. doi:10.2169/internalmedicine.44.818
- Wang H, Xi S, Liu Z, et al. Arsenic methylation metabolism and liver injury of acute promyelocytic leukemia patients undergoing arsenic trioxide treatment. Environ Toxicol. 2013;28(5):267–275. doi:10.1002/tox.20717
- Zhou J, Zhang Y, Li J, et al. Single-agent arsenic trioxide in the treatment of children with newly diagnosed acute promyelocytic leukemia. Blood. 2010;115(9):1697–1702. doi:10.1182/blood-2009-07-230805
- Flanagan B, Keber B, Mumford J, et al. Hematologic conditions: leukocytosis and leukemia. FP Essent. 2019;485:17–23.
- Mathews V, Desire S, George B, et al. Hepatotoxicity profile of single agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia, its impact on clinical outcome and the effect of genetic polymorphisms on the incidence of hepatotoxicity. Leukemia. 2006;20:881–883. doi:10.1038/sj.leu.2404165
- Maimaitiyiming Y, Zhu H, Yang C, et al. Biotransformation of arsenic trioxide by AS3MT favors eradication of acute promyelocytic leukemia: revealing the hidden facts. Drug Metab Rev. 2020;52(3):425–437. doi:10.1080/03602532.2020.1791173
- Maimaitiyiming Y, Wang C, Xu S. Role of arsenic (+3 oxidation state) methyltransferase in arsenic mediated APL treatment: an in vitro investigation. Metallomics. 2018;10(6):828–837. doi:10.1039/C8MT00057C
- Sui M, Zhang Z, Zhou J. Inhibition factors of arsenic trioxide therapeutic effects in patients with acute promyelocytic leukemia. Chin Med J (Engl). 2014;127(19):828–837. doi:10.1039/C8MT00057C
- Khairul I, Wang QQ, Jiang YH, et al. Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget. 2017;8(14):23905–23906. doi:10.18632/oncotarget.14733
- Iriyama N, Yoshino Y, Yuan B, et al. Speciation of arsenic trioxide metabolites in peripheral blood and bone marrow from an acute promyelocytic leukemia patient. J Hematol Oncol. 2012;5:1. doi:10.1186/1756-8722-5-1
- Zhang Z, Chen Y, Meng H, et al. Determination of arsenic metabolites in patients with newly diagnosed acute promyelocytic leukemia treated with arsenic trioxide. Leuk Lymphoma. 2013;54(9):2041–2046. doi:10.3109/10428194.2013.769222
- Šlejkovec Z, Podgornik H, Černelč P, et al. Exceptions in patterns of arsenic compounds in urine of acute promyelocytic leukaemia patients treated with As2O3. Biometal. 2016;29(1):107–118. doi:10.1007/s10534-015-9901-5
- Akao Y, Yamada H, Nakagawa Y. Arsenic-induced apoptosis in malignant cells in vitro. Leuk Lymphoma. 2000;37(1):53–63. doi:10.3109/10428190009057628
- Mathews VV, Paul MS, Abhilash M, et al. Mitigation of hepatotoxic effects of arsenic trioxide through omega-3 fatty acid in rats. Toxicol Ind Health. 2014;30(9):806–813. doi:10.1177/0748233712463778
- Rech VC, Mezzomo NJ, Athaydes GA, et al. Thiol/disulfide status regulates the activity of thiol-containing kinases related to energy homeostasis in rat kidney. An Acad Bras Cienc. 2018;90(1):99–108. doi:10.1590/0001-3765201720160348
- Jung K, Kwak M. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15(10):7266–7291. doi:10.3390/molecules15107266
- Maimaitiyiming Y, Wang QQ, Yang C, et al. Hyperthermia selectively destabilizes oncogenic fusion proteins. Blood Cancer Discov. 2021;2(4):388–401. doi:10.1158/2643-3230.BCD-20-0188
- Kitamura K, Minami Y, Yamamoto K, et al. Involvement of CD95-independent caspase 8 activation in arsenic trioxide-induced apoptosis. Leukemia. 2000;14:1743–1750. doi:10.1038/sj.leu.2401900
- Kiguchi T, Yoshino Y, Yuan B, et al. Speciation of arsenic trioxide penetrates into cerebrospinal fluid in patients with acute promyelocytic leukemia. Leuk Res. 2010;34(3):403–405. doi:10.1016/j.leukres.2009.08.001
- Kann S, Estes C, Reichard JF, et al. Butylhydroquinone protects cells genetically deficient in glutathione biosynthesis from arsenite-induced apoptosis without significantly changing their prooxidant status. Toxicol Sci. 2005;87(2):365–384. doi:10.1093/toxsci/kfi253
- Akao Y, Nakagawa Y, Akiyama K. Arsenic trioxide induces apoptosis in neuroblastoma cell lines through the activation of caspase3 in vitro. FEBS Lett. 1999;455(1):59–62. doi:10.1016/s0014-5793(99)00841-8
- Gribble MO, Tang WY, Shang Y, et al. Differential methylation of the arsenic (III) methyltransferase promoter according to arsenic exposure. Arch Toxicol. 2014;88(2):275–282. doi:10.1007/s00204-013-1146-x
- Howe CG, Niedzwiecki MM, Hall MN, et al. Folate and cobalamin modify associations between S-adenosylmethionine and methylated arsenic metabolites in arsenic-exposed Bangladeshi adults. J Nutr. 2014;144(5):690–697. doi:10.3945/jn.113.188789
