ABSTRACT
Objectives: Allogeneic hematopoietic stem cell transplantation (HSCT) remains the most important curative modality for several hematologic malignancies, but an HLA-matched sibling or unrelated donor is not always available, particularly for ethnic minorities and multiethnic families. We have shown that Haplo-HSCT can be conducted safely on an outpatient basis, using peripheral blood stem cells; this leading into substantial decreases in the costs. Methods: In this study twenty-one patients prospectively received the conventional dose of post-transplantation cyclophosphamide (PTCy): (50 mg/Kg on days 3 and 4), whereas 10 were given reduced doses of the drug (25 mg/Kg on days 3 and 4). Results: According to the statistical analysis, the two comparative groups (PTCy 50 mg/kg vs PTCy 25 mg/kg) had no significant difference in terms of age, sex, hematological recovery, and type of conditioning regimen. The median OS for the group PTCy 50 mg/kg is 5.7 months meanwhile for the group PTCy 25 mg/kg the median is 6.4 months. The median follow up for entire group is 4.5 months (IQR: 1.1–18.9 mo). Conclusion: These results could indicate that the Cy-dependent hematological toxicity can be reduced without compromising its effectivity. This preliminary observation may be considered as an idea to conduct prospective randomized studies to explore the possibility of significantly reducing the doses of PT-Cy in the setting of Haplo-HSCT.
Trial registration: ClinicalTrials.gov identifier: NCT05780554.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) remains the most important curative modality for several hematologic malignancies, but an HLA-matched sibling or unrelated donor is not always available, particularly for ethnic minorities and multiethnic families [Citation1]. This problem has led to the expansion of the donor pool to include alternative donor sources such as HLA-haploidentical (Haplo) relatives, HLA-mismatched unrelated donors, and HLA-matched or mismatched cord blood. Post-transplantation cyclophosphamide (PTCy)-containing graft-versus-host disease (GVHD) prophylaxis, pioneered at Johns Hopkins [Citation2], revolutionized Haplo-HSCT with acceptable rates of engraftment, GVHD, relapse, and survival [Citation3]. We [Citation4] and others [Citation5] have shown that Haplo-HSCT can be conducted safely on an outpatient basis, using peripheral blood stem cells; this leading into substantial decreases in the costs. Outpatient-based Haplo-HSCT has turned into the solution of the HSCT most frequent problems in low and middle income countries (LMIC): Cost and donor availability [Citation1,Citation4,Citation5]. The employed doses of PTCy are 50 mg/Kg. on days +3 and +4 of the HSCT (4–5) and can lead into hematological, cardiac and other toxicities [Citation1,Citation4,Citation5]. There is preliminary information about diminished doses of PTCy, might being equally effective in the prevention of GVHD and substantially less toxic [Citation6,Citation7]. We report here the results of employing reduced doses of PTCy (25 mg/Kg). On days 3 and 4, in a group of 10 patients.
Material and methods
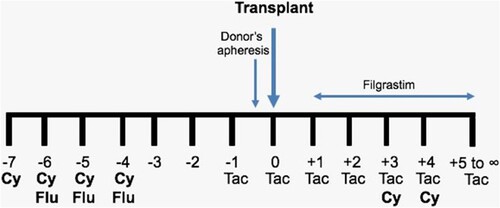
Subjects. All patients given a Haplo-HSCT in the Centro de Hematología y Medicina Interna in Puebla, México were prospectively included, employing the ‘Mexican’ method to conduct Haplo-HSCT on an outpatient basis [Citation4]. Between March 2013 and July 2016, the transplants were done using the conventional doses of PTCy (50 mg/Kg) on days 3 and 4, whereas after July 2016, the allografts were done using 50% of the dose of Cy (25 mg/Kg) on days 3 and 4. The depicts the employed protocol, registered in www.clinicalTrials.gov, identifier NCT05780554. Twenty-one patients received the conventional dose of PTCy (50 mg/Kg on days 3 and 4), whereas 10 were given reduced doses of the drug (25 mg/Kg on days 3 and 4). All grafts were started as outpatients and could be completed as outpatients in 71 and 60% of cases respectively, for the groups of PTCy 50 and 25 mg/Kg.
Figure 1. Scheme of the ‘Mexican Method’ to conduct haploidentical hematopoietic stem cell transplants on an outpatient basis. Cy = cyclophosphamide; pre-transplant Cy: 500 mg/m2; post-transplant Cy: either 25 or 50 mg/Kg. Flu = fludarabine, 25 mg/m2. Tac = tacrolimus.
Statistical analysis of data. The IBM Statistical Package for Social Sciences version 25.0 was used for data analysis (Armonk, NY: IBM Corp). The qualitative variables were described using ratios and percentages. According to the Shapiro–Wilk test, our variables were not normally distributed and were described using median and interquartile ranges (IQR). The level of statistical significance was set at p 0.05. Relapse-free survival (RFS) was defined as the interval from HSCT to relapse or death from any cause. Survival was defined as the interval from HSCT to death.
