ABSTRACT
Introduction
An effective salvage regimen for the reinduction of remission is lacking for refractory or relapsed primary central nervous system lymphoma (r/r PCNSL). This study aimed to evaluate the efficacy and safety of cytarabine plus temozolomide in treating r/r PCNSL and to explore the associated prognostic factors.
Methods
A single-center retrospective cohort study was conducted to assess the efficacy and safety of cytarabine and temozolomide (AT) in r/r PCNSL patients. KIR and HLA genotyping was performed on peripheral blood samples.
Results
Thirty PCNSL patients receiving an AT regimen (cytarabine 3 g/m2 for 2 days combined with temozolomide 150 mg/m2 for 5 days) in our institution were analyzed. The median age was 65 years (range 25–79 years). A total of 43.4% of patients (13/30) achieved an overall response within a median follow-up of 16 months (95% confidence interval [CI]: 11–23 months). The median PFS and OS of the cohort were 1.5 months (95% CI: 1–4 months) and 19.5 months (95% CI: 11 months to not calculable), respectively. Patients harboring KIR3DL1/HLA-B genotypes predicting low affinity had a higher response rate (p = 0.042) and longer median PFS (3 months) than those with KIR3DL1/HLA-B genotypes predicting high affinity (1 month) (p = 0.0047). Cox regression analysis indicated that KIR/HLA-B genotypes were independently associated with PFS (p = 0.043). However, KIR/HLA-B genotypes had no impact on the OS of the cohort. The toxicity of AT treatment was mild and manageable.
Conclusion
The AT regimen was well tolerated, and patients with specific KIR-HLA genotypes may benefit from this regimen.
Introduction
Primary central nervous system lymphoma (PCNSL) is an uncommon subtype of lymphoma and accounts for up to 6.4% of extranodal diffuse large B-cell lymphoma (DLBCL) cases in China [Citation1]. RCHOP (rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisone) as the most commonly used treatment for DLBCL has shown poor efficacy for PCNSL, as most of these drugs cannot penetrate the blood–brain barrier [Citation2,Citation3]. Although the prognosis of PCNSL has been greatly improved with high-dose methotrexate (MTX)-based treatment, the 5-year overall survival (OS) of PCNSL is only 24%–44% for patients in European countries [Citation4,Citation5] and 26.9% for patients in China [Citation1], which is much lower compared to systemic DLBCL, the 5-year OS of which has been reported to achieve 77.7% [Citation6].
As previously reported, the overall response rate (ORR) of high-dose MTX-based therapies was 50%–80% [Citation7–9]. The most common regimens used along with high-dose MTX include temozolomide [Citation10], cytarabine [Citation11], rituximab [Citation12], and thiotepa [Citation13]. Although a high response rate has been achieved since the use of MTX, up to 29% of patients were chemoresistant to MTX, and 16.5% of patients relapsed after treatment [Citation14]. The median OS calculated from disease progression was only 7.2 months in patients with refractory disease [Citation15]. Thiotepa-based myeloablative therapy followed by autologous stem cell transplantation (ASCT) has been applied as a consolidative treatment for PCNSL with a 5-year OS rate of 70%–80% and as salvage treatment with an ORR of 85% [Citation16,Citation17]. However, due to the fragile physical conditions of patients caused by disease and age, ASCT is not indicated for all patients. Some other regimens have been evaluated for use in refractory or relapsed PCNSL (r/r PCNSL). For example, high-dose cytarabine presented an ORR of 36% in r/r PCNSL with a median progression-free survival (PFS) of 3 months [Citation18]. Pemetrexed was reported to have an ORR of 64.7% and a median PFS of 5.8 months [Citation19]. Other regimens, including lenalidomide [Citation20], pomalidomide [Citation21], ibrutinib [Citation22], and checkpoint inhibitors [Citation23] have also been evaluated. These new regimens have not routinely been used, and their efficacy needs to be further evaluated.
Natural killer (NK) cells play an important role in tumor immune surveillance. The activity of NK cells is determined through signals transduced by activating receptors and membrane inhibitory receptors, such as the killer cell immunoglobulin-like receptor (KIR) family, which recognizes human leukocyte antigen class I (HLA-I) [Citation24]. The binding affinity between donor KIRs and recipient HLA-I had an impact on the outcomes of patients with myeloid neoplasms receiving allogeneic stem cell transplantation [Citation25,Citation26], in which NK cells were educated by weak inhibition of HLA ligands. Such effects were also reported in autologous stem cell transplantation settings, in which patients harboring KIR genotypes along with low-affinity HLA ligands had lower relapse rates [Citation27].
In nonhematopoietic stem cell transplantation settings, NK-cell activity was found to be correlated with the outcomes of immunotherapy because the antitumor effects of immunotherapy are partly exerted by NK cells due to the antibody-dependent cell-mediated cytotoxicity (ADCC). For example, follicular lymphoma patients with KIR3DL1/HLA-Bw4 genotypes could benefit from rituximab maintenance therapy [Citation28]. Patients with neuroblastoma harboring specific KIR3DL1/HLA-B genotypes associated with a noninteraction pattern had improved OS and PFS compared with those with a strong or weak interaction pattern [Citation29].
In this report, we retrospectively evaluated the efficacy of high-dose cytarabine combined with temozolomide (AT) treatment in patients with r/r PCNSL at our institution. KIR and HLA-I genotypes were analyzed. The results indicated that specific KIR and HLA-I pairs could, to some level, predict the outcomes of treatment.
Materials and methods
Patient cohort
Patients with histologically confirmed PCNSL at our institution from June 2017 to June 2020 were screened for eligibility. Patients were included if they demonstrated disease progression after methotrexate (3.5 g/m2∼8.0 g/m2)-based treatments and received high-dose cytarabine and temozolomide. All chosen patients had a disease status that was measurable with contrast-enhanced MR scans. Those who had previously received high-dose cytarabine or temozolomide were excluded. The medical records of each patient were collected and checked by two physicians. Written informed consent was obtained from each patient. The study protocol was approved by the ethics committee of Huashan Hospital, Fudan University (ChiCTR2100054482).
Treatment and assessment
During the treatment period, patients received cytarabine 3.0 g/m2 on days 1–2 and temozolomide 150 mg/m2 on days 1–5. Cytarabine was administered as a 3-h infusion, and temozolomide was given orally. The dosage of cytarabine was reduced by 70% for patients who were 70 years old or older. In the case of meningeal involvement, intrathecal injection of cytarabine (50 mg) was added at each chemotherapy phase. Intravitreal methotrexate therapy was applied in addition to the base treatment if patients were diagnosed with intraocular lymphoma. The treatment was administered every four weeks. Treatment response was assessed by the outcome of gadolinium-enhanced brain MRI scans three weeks after previous AT treatment in accordance with international guidelines [Citation30]. In brief, patients achieving complete remission (CR), complete remission unconfirmed (CRu), or partial remission (PR) were categorized as responders, and those with stable disease (SD) or progressive disease (PD) were regarded as non-responders. Responders continued to receive 8 cycles of treatment until their disease progressed, while non-responders were switched to different chemotherapy or whole-brain radiation therapy. All patients participated in a follow-up program with MRI controls every 3 months for 2 years. PFS was defined as the time from AT treatment to disease progression or death of any cause if progression was not determined. OS was calculated from the date of AT treatment to death from any cause or the last date of follow-up. The toxicity of treatment was assessed according to the Common Toxicity Criteria (CTCAE, version 5.0).
KIR and HLA class I genotyping
Genomic DNA was extracted from peripheral blood samples. KIR genotyping was performed using PCR with sequence-specific primers [Citation31], and HLA class I genotyping was performed using sequencing-based typing [Citation32]. HLA-Bw4 alleles are predicted to have high affinity for KIR3DL1 receptors if isoleucine is at position 80 (HLA-Bw4-80I); on the other hand, a threonine present at position 80 (HLA-Bw4-80 T) can predict that HLA-Bw4 alleles have low affinity for KIR3DL1 receptors. HLA-C1 alleles are predicted to have a low affinity for KIR2DL2/3, whereas HLA-C2 alleles have a high affinity for KIR2DL1. HLA-A*23:01, HLA-A*24:02, and HLA-A*32:01 alleles are considered high-affinity KIR3DL1 ligands if HLA-Bw4-80I alleles are present [Citation27]. Binding affinities between specific KIRs and corresponding HLA-I ligands were obtained from the Immuno Polymorphism Database [Citation33]. We developed a scoring system predicting the affinity between KIR3DL1 and HLA-B. The HLA-Bw6 genotype (predicting mismatch) was scored 0, the HLA-Bw4-80 T genotype (predicting low-affinity interactions) was scored 1, and the HLA-Bw4-80I genotype (predicting high-affinity interactions) was scored 2. Patients were categorized into the HLA-B high-affinity group if the score of two HLA-B alleles was ≥2 and the HLA-B low-affinity group if the score of two HLA-B alleles was <2.
Statistical analysis
Survival was estimated using the Kaplan–Meier method. Treatment response, as measured by the proportion of responders, was compared between each group using Fisher’s exact test. Log-rank test was used for univariate analysis. Multivariate analysis was conducted by Cox regression analysis. A P value less than 0.05 was considered statistically significant. The statistical analysis was performed using the GraphPad Prism 9.0 and STATA 15 software package.
Results
Patient characteristics
A total of 31 patients with PCNSL received AT treatment from June 2017 to June 2020 at our institution. One patient whose blood sample was not available was excluded from the study. The analysis included 30 patients (). Two patients were lost to follow-up when calculating OS. As shown, the median age and ECOG performance status of all patients were 65 years (range 25–79) and 3 (range 1–4), respectively. Eighty percent of patients (24/30) switched to AT therapy due to disease progression after high-dose MTX-based therapy, and the remaining 20% of patients (6/30) received AT therapy as they relapsed after having achieved CR. Based on the medical records, 33.3% of patients (10/30) presented two or more lesions in the brain prior to AT treatment, and 66.7% of them (20/30) had tumors involving deep structures, including the periventricular regions, basal ganglia, corpus callosum, brainstem and cerebellum. The median longest lesion diameter on MR images was 2.3 cm (range: 0.5–5.1). Furthermore, 16.7% of patients (5/30) had lymphoma cells in the cerebrospinal fluid (CSF), and 3.3% (1/30) had lymphoma cells in vitreous fluid. All patients had received a median of 9 g MTX at least once prior to AT treatment. Some patients had previously received other regimens, including idarubicin, rituximab, pemetrexed, and lenalidomide. Other than chemotherapy, 6.7% of patients (2/30) had previously received whole-brain radiotherapy.
Table 1. Patient characteristics.
Treatment assessment and toxicity
A total of 43.4% of patients (13/30) achieved an overall response (8 with CR or CR unconfirmed and 5 with PR). Forty percent of the patients (12/30) developed disease progression. The median follow-up was 16 months (95% CI: 11–23 months). The median PFS was 1.5 months (95% CI: 1–4 months), and the median OS was 19.5 months (95% CI: 11 months to not calculable). The response assessment to AT treatment based on patient characteristics is listed in . Patients in the HLA-B low-affinity group had a higher response rate than those in the HLA-B high-affinity group (57.1% vs 11.1%, p = 0.042). Other factors, such as KIR/HLA-A or KIR/HLA-C pairs, age, sex, ECOG performance status, and tumor characteristics, including the number, location, volume of lesions, and CSF involvement or not had no impact on treatment response. The side effects of AT treatment were mild and manageable (). In brief, 23.3% of patients (7/30) showed neutropenia, and three of them developed grade 3 neutropenia; all of these patients recovered soon with or without the administration of granulocyte-colony stimulating factors. Twenty percent of patients (6/30) developed thrombocytopenia; among them, two patients developed grade 3 thrombocytopenia. All patients with thrombocytopenia recovered without platelet transfusion. A total of 13.3% of patients (4/30) were diagnosed with anemia; only one patient received red blood cell transfusion. The 10% of patients (3/30) who had infections (diarrhea, urinary tract infection, and sepsis) recovered after antibiotic administration.
Table 2. Response to AT treatment according to patient characteristics.
Table 3. Toxicity of treatment.
Impact of KIR and HLA-I pairs on PFS and OS
The KIR and HLA-I alleles are listed in . Patients in the HLA-B low-affinity group appeared to have a longer median PFS (3 months) than those in the HLA-B high-affinity group (1 month) (p = 0.0079; A). KIR3DL1 and specific HLA-A alleles, such as HLA-A*23:01, HLA-A*24:02, and HLA-A*32:01, which were predicted to have high affinity for KIR3DL1, did not have an impact on PFS (p = 0.0920; B). No significant difference in PFS was found between patients with KIR2DL1 and HLA-C2 alleles (high affinity) and those possessing KIR2DL2/3 and HLA-C1 alleles (low affinity) (p = 0.7188; C). The HLA-B (p = 0.9066), HLA-A (p = 0.6409), and HLA-C (p = 0.5914) alleles had no impact on OS in the cohort.
Figure 1. Kaplan–Meier curve of patients with specific KIR-HLA genotype groups. (A) PFS of patients with KIR3DL1 and HLA-B (high affinity) and patients with KIR3DL1 and HLA-B (low affinity). (B) PFS of patients with KIR3DL1 and HLA-A (high affinity) and patients with KIR3DL1 and HLA-A (low affinity). (C) PFS of patients with KIR2DL1 and HLA-C2 (high affinity) and patients with KIR2DL2/3 and HLA-C1 (low affinity).
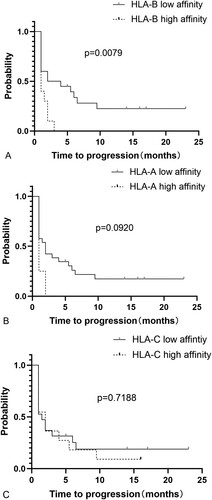
Table 4. KIR ligand assignments.
Prognostic factors
Age had no impact on the cohorts’ PFS (p = 0.2076) or OS (p = 0.2970). In addition, there was no sex-related impact on PFS (p = 0.6170) or OS (p = 0.2144). An ECOG performance status ≥3 was found to be an independent unfavorable prognostic marker for PCNSL [Citation34]; however, it did not demonstrate shorter PFS (p = 0.8252) or OS (p = 0.3564) in this cohort. Patients with multifocal lesions had similar median PFS (p = 0.0817) or OS (p = 0.9522) to those with a solitary lesion. Patients with deep structure involvement, including the periventricular regions, basal ganglia, corpus callosum, brainstem, and cerebellum, which was regarded as an unfavorable factor, had a longer PFS (2 months) than those with pallium involvement only (1 month) (p = 0.0134); however, no significantly longer OS was observed between the two groups (p = 0.4682). Patients with larger tumor volumes had a similar median PFS (p = 0.4656) or OS (p = 0.1509) to their counterparts. Cerebrospinal fluid involvement showed no further impact on PFS (p = 0.5840) or OS (p = 0.6239) in this cohort (Supplementary Table 1). Multivariate analysis by Cox regression indicated that KIR3DL1/HLA-B pairs predicting low affinity (score 0 or 1) were independently associated with a longer PFS (p = 0.043; ). There was no significant difference in the distribution of KIR3DL1/HLA-B pairs between each subgroup (Supplementary Table 2).
Table 5. Multivariate analysis by Cox regression for PFS (n = 30).
Discussion
The prognosis of PCNSL has improved since the use of high-dose MTX. However, treatment options are limited for patients whose tumors are resistant to MTX or those who are too fragile to tolerate high-dose MTX. Salvage radiotherapy has been reported to achieve an ORR of 80% and a 2-year OS of 57% for refractory or recurrent PCNSