ABSTRACT
Cardiotoxicity of antitumor therapy results in declining survival rates. More specifically, cardiotoxicity is positively correlated with cumulative dose of anthracyclines and eventually develops from reversible to irreversible. In this context, early monitoring methods should be explored for the timely detection of cardiotoxicity and cardioprotective therapy should be performed in patients under consideration for potentially cardiotoxic therapy. This paper reports a 22-year-old male patient with acute myeloid leukemia who underwent whole-course cardiac monitoring after receiving antileukemia therapy. After the early detection of an asymptomatic decrease in left ventricular ejection fraction (LVEF), along with a significant decrease in global longitudinal strain (GLS), the patient was treated with sacubitril/valsartan (Sac/Val). Finally, the patient completed four courses of chemotherapy and subsequent hematopoietic stem cell transplantation as planned. The measurements of LVEF and GLS also recovered after 2 months treatment of Sac/Val. Therefore, the early identification and protection of patients with cardiotoxicity are of paramount importance and future prospective studies are expected to develop the management and treatment of cancer treatment-related cardiac dysfunction.
Introduction
Over the past several decades, the development and renewal of antitumor drugs and improved supportive care have led to prolonged survival in patients with malignant neoplastic disease. However, the cardiotoxicity of antitumor therapy increased overall mortality. Cancer treatment-related cardiac dysfunction (CTRCD) may lead to heart failure (HF) and death if left untreated [Citation1]. Despite the formulation of the corresponding diagnostic criteria for CTRCD, sensitive monitoring methods and effective preventive and therapeutic measures still require further investigations. In contrast, speckle tracking imaging (STI) techniques are considered more sensitive and reproducible for monitoring early cardiotoxicity. Meanwhile, the global longitudinal strain (GLS) measured by STI can quantitatively evaluate the left ventricular systolic function [Citation2]. While β blockers and ACEI/ARBs are most commonly used for CTRCD, the treatment of sacubitril/valsartan (Sac/Val) offers a new option. Hence, this paper utilizes the reported case to generate new possibilities for early cardiotoxic management.
Case report
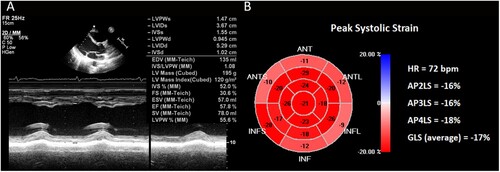
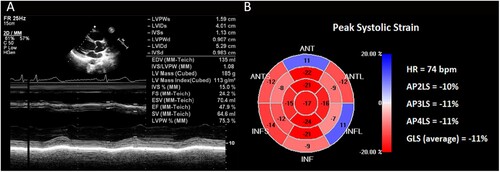
Our patient, a 22-year-old Chinese male, was referred due to anemia, pancytopenia, and abnormal blood smear with no significant past medical history. Bone marrow examination was reported as acute myeloid leukemia (AML) M2 subtype in the FAB classification. Translocation (8;21)(q22;q22) which produced the fusion gene RUNX1-RUNX1T1 (AML1/ETO), displayed a characteristic immunophenotype, with a subpopulation of blast cells showing high-intensity expression of CD34, CD117, CD38, CD13, CD 33 and HLA-DR, but relatively weak expression of CD 56. The patient had a 5-year smoking history, no previous history of cardiovascular disease, and presented favorable physical examination results. We conducted comprehensive and standardized management on his antileukemia treatment during hospitalization. In order to monitor subclinical cardiotoxicity, we routinely used the two-dimensional speckle tracking imaging (2D-STI) technology for cardiac assessment. The left ventricular ejection fraction (LVEF) and GLS variables were 58% ((A)) and −17% ((B)) at the initial diagnosis of AML. As the bone marrow revealed residual disease after the IA regimen (50 mg idarubicin total dose; 200 mg cytarabine, 1 time/day, days 1-7) induction chemotherapy, indicating that the patient did not achieve complete remission after the first course, the patient was adjusted to the CLAG regimen (7.9 mg cladribine and 2.5 g cytarabine, 1 time/day, days 1-5; G-CSF 300 mg, 1 time/day, days 0-5) for a 4-week cycle for the following 3 courses. Prior to each course, we evaluated the cardiac function. The patient had no precordial discomfort, and no severe arrhythmia was found by the electrocardiogram. However, before the fourth course, we monitored LVEF decreased to 48% ((A)) and GLS decreased to −11% ((B)), while the serum cardiac troponin (cTn), creatine kinase isoenzyme MB (CK-MB) and N-terminal pro-brain natriuretic peptide (NT-pro-BNP) were detected as normal. According to the established criteria, the patient was diagnosed with CTRCD [Citation3]. As a result, the patient was advised to take Sac/Val 25 mg b.i.d.
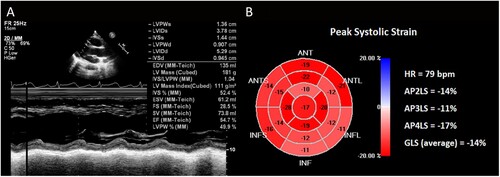
According to his blood pressure, renal function and serum potassium, the dose of the drug was gradually increased under safe conditions. Sac/Val was administered 100 mg daily and 50 mg nightly after the completion of the fourth course and while waiting for allogeneic hematopoietic stem cell transplantation (allo-HSCT). After 2 months, LVEF recovered to 55% ((A)) and GLS recovered to -14% ((B)). Dynamic electrocardiogram also demonstrated no obvious severe arrhythmia. Since the patient and his father were of human leukocyte antigen (HLA) haploidentical, the hematologist recommended him to undergo conditioning chemotherapy regimen of FLAG combined with modified BUCY (fludarabine, 30 mg/m2, days 1–5; cytarabine, 2 g/m2, days 1–5; G-CSF 300 mg, days 0–5; busulfan, 0.8 mg/kg every 6 h, days 6–9). To prevent acute graft-versus-host disease (GVHD), the patients received intravenous cyclosporine at a dose of 3 mg/kg/day in two split administration, with adjusted trough plasma level of 100–250 ug/L. Rabbit anti-thymocyte globulin (ATG) was administered at a total dose of 4–6 mg/kg on day −2 and day −1. Short-term methotrexate was infused on the first (15 mg/m2), third, sixth, and eleventh (10 mg/m2, respectively) days after transplantation. The Sac/Val was combined with diuretics to prevent HF, given the possibilities of aggravating cardiotoxicity with the conditioning regimen and related treatments to HSCT. Ultimately, the patient successfully completed allo-HSCT without HF and signs of elevated cTn and NT-pro-BNP. Analysis of short tandem repeats (STR) following allo-HSCT determined that the patient achieved early (<30 days) and sustained full donor chimerism (FDC). The patient continued the treatment of Sac/Val for 6 months after allo-HSCT, no symptoms or manifestations of water-sodium retention, hypotension, renal insufficiency, or hypokalemia were observed.
Discussion
The backbone of therapy for AML is a combination of cytarabine- and anthracycline-based regimens with HSCT for eligible candidates. Chemotherapy agents act by destroying malignant cells, but they may also have the adverse effects to cardiac function. Cardiotoxicity has been proved to be positively correlated with the cumulative dose of anthracyclines [Citation4]. In addition, bacterial sepsis occurs frequently during AML treatment and contributes to the development of infection-associated left ventricular systolic dysfunction (LVSD) [Citation5]. With the improved survival by advanced therapy strategies, cardiotoxicity becomes more clinically relevant. The AAML0531 Clinical Trial has demonstrated that the occurrence of early cardiotoxicity was associated with statistically significant and clinically meaningful reductions in event-free survival (EFS) and overall survival (OS) [Citation6]. In patients undergoing HSCT, reduced LVEF (<50%) is associated with an increased risk of nonrelapse mortality (NRM) and GVHD [Citation7]. Therefore, early detection of cardiotoxicity and initiation of cardioprotective therapy (CPT) are extremely important.
Traditional surveillance of LVEF often identifies myocardial dysfunction, and it can be measured by different cardiac imaging techniques. Cardiac magnetic resonance (CMR) imaging is a non-invasive imaging technique that can accurately quantify the cardiac structure and function. However, the use of CMR is limited by its complex technology, high price, noise effects, and unavailability for patients with metal implants. Echocardiography is currently the first line investigation for the evaluation of LVEF. Despite its effectiveness, it has shown that serial measurements of LVEF are unable to detect subtle regional changes and its reduction may become manifest at a later stage of the disease process when significant toxicity has already occurred [Citation8]. Generally, endocardial damage does not cause a decrease in stroke volume and LVEF under the compensation of epicardium [Citation3]. A growing body of work suggests that GLS measured by STI can reflected the ultrastructural function of the myocardium and is the first dimension of myocardial tension damage that can evaluate endocardial damage [Citation9]. The decline of GLS often occurs prior to a reduced LVEF [Citation10]. Thus, it can identify subclinical myocardial dysfunction early and prognosticate subsequent CTRCD or HF. Furthermore, STI can combine left ventricular pressure (LVP) and GLS for the non-invasive and quantitative evaluation of the left ventricular myocardial work (LVMW). By establishing the relationship between pressure and strain, the left ventricular pressure-strain loop (LVPSL) is used to evaluate segmental and global LVMW [Citation11]. Compared with GLS, it reduced the influence of heart loading conditions on the strain and also serves as a parameter for early monitoring of cardiac function [Citation12].
Based on the available clinical evidence, we suggest that a baseline cardiological assessment should be performed in patients under consideration for potentially cardiotoxic therapies, particularly those with risk factors or known cardiovascular disease. In addition, these patients should undergo a detailed echocardiographic follow-up including measurements of LVEF, GLS and diastolic function every 3 course of chemotherapy, pre- and post-HSCT, and between 6 and 12 months after completion of treatment [Citation13]. If the LVEF falls by 10% to below 50% or GLS falls by 15% compared to baseline, early initiation of standard HF treatment may be beneficial and repeat echocardiograms should be performed every month during CPT [Citation14].
β-blockers have been proved to improve the significant decrease of LVEF and reduce the cardiovascular mortality in anthracyclines-treated patients [Citation15–17]. ACEI/ARBs can also effectively prevent myocardial injury as detectable by troponin increase were observed [Citation18–20]. The OVERCOME study has shown that the concomitant treatment with enalapril and carvedilol may prevent LVSD in patients with malignant hemopathies treated with high-dose chemotherapy regimens [Citation21]. However, there is no consensus on the standard treatment for CTRCD. Sac/Val, the first angiotensin receptor-neprilysin inhibitor (ARNI), can inhibit vasoconstriction and aldosterone secretion, maintain water and sodium balance, protect target organs and reverse myocardial remodeling [Citation22]. In 2015, Sac/Val gained its FDA approval for the treatment of HF with reduced EF (HFrEF), which means LVEF ≤ 40%. The PARADIGM-HF study exhibits a significant decrease in cardiovascular death or HF hospitalization rate after treatment with Sac/Val compared to enalapril [Citation23]. Same results have also been studied in the subgroup of HF with mid-range EF (HFmrEF) (45 ≤ LVEF ≤ 57%) in the succeeding study [Citation24]. In addition, the GLS of HFrEF patients was elevated after 3 months and 1 year of treatment with Sac/Val, but not in the ACEI/ARB group [Citation25,Citation26]. In view of the potent anti-heart failure effects of Sac/Val, its clinical application in cardio-oncology has also received widespread attention. For cancer patients with HFrEFA, short-term treatment of Sac/Val can significantly improve the ultrasound parameters, including LVEF, left ventricle internal diameter in diastole (LVIDD) and diastolic function [Citation27]. Another retrospective study also shows that Sac/Val can increase LVEF and functional class, and decreased NT-pro-BNP in patients with CTRCD [Citation28].
It is worth noting that the prevention of cardiotoxicity is even more important than CPT, and it's still necessary to standardize monitoring strategies to early identify CTRCD for timely clinical intervention. Future prospective studies are expected to develop the management and treatment of CTRCD.
Conclusion
The whole-course monitoring demonstrated the importance of the early detection of cardiotoxicity, providing evidence for early intervention that reversed cardiotoxicity and enabled the patient to undergo subsequent transplantation. This case was significant because 2D-STI was used for the first time to evaluate the efficacy of Sac/Val in the treatment of subclinical cardiotoxicity associated with tumor therapy. Thus, exploring other potential monitoring methods and protective agents must be done for early cardiotoxicity.
Statement of ethics
The patient performed all necessary examinations and provided written informed consent to use these examinations for research purposes and publication. This case report was issued in accordance with the World Medical Association Declaration of Helsinki.
Acknowledgements
MW and TJ designed the study and collected the data. MW drafted the manuscript. TJ revised the article and did the final editing. QD, and NZ contributed to the acquisition of data and critically reviewed of the manuscript. XT assisted in study design and critically reviewed the article. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Brady B, Murphy R. Cancer treatment-related cardiac dysfunction. BMJ Support Palliat Care. 2022 Jul 26. 13(1):53–56. doi:10.1136/spcare-2022-003832
- Oikonomou EK, Kokkinidis DG, Kampaktsis PN, et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. 2019 Oct 1;4(10):1007–1018. doi:10.1001/jamacardio.2019.2952
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015 Mar;16(3):233–270. doi:10.1093/ehjci/jev014
- Fujiwara SI, Murahashi R, Nakashima H, et al. Effect of cumulative daunorubicin dose on cardiotoxicity after allogeneic stem cell transplantation. Leuk Res. 2022 Oct;121:106951. doi:10.1016/j.leukres.2022.106951
- Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care. 2009 Oct;15(5):392–397. doi:10.1097/MCC.0b013e3283307a4e
- Getz KD, Sung L, Ky B, et al. Occurrence of treatment-related cardiotoxicity and its impact on outcomes among children treated in the AAML0531 clinical trial: a report from the children's oncology group. J Clin Oncol: Official J Am Soc Clin Oncol. 2019 Jan 1;37(1):12–21. doi:10.1200/jco.18.00313
- Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005 Oct 15;106(8):2912–2919. doi:10.1182/blood-2005-05-2004
- Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010 Jan 19;55(3):213–220. doi:10.1016/j.jacc.2009.03.095
- Collier P, Phelan D, Klein A. A test in context: myocardial strain measured by speckle-tracking echocardiography. J Am Coll Cardiol. 2017 Feb 28;69(8):1043–1056. doi:10.1016/j.jacc.2016.12.012
- Kang Y, Xu X, Cheng L, et al. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur J Heart Fail. 2014 Mar;16(3):300–308. doi:10.1002/ejhf.8
- Papadopoulos K, Ozden Tok O, Mitrousi K, et al. Myocardial work: methodology and clinical applications. Diagnostics (Basel). 2021 Mar 22;11(3):573. doi:10.3390/diagnostics11030573
- Roemer S, Jaglan A, Santos D, et al. The utility of myocardial work in clinical practice. J Am Soc Echocardiogr. 2021 Aug;34(8):807–818. doi:10.1016/j.echo.2021.04.013
- Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol: Official J Am Soc Clin Oncol. 2017 Mar 10;35(8):893–911. doi: 10.1200/JCO.2016.70.5400.
- Thavendiranathan P, Negishi T, Somerset E, et al. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol. 2021 Feb 2;77(4):392–401. doi:10.1016/j.jacc.2020.11.020
- Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018 May 22;71(20):2281–2290. doi:10.1016/j.jacc.2018.02.049
- Menna P, Salvatorelli E. Primary prevention strategies for anthracycline cardiotoxicity: a brief overview. Chemotherapy. 2017;62(3):159–168. doi:10.1159/000455823.
- Nabati M, Janbabai G, Baghyari S, et al. Cardioprotective effects of carvedilol in inhibiting doxorubicin-induced cardiotoxicity. J Cardiovasc Pharmacol. 2017 May;69(5):279–285. doi:10.1097/FJC.0000000000000470.
- Janbabai G, Nabati M, Faghihinia M, et al. Effect of enalapril on preventing anthracycline-induced cardiomyopathy. Cardiovasc Toxicol. 2017 Apr;17(2):130–139. doi:10.1007/s12012-016-9365-z
- Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 ( 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016 Jun 1;37(21):1671–1680. doi:10.1093/eurheartj/ehw022
- Cardinale D, Ciceri F, Latini R, et al. Anthracycline-induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: the international CardioOncology society-one trial. Eur J Cancer. 2018 May;94:126–137. doi:10.1016/j.ejca.2018.02.005
- Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (Prevention of left ventricular dysfunction with enalapril and caRvedilol in patients submitted to intensive chemotherapy for the treatment of malignant hEmopathies). J Am Coll Cardiol. 2013 Jun 11;61(23):2355–2362. doi:10.1016/j.jacc.2013.02.072
- Docherty KF, Vaduganathan M, Solomon SD, et al. Sacubitril/valsartan: neprilysin inhibition 5 years after PARADIGM-HF. JACC Heart Fail. 2020 Oct;8(10):800–810. doi:10.1016/j.jchf.2020.06.020
- McMurray JJ, Packer M, Desai AS, et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Eur J Heart Fail. 2013 Sep;15(9):1062–1073. doi:10.1093/eurjhf/hft052
- Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-Neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019 Oct 24;381(17):1609–1620. doi:10.1056/NEJMoa1908655
- De Vecchis R, Paccone A, Di Maio M. Sacubitril/valsartan therapy for 14 months induces a marked improvement of global longitudinal strain in patients with chronic heart failure: a retrospective cohort study. Cardiol Res. 2019 Oct;10(5):293–302. doi:10.14740/cr910
- Mirić D, Baković D, Eterović D, et al. Left-ventricular function after 3 months of sacubitril-valsartan in acute decompensated heart failure. J Cardiovasc Transl Res. 2021 Apr;14(2):290–298. doi:10.1007/s12265-020-10041-4
- Gregorietti V, Fernandez TL, Costa D, et al. Use of sacubitril/valsartan in patients with cardio toxicity and heart failure due to chemotherapy. Cardiooncology. 2020 Nov 5;6(1):24. doi:10.1186/s40959-020-00078-4
- Martin-Garcia A, Lopez-Fernandez T, Mitroi C, et al. Effectiveness of sacubitril-valsartan in cancer patients with heart failure. ESC Heart Fail. 2020 Apr;7(2):763–767. doi:10.1002/ehf2.12627