?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objective
To explore the effects of preoperative autologous whole blood donation and autologous pure red blood cell (RBC) donation on hematopoietic stem cells (HSC), clarify the effects of transfusion by different blood components on HSC, and improve the treatment effect of autotransfusion.
Methods
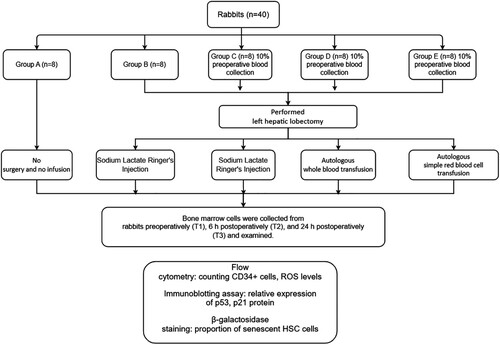
Forty healthy male New Zealand rabbits were divided into five groups (n = 8) at random: control (Group A), surgery alone (Group B), surgery + blood sampling (Group C), surgery + preoperative autologous whole blood autotransfusion (Group D), and surgery + preoperative autologous pure RBC autotransfusion (Group E). The rabbits’ bone marrow was collected before surgery (T1), 6 h after surgery (T2), and 24 h after surgery (T3) to detect the CD34+ cell count, the reactive oxygen species (ROS) concentration, the ratio of senescent cells, and the expression of HSC-related proteins (p53 and p21).
Results
Compared with groups A and B, the percentage of CD34+ cells in groups D and E at each time point was significantly increased, while the proportion of senescent cells, ROS, p53 and p21 were significantly decreased (P<0.05). Compared with Group C, the percentage of CD34+ cells at T2 and T3 rose significantly, while the ratio of senescent cells, the ROS concentration, and the content of p53 and p21 declined significantly in Groups D and E (P < 0.05). Compared with Group D, the ratio of senescent cells at T2 declined significantly, the percentage of CD34+ cells at T3 rose significantly, and the ROS concentration at T2 and T3 declined significantly in Group E (P < 0.05).
Conclusion
From the perspective of HSC, collection and transfusion of pure RBC is more beneficial to postoperative recovery of patients than whole blood transfusion.
1. Introduction
In recent years, transfusion safety and insufficient blood supply has become increasingly prominent, and autotransfusion has gradually attracted attention. Autotransfusion is a strategic blood supply method to compensate for surgical bleeding. It is a safe transfusion method in which a patient's own blood or blood components are collected and transfused back to them during or following surgery after storage or certain treatment [Citation1]. This method avoids transfusion-related complications, such as blood-borne disease transmission and immunosuppression [Citation2]. The patients who only receive autologous blood can eliminate risks including viral transmission, immune-mediated hemolysis, fever, and anaphylactic reaction and relieve the blood shortage [Citation3]. Common clinical autotransfusion types include preoperative autologous blood donation (PABD), acute normovolemic hemodilution, and salvaged blood autotransfusion.
The process of PABD is to collect the patient's blood and/or blood components before surgery and to store them until they are transfused back to the patient if transfusion treatment is required. For patients receiving PABD, repeated blood drawing prior to surgery, like voluntary blood donation, can stimulate the proliferation and differentiation of bone marrow hematopoietic cells, accelerate hematopoiesis, and promote their recovery [Citation4].
Blood component transfusion is when effective components in the blood are separated into high-concentration and high-potency blood products, and targeted blood compositions are subsequently transfused according to patients’ actual needs. Blood component transfusion relieves patients’ conditions by reducing the overall transfusion volume, the need for allogeneic blood, and transfusion-related complications. Reducing the frequency and volume of allogeneic blood transfusion benefits patients [Citation5,Citation6]. Also, the reduction of blood product consumption significantly lowers the cost of medical treatment [Citation7].
Hematopoietic stem cells (HSC) refer to a type of mature stem cells with self-renewal and self-differentiation abilities. They are the origin of all blood and immune cells and can be differentiated into a variety of mature blood cells. In the whole life process, the steady state and regeneration of the blood system depend on HSC activity [Citation8]. In typical cases, most HSCs are in the G0 phase of the cell cycle and maintain a quiescent state. Quiescence contributes to HSC sustainability because it protects the HSC pool from premature failure caused by various stresses [Citation9]. In case of chemotherapy injury, infection, and other stimuli, HSC are activated and start to proliferate and differentiate in quantity [Citation10].
Many factors cause HSC senescence, including DNA injury, reactive oxygen species (ROS) increase, epigenetic change, and DNA methylation [Citation11,Citation12]. Others include telomere dysfunction, polarity loss, exogenous factors, and changes in the hematopoietic microenvironment [Citation13].
Whether different autologous blood compositions affect HSC functioning after storage and transfusion is unknown. The present study aims to explore the effects of blood components in PABD on HSC proliferation and differentiation. In this study, 40 healthy male New Zealand rabbits aged 3–6 months, with a weight of 2.5–3.0 kg, were chosen as the subjects to compare the CD34+ cell count, the ROS concentration, the ratio of senescent cells, and the expression of HSC-related proteins in the bone at different periods. The results would further define the relationship between autotransfusion components, bone marrow HSC proliferation and differentiation, and improve the quality of autotransfusion.
2. Experimental materials and methods
2.1. Experimental materials
Pentobarbital sodium (Beijing Propbs Biotechnology Company); sodium lactate Ringer's solution (Cisen Pharmaceutical Co., Ltd.); sodium chloride injection (Shanghai Baite Medical Supplies Company); heparin (Wanbang Biopharma); lymphocyte separation fluid (GE Healthcare Life Sciences); RIPA lysis buffer (Lot number:R0010, Beijing Solaibao Technology Co., LTD.); CD34 antibody (Shanghai Abcam Trading Co., Ltd.); ROS kit (Beyotime Biotechnology Institute); radioimmunoprecipitation assay lysis buffer, phenylmethylsulfonyl fluoride, phosphate-buffered saline (PBS) solution, β-galactosidase staining kit (Beijing Solarbio Technology Co., Ltd.); p53 antibody and p21 antibody (Shanghai Abcam Trading Co., Ltd.); disposable blood bag (Lot number: H31022624, Shanghai Blood Transfusion Technology Co., Ltd.); centrifuge tube (Pall Corporation); disposable bone marrow puncture needle (Shanghai Jumu Medical Devices Co., Ltd.); electronic scale (Hochoice); Eppendorf® Centrifuge 5415D small high-speed centrifuge (Eppendorf®); FACSCalibur flow cytometer (BD Biosciences); DYY-7C electrophoresis apparatus (Beijing Liuyi); Gel Doc XR+ gel imaging system and Quantity One image analysis software (Bio-Rad); and a BX51 ordinary optics microscope (Olympus).
2.2. Experimental methods
Forty healthy male New Zealand rabbits aged 3–6 months, with a weight of 2.5–3.0 kg (Shanghai Songlian Center for Experimental Animals, batch No. SCXKH 2018-0012), were fed separately in clear animal cages under the following conditions: indoor temperature 24°C–28°C, relative humidity 60%–70%, day–night cycle 12 h, fasting 6 h before surgery. They were randomly divided into five groups (n = 8): Group A(control): No treatment was taken after the rabbits were anaesthesia; Group B(surgery alone): After the rabbits were anaesthesia, the left lateral lobe of the liver was surgically resected under open abdomen, and the blood volume was supplemented by infusion of sodium lactate Ringer's solution without transfusion; Group C(surgery + blood sampling): 10% of rabbit whole blood was collected and stored one week before surgery. Then the left lateral lobe of liver was surgically removed, and the blood volume was supplemented by infusion of sodium lactate Ringer's solution without transfusion; Group D(surgery + preoperative whole blood cell autotransfusion): 10% of rabbit whole blood was collected and stored one week before surgery. The left lateral lobe of liver was surgically removed, and the stored autologous whole blood was transfused back during the operation; Group E(surgery + preoperative pure RBC autotransfusion): 10% of rabbit whole blood was collected one week before surgery and made into suspended red blood cells for storage. One week later, the left lateral lobe of the liver was surgically removed and the stored autored blood cells were transfused back during the operation. The bone marrow samples were collected before surgery (T1), 6 h after surgery (T2), and 24 h after surgery (T3) ().
2.3. Preparation of autologous red blood cells and collection of bone marrow
Before surgery, 10% of the total blood volume, which amounts to 7%–8% of the rabbit weight, was collected from the rabbits. Whole blood drawn from the right femoral vein of rabbits was placed in the ACD blood storage bag, labeled with the group number, blood collection volume, and blood collection date, and stored in the refrigerator at 4°C to 6°C. Whole blood collected from group E was centrifuged at 4000r/min, and the plasma and white blood cell components were filtered and then made into suspended red blood cells for preservation. The skin of the rabbits at 1 cm under the tibial plateau was disinfected, and the bone marrow puncture needle was slowly inserted vertically. The needle core was pulled out when there was a clear sense of falling and 2 ml of bone marrow was slowly drawn from the rabbits. After filtration by the blood transfusion apparatus, the filtered bone marrow was collected into an injection syringe containing 125 U heparin and 1 ml normal saline for subsequent detection.
2.4. CD34+ cell sorting and counting with a flow cytometer
The 0.5 ml of bone marrow fluid was dripped in PBS solution (containing 1000 U heparin sodium) to be diluted to 10 ml. After centrifuging at 1,000 r/min for 5 min, the supernatant was discarded. The RIPA lysis buffer was added for lysis for 5 min. Then, the solution was centrifuged again at 1,000 r/min for 5 min, and the supernatant was discarded. A volume of PBS solution was added to resuspend the cells at a concentration of 107/mL. The 100 µL of bone marrow cell suspension was added to the CD34 antibody, incubated for 15 min at indoor temperature, and the solution was centrifuged again at 1,000 r/min for 5 min. After the supernatant was discarded, PBS was added for rinsing. The samples were placed in the flow cytometer for sorting, and FlowJo software was used to analyze the percentage of CD34+ cells before and after sorting.
2.5. Detection of reactive oxygen species activity
The serum-free culture solution was used to dilute dichloro-dihydro-fluorescein diacetate (DCFH-DA) (ratio 1:1000) with a concentration of 10 µmol/L. The 0.5 mL of bone marrow fluid was added to the diluted DCFH-DA, and the cell concentration was adjusted to 106/mL. The solution was incubated for 20 min at 37°C. After this, the serum-free culture solution was used at least three times to remove the DCFH-DA which had not entered the cells. The flow cytometer was used to detect the intensity of fluorescence at every time point (laser wavelength 488 nm, emission wavelength 525 nm), and Diva software was used for the data analysis.
2.6. Detection of p53 and p21 content
The bone marrow cells collected were rinsed three times with PBS and centrifuged at 1,000 r/min for 5 min. The supernatant was discarded, and the lysis buffer was added. The solution obtained was placed on ice for 20 min, then centrifuged for 15 min at 4°C (at 12,000 r/min), and the supernatant was taken. The bovine serum albumin standard substances with a concentration of 2 µg/µl were diluted with PBS to six gradients and added to enzyme-linked immunosorbent assay (ELISA) plate wells. The protein samples were diluted 10 times with PBS and then added to each ELISA plate well. Then, 200 µl BCA working solution was added to each ELISA plate well, mixed and incubated for 30 min at 37°C. After cooling to indoor temperature, the ELISA analyzer was used to determine the absorbance at 562 nm.
The protein concentration in the supernatant was calculated by comparing the standard curve. According to the protein quantification result, 30 μg of the sample was taken into the polymerase chain reaction tube, and 5 × sodium dodecyl sulfate buffer solution was added. After full mixing, the solution was boiled for 5 min at 95°C. After electrophoresis, the protein was transferred to the polyvinylidene difluoride membrane with the wet transfer method and was closed with 5% skim milk powder for 1 h at indoor temperature. The primary antibodies p53 (1:2,000) and p21 (1:1,000) were incubated overnight at 4°C.
The primary antibodies were rinsed with tris-buffered saline with 0.1% Tween® 20 detergent buffer solution three times, 15 min each time. Then, the second antibody marked with horseradish peroxidase diluted at 1:5,000 was added for incubation for 45 min at indoor temperature. After color development with enhanced chemiluminescence reagent, Quantity-One image analysis software was used to determine the gray value of the target strip. The ratio of this to the GAPDH stripe was the relative expression level of the target protein.
2.7. Detection of senescent cells with senescence-associated β-galactosidase staining
Senescence-associated β-galactosidase is related to cell senescence, which has the characteristic of accumulation in lysosomes of senescent cells and high expression, and can be used to determine the degree of cell senescence. It is the most widely used biological marker of senescent cells [Citation14]. Bone marrow cells were collected in a 1.5 mL centrifuge tube and rinsed with PBS. Then, 1 mL of β-galactosidase was added for staining and fixing for 15 min at indoor temperature. During fixation, the solution was slowly shaken to avoid cell clumping. After centrifugation, the fixative was absorbed, and the solution was rinsed three times with PBS (3 min each time). The solution was centrifuged again to remove the PBS, and 1 mL staining working fluid (β-galactosidase staining solution A 10 μL, β-galactosidase staining solution B 10 μL, β-galactosidase staining solution V 930 μL, and X-Gal solution 50 μL) was added to each tube. They were incubated and stained overnight at 37°C without CO2. Some stained cells were dripped on the glass slide, and an ordinary light microscope (OLYMPUS BX51) was used to observe, take photos, and count the stained cells.
2.8. Statistical analysis
SPSS™ Statistics v22.0 software was used for statistical analysis. Normal distribution measurement data was expressed with mean ± standard deviation (). One-way analysis of variance (ANOVA) was adopted for intergroup comparison, and repeated ANOVA was used for intragroup comparison. A value of P < 0.05 indicated a difference was statistically significant.
3. Results
3.1. Comparison of the general data of the rabbits
There was no statistical significance in the weight and total dosage of narcotics among the five groups. There was no statistical significance in the intraoperative blood loss among Groups B–E (P > 0.05) ().
Table 1. Comparison of general data of rabbits among all groups.
3.2. Percentage of CD34+ cells
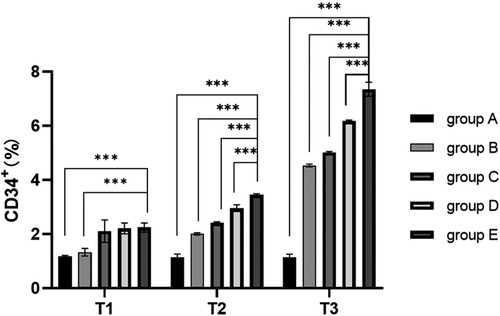
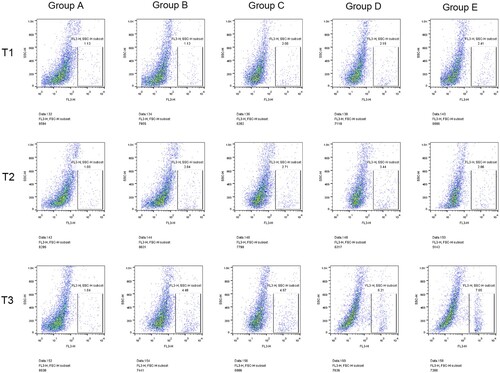
The percentage of CD34+ cells in the B-E group after hepatectomy was significantly higher than before surgery (P < 0.05), indicating that hepatectomy could increase the percentage of CD34+. Compared with group A, the percentage of CD34+ cells in preoperative blood collection group (group C-E) was significantly increased at all time points (P < 0.05). There was a significant difference between group B and group C after preoperative blood collection (P < 0.05), indicating that preoperative blood collection could increase the percentage of CD34+. Compared with groups B and C, the percentage of CD34+ cells in groups D and E after operation was significantly increased (P < 0.05), indicating that blood transfusion (whole blood /RBC) could significantly increase the percentage of CD34+. In addition, the percentage of CD34+ cells in the transfusion group of pure red blood cells was significantly higher than that in the whole blood transfusion group at 24 h after surgery (P < 0.05) (, ).
Figure 1. Comparison of percentage of CD34+ cells at different time among all groups. Note: T1: Percentage of CD34+ cells in different groups of rabbits before surgery; T2: Percentage of CD34+ cells in different groups 6 h after surgery; T3: Percentage of CD34+ cells in different groups 24 h after surgery; Group A: No treatment was taken after the rabbits were anaesthesia; Group B:After the rabbits were anaesthesia, the left lateral lobe of the liver was surgically resected under open abdomen, and the blood volume was supplemented by infusion of sodium lactate Ringer's solution without transfusion; Group C:10% of rabbit whole blood was collected and stored one week before surgery. Then the left lateral lobe of liver was surgically removed, and the blood volume was supplemented by infusion of sodium lactate Ringer's solution without transfusion; Group D: 10% of rabbit whole blood was collected and stored one week before surgery. The left lateral lobe of liver was surgically removed, and the stored autologous whole blood was transfused back during the operation; Group E: 10% of rabbit whole blood was collected one week before surgery and made into suspended red blood cells for storage. One week later, the left lateral lobe of the liver was surgically removed and the stored autored blood cells were transfused back during the operation.
3.3. Detection of reactive oxygen species in bone marrow hematopoietic stem cells
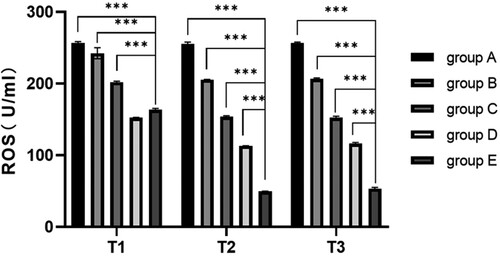
Compared with pre-operation, ROS concentration in the hepatectomy group (B-E group) decreased significantly at 6 and 24 h after surgery (P < 0.05), while ROS concentration in the hepatectomy group (B-E group) had no significant difference between 6 and 24 h after surgery (P > 0.05), indicating that hepatectomy could reduce ROS concentration. Compared with groups A and B, ROS concentration in preoperative blood collection group (group C-E) was significantly decreased at each time point (P < 0.05), indicating that blood collection could also indirectly reduce ROS concentration.
Compared with group C, ROS concentration in groups D and E was significantly decreased after surgery (P < 0.05), indicating that blood transfusion could reduce ROS concentration. Compared with whole blood, ROS concentration decreased significantly after pure RBC transfusion (P < 0.05) (, ).
Figure 2. ROS concentration of HSC cells at different time. Note: T1: ROS concentration in HSC cells of different groups before surgery; T2: ROS concentration in HSC cells of different groups 6 h after surgery; T3: ROS concentration in HSC cells of different groups 24 h after surgery; Group A: No treatment was taken after the rabbits were anaesthesia; Group B:After the rabbits were anaesthesia, the left lateral lobe of the liver was surgically resected under open abdomen, and the blood volume was supplemented by infusion of sodium lactate Ringer's solution without transfusion; Group C:10% of rabbit whole blood was collected and stored one week before surgery. Then the left lateral lobe of liver was surgically removed, and the blood volume was supplemented by infusion of sodium lactate Ringer's solution without transfusion; Group D: 10% of rabbit whole blood was collected and stored one week before surgery. The left lateral lobe of liver was surgically removed, and the stored autologous whole blood was transfused back during the operation; Group E: 10% of rabbit whole blood was collected one week before surgery and made into suspended red blood cells for storage. One week later, the left lateral lobe of the liver was surgically removed and the stored autored blood cells were transfused back during the operation.
3.4. Detection of senescent bone marrow hematopoietic stem cells with senescence-associated β-galactosidase staining
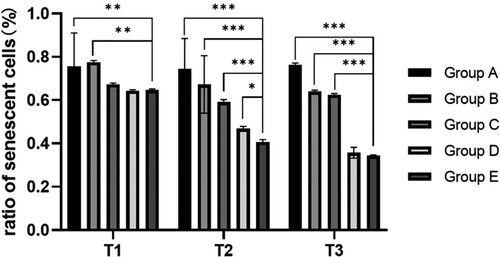
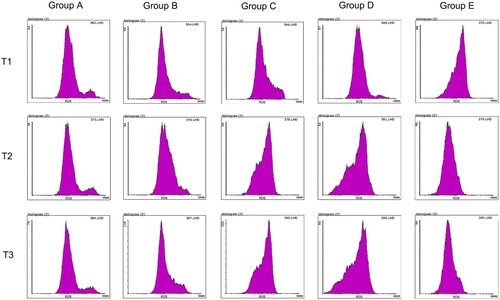
Compared with pre-operation, the ratio of senescent cells in the hepatectomy group (B-E group) decreased significantly at 6 and 24 h after surgery (P < 0.05). Compared with group A, the ratio of senescent cells in the hepatectomy group (B-E group) was significantly decreased at 6 and 24 h after surgery (P < 0.05), indicating that hepatectomy could reduce the ratio of senescent cells. Compared with group C, the ratio of senescent cells in groups D and E at 6 and 24 h after operation was significantly decreased (P < 0.05), indicating that blood transfusion (whole blood /RBC) could reduce the ratio of senescent cells. The ratio of senescent cells was significantly lower after 6 h of pure red blood cells transfusion than that of whole blood transfusion (P < 0.05) (, ).
Figure 3. Comparison of ratio of senescent cells at different time. Note: T1: Ratio of senescent cells of different groups before surgery; T2: Ratio of senescent cells of different groups 6 h after surgery; T3: Ratio of senescent cells of different groups 24 h after surgery; Group A: No treatment was taken after the rabbits were anaesthesia; Group B: After the rabbits were anaesthesia, the left lateral lobe of the liver was surgically resected under open abdomen, and the blood volume was supplemented by infusion of sodium lactate Ringer's solution without transfusion; Group C: 10% of rabbit whole blood was collected and stored one week before surgery. Then the left lateral lobe of liver was surgically removed, and the blood volume was supplemented by infusion of sodium lactate Ringer's solution without transfusion; Group D: 10% of rabbit whole blood was collected and stored one week before surgery. The left lateral lobe of liver was surgically removed, and the stored autologous whole blood was transfused back during the operation; Group E: 10% of rabbit whole blood was collected one week before surgery and made into suspended red blood cells for storage. One week later, the left lateral lobe of the liver was surgically removed and the stored autored blood cells were transfused back during the operation.
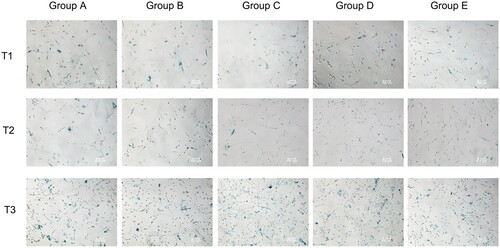
3.5. Content of the p53 and p21 proteins
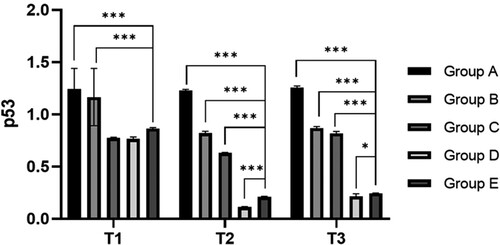
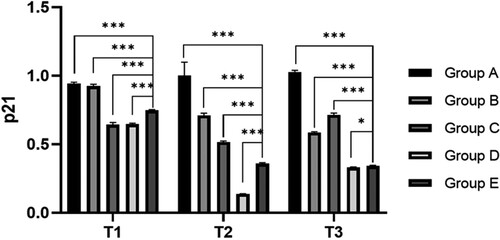
Compared with the preoperative results, the expression levels of p53 and p21 in the hepatectomy group (B-E group) were significantly decreased at 6 h after surgery (P < 0.05). Compared with group A, the expression levels of p53 and p21 in preoperative blood collection group (C-E group) were significantly decreased at all time points (P < 0.05), indicating that blood collection could reduce the expression of p53 and p21. Compared with group C, the expression levels of p53 and p21 in groups D and E at 6 and 24 h after surgery were significantly decreased (P < 0.05), indicating that blood transfusion (whole blood /RBC) could reduce the ratio of senescent cells (, ).
Figure 4. Relative expression level of p53 and p21 at different time. Note: T1: Protein expression of p53 and p21 in different groups before surgery; T2: Protein expression of p53 and p21 in different groups 6 h after surgery; T3: Protein expression of p53 and p21 in different groups after surgery; Group A: No treatment was taken after the rabbits were anaesthesia; Group B:After the rabbits were anaesthesia, the left lateral lobe of the liver was surgically resected under open abdomen, and the blood volume was supplemented by infusion of sodium lactate Ringer's solution without transfusion; Group C:10% of rabbit whole blood was collected and stored one week before surgery. Then the left lateral lobe of liver was surgically removed, and the blood volume was supplemented by infusion of sodium lactate Ringer's solution without transfusion; Group D: 10% of rabbit whole blood was collected and stored one week before surgery. The left lateral lobe of liver was surgically removed, and the stored autologous whole blood was transfused back during the operation; Group E: 10% of rabbit whole blood was collected one week before surgery and made into suspended red blood cells for storage. One week later, the left lateral lobe of the liver was surgically removed and the stored autored blood cells were transfused back during the operation.

4. Discussion
The process of PABD requires preoperative blood collection and storage, which can stimulate bone marrow HSC to enter the cell cycle from the quiescence phase and start to proliferate and differentiate, thus accelerating postoperative recovery of RBC. The origin of all RBCs and immune cells is HSC, and it remains in the steady state of the blood system.
The CD34 protein is an important marker of HSC surface. The presence of CD34+ protein on the cell surface is often used clinically as a marker to screen and count HSC [Citation15]. The research of Pala et al [Citation16], showed that autologous voluntary blood donation could make the quantity of CD34+ cells in the peripheral blood reach a peak 6 h after blood collection and decline to a normal level within 24 h after blood collection. But in this study, HSC change after transfusion of the stored blood was not observed, and the indicators related to HSC activity were not detected.
The results of this study showed that, from preoperation to 24 h postoperation, the count of CD34+ cells continued to rise in Groups B–E. This indicates that blood collection and surgical wounds may cause bone marrow HSC to enter the active proliferation phase from the quiescence phase, thus leading to the rise of HSC quantity in the bone marrow. The decline of HSC is related to its senescence [Citation17]. Changes related to internal and external HSC senescence change HSC functions with age. Under stress conditions, HSC rebuilding and proliferation potential are lost, and the reduction of self-renewal ability and apoptosis increases eventually leads to functional failure [Citation18].
The results of this study show that the proportion of senescent HSC stored before surgery was significantly lower than in the nonblood collection group, and the proportion of senescent HSC in group D and E decreased further at 6h and 24h after operation. The proportion of senescent HSC in group E was lower than that in group D at 6h after operation. This suggests that autologous blood storage and transfusion can promote HSC rebirth, thereby promoting bone marrow hematopoietic function. Compared with whole blood, transfusion of pure RBC could further reduce the proportion of senescent HSC.
The protein p53 ensures long life by inhibiting tumors and has the effect of promoting senescence. However, excessive activation of p53 and p16 accumulation in senescent HSC causes DNA injury [Citation19,Citation20]. If HSC lacking p21 protein proliferates excessively, its self-renewal and multiple differentiation abilities fail [Citation21]. The results of this study show that from preoperation to 24 h after surgery, the expression level of p53 and p21 in the blood stored before surgery presented a rising trend after a decline in each group. Preoperative blood collection, surgery, and other stimuli made activated p53 induce cyclin-dependent kinase inhibitor p21, and histocyte cycle entered the S phase of the cell cycle, promoting DNA injury repair [Citation9].
The ROS produced by normal cell metabolism such as super-oxygen ion and hydrogen peroxide will induce all kinds of DNA injuries, like affecting mitochondrial DNA replication and leading to the weakening of mitochondrial function [Citation22]. In the senescent HSC, the increase of ROS inhibits the steady state and function maintenance of HSC [Citation23]. Maintaining a low level of ROS in HSC is of great significance for the long-term self-renewal ability of HSC. The results of this study showed that, after partial hepatectomy (Groups B–E), the ROS concentration in the bone marrow HSC declined significantly, and the ROS concentration in Group E was lower than in Group D. Surgery, blood collection, transfusion, and other stimuli may promote HSC renewal, increase the proportion of new HSC in the bone marrow HSC pool, and lead to a decrease in the mean of ROS in HSC specimens. Compared with whole blood, transfusion of pure RBC components can further reduce ROS in HSC, which may be related to the oxidative stress of cells [Citation24]. Mature RBC lacks a nucleus and organelle, cannot carry out aerobic metabolism, and mainly depends on anaerobic glycolysis to generate energy. Although the blood is kept at 4°C, cell metabolism does not stop completely. In addition to RBC, there are also a lot of white blood cells and platelets in the whole blood. Due to all kinds of stimuli during storage, such as cell activation, apoptosis, and injury [Citation25], the transfusion of particles into the body intensifies the oxidative stress of cells [Citation26], increasing ROS in HSC. Studies show that particles in healthy people mostly come from platelets, and particle release is highly correlated to adverse reactions of transfusion. This is comparable with the present research results.
Conclusion
In conclusion, through down-regulating ROS in HSC and the expression level of p53 and p21, PABD can reduce the impacts of oxidative stress on its functions, activate HSC, promote the generation of CD34+ cells, stimulate the proliferation and differentiation of bone marrow HSC, and reduce the proportion of senescent cells to cope with blood loss stimulus, thus stimulating hematopoiesis.
From the perspective of HSC, collection and transfusion of RBC components is more beneficial than whole blood. This study neither deeply explored the detailed molecular mechanism of PABD on HSC influence, nor observed long-term hematopoiesis of experimental animals. This may be the future research focus.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sugimoto T, Hashimoto M, Hayakawa I, et al. Alterations in HbA1c resulting from the donation of autologous blood for elective surgery in patients with diabetes mellitus. Blood Trans. 2014 Jan;12(Suppl. 1):209–213. DOI:10.2450/2013.0271-12.
- Zhou J. A review of the application of autologous blood transfusion. Braz J Med Biol Res. 2016 Aug 1;49(9):e5493. DOI:10.1590/1414-431x20165493
- Sharma S, Boston SE, Kotlowski J, et al. Preoperative autologous blood donation and transfusion in dogs undergoing elective surgical oncology procedures with high risk of hemorrhage. Vet Surg. 2021;50(3):607–614. DOI:10.1111/vsu.13598
- Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20(8):833–846. DOI:10.1038/nm.3647
- Vranken NPA, Weerwind PW, Barenbrug PJC, et al. The role of patient’s profile and allogeneic blood transfusion in development of post-cardiac surgery infections: a retrospective study. Int Cardiovasc Thorac Surg. 2014;19:232–238. DOI:10.1093/icvts/ivu096
- Santos AA, Sousa AG, Piotto RF, et al. Mortality risk is dose-dependent on the number of packed red blood cell transfused after coronary artery bypass graft. Rev Bras Cir Cardiovasc. 2013;28(4):509–517. DOI:10.5935/1678-9741.20130083
- Murphy GJ, Reeves BC, Rogers CA, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–2552. DOI:10.1161/CIRCULATIONAHA.107.698977
- Tian X, Yang F, Wu Y. Advances in research and application of hematopoietic stem cells. Chin J Cell Biol. 2019;41(6):1003–1011.
- Yamashita M, Nitta E, Suda T. Regulation of hematopoietic stem cell integrity through p53 and its related factors. Ann N Y Acad Sci. 2016;1370(1):45–54. DOI:10.1111/nyas.12986
- Ito K, Ito K. Hematopoietic stem cell fate through metabolic control. Exp Hematol. 2018 Aug;64:1–11. DOI:10.1016/j.exphem.2018.05.005
- Adelman ER, Figueroa ME. Human hematopoiesis: aging and leukemogenic risk. Curr Opin Hematol. 2021;28(1):57–63. DOI:10.1097/MOH.0000000000000622
- Cakouros D, Gronthos S. Epigenetic regulation of bone marrow stem cell aging: revealing epigenetic signatures associated with hematopoietic and mesenchymal stem cell aging. Aging Dis. 2019;10(1):174–189. DOI:10.14336/AD.2017.1213
- Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019 May;20(5):303–320. DOI:10.1038/s41580-019-0103-9.
- Mohamad Kamal NS, Safuan S, Shamsuddin S, et al. Aging of the cells: insight into cellular senescence and detection methods. Eur J Cell Biol 2020;99(6):151108. DOI:10.1016/j.ejcb.2020.151108
- Lin CS, Ning H, Lin G, et al. Is CD34 truly a negative marker for mesenchymal stem cells? Cytotherapy. 2012;14(10):1159–1163. DOI:10.3109/14653249.2012.729817
- Pala C, Mumcuoglu H, Kurnaz F, et al. Changes in CD34 + cell count in peripheral blood after whole blood donation. Transfus Apher Sci. 2013;49(2):259–262. DOI:10.1016/j.transci.2013.04.038
- Schultz MB, Sinclair DA. When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development. 2016;143(1):3–14. DOI:10.1242/dev.130633
- Rossi DJ, Bryder D, Seita J, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–729. DOI:10.1038/nature05862
- Dumble M, Moore L, Chambers SM, et al. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood. 2007 Feb 15;109(4):1736–1742. DOI:10.1182/blood-2006-03-010413.
- Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443(7110):421–426. DOI:10.1038/nature05159
- Van Os R, Kamminga LM, Ausema A, et al. A limited role for p21Cip1/Waf1 in maintaining normal hematopoietic stem cell functioning. Stem Cells. 2007;25(4):836–843. DOI:10.1634/stemcells.2006-0631
- Almeida M, O’Brien CA. Basic biology of skeletal aging: role of stress response pathways. J Gerontol A Biol Sci Med Sci. 2013;68(10):1197–1208. DOI:10.1093/gerona/glt079
- An R, Yi W, Ju Z. Progress in hematopoietic stem cell ageing research. Progr Biochem Biophys. 2014;41(3):238–246. DOI:10.3724/SP.J.1206.2014.00008
- Koralkova P, Van Solinge WW. Rare hereditary red blood cell enzymopathies associated with hemolytic anemia–pathophysiology, clinical aspects, and laboratory diagnosis. Int J Lab Hematol. 2014;36(3):388–397. DOI:10.1111/ijlh.12223
- Simak J, Gelderman MP. Cell membrane microparticles in blood and blood products: potentially pathogenic agents and diagnostic markers. Transfus Med Rev. 2006;20(1):1–26. DOI:10.1016/j.tmrv.2005.08.001
- Angelillo-Scherrer A. Leukocyte-derived microparticles in vascular homeostasis. Circ Res. 2012;110(2):356–369. DOI:10.1161/CIRCRESAHA.110.233403