Results
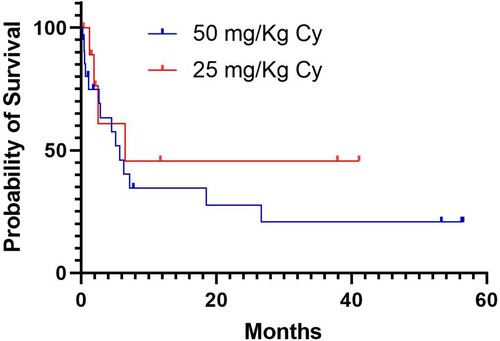
According to the statistical analysis, the two comparative groups (PTCy 50 mg/kg vs PTCy 25 mg/kg) have no significant difference in terms of age, sex, hematological recovery, and type of conditioning regimen. Cytokine release syndrome and hematological toxicity were less frequent in the group of persons given diminished dose of PTCy, whereas the prevalence of both acute and chronic graft versus host disease was similar in the two groups (). The median OS for the group PTCy 50 mg/kg is 5.73 months meanwhile for the group PTCy 25 mg/kg is 6.46 months. The depicts the Kaplan-Meier OS plots for the two subsets of patients, which were not statistically different. The RFS were better in the patients grafted with the PTCy 25 mg/kg (2.13 mo vs 5.13 mo). The median follow up for entire group is 4.53 months (IQR: 1.1–18.9 mo).
Table 1. Salient features of the two groups of patients given a haploidentical stem cell transplantation.
Figure 2. Overall survival of the patients given haploidentical hematopoietic stem cell transplants employing post-transplant cyclophosphamide (Cy) at either 25 or 50 mg/Kg/day on days 3 and 4. Overall survival (OS) was defined as the interval from date of transplant to death and relapse free survival (RFS) was defined as the interval from starting therapy to relapse or death from any cause. The probability of OS and RFS was estimated using the Kaplan–Meier method. The log-rank test was used to compare groups.
Discussion
Having shown that Haplo-HSCT renders similar results to those obtained with cord-blood allografting, this method is proving to be the best option for persons needing an allograft and not having a suitable donor [Citation1,Citation3]; conducting the procedure on an outpatient basis further simplifies the method and reduces costs [Citation4,Citation5]. In our experience, the two main reasons to accept into the hospital a patient given an Haplo-HSCT on an outpatient basis are complications of the hematological toxicity and/or cytokine-release syndrome (CRS) [Citation4,Citation5]. If the Cy-dependent hematological toxicity can be reduced without compromising its effectivity, the possibility of completing an Haplo-HSCT fully on an outpatient basis should result in reduction of costs and in turn, in more allografts being conducted. There are few papers dealing with the employment of reduced doses of PTCy in the setting on Haplo-HSCT; decreases from the conventional total dose of PTCY (100 mg/Kg) to 80 mg [Citation8,Citation9]. Our work has limitations, mainly the small sample size; however, we have shown the results of further decreasing the total dose to 50 mg/Kg without significantly compromising the efficacy, safety and results of the procedure. This preliminary observation may be considered as an idea to conduct a prospective clinical trial in a larger sample to explore the possibility of significantly reducing the doses of PT-Cy in the setting of Haplo-HSCT.
All subjects provided written informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Centro de Hematología y Medicina Interna, Clínica Ruiz.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ruiz-Argüelles GJ, Ruiz-Delgado GJ, González-Llano O, et al. Haploidentical bone marrow transplantation in 2015 and beyond. Curr Oncol Rep. 2015 Dec;17(12):57. doi:10.1007/s11912-015-0482-9. PMID: 26467275.
- Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008 Jun;14(6):641–650. doi:10.1016/j.bbmt.2008.03.005. PMID: 18489989; PMCID: PMC2633246.
- Chang Y-J, Ding Q, Hwang WYK, et al. Editorial: recent developments in haploidentical hematopoietic cell transplantation: therapy and complications. Front Immunol. 2021 Aug 17;12:746221. doi:10.3389/fimmu.2021.746221. PMID: 34484249; PMCID: PMC8416152.
- Murrieta-Álvarez I, Ruiz-Argüelles GJ. Bien plus encore: haplos indeed can be completed on an outpatient basis. Transplant Cell Ther. 2021 Jun;27(6):519–520. doi:10.1016/j.jtct.2021.03.009. Epub 2021 Mar 8. PMID: 33819480.
- Colunga-Pedraza PR, Gómez-De León A, Rodríguez-Roque CS, et al. Outpatient haploidentical stem cell transplantation using post-transplant cyclophosphamide Is safe and feasible. Transplant Cell Ther. 2021 Mar;27(3):259.e1–259.e6. doi:10.1016/j.jtct.2020.12.006. Epub 2020 Dec 16. PMID: 33781529.
- Duléry R, Goudet C, Mannina D, et al. Reduced post-transplant cyclophosphamide doses in haploidentical hematopoietic cell transplantation for elderly patients with hematological malignancies. Bone Marrow Transplant. 2022 Dec 30;58:386–392. doi:10.1038/s41409-022-01908-y. Epub ahead of print. PMID: 36585459.
- Nakamae H, Okamura H, Hirose A, et al. A prospective study of an HLA-haploidentical peripheral blood stem cell transplantation regimen based on modification of the dose of posttransplant cyclophosphamide for poor prognosis or refractory hematological malignancies. Cell Transplant. 2022 Jan–Dec;31:096368972211120. doi:10.1177/09636897221112098. PMID: 35906755; PMCID: PMC9340897.
- Sugita J, Kamimura T, Ishikawa T, et al. Reduced dose of posttransplant cyclophosphamide in HLA-haploidentical peripheral blood stem cell transplantation. Bone Marrow Transplant. 2021 Mar;56(3):596–604. doi:10.1038/s41409-020-01065-0. Epub 2020 Sep 24. PMID: 32973350.
- Wang Y, Wu D-P, Liu Q-F, et al. Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J Hematol Oncol. 2019 Sep 3;12(1):88. doi:10.1186/s13045-019-0781-y. PMID: 31481121; PMCID: PMC6724335.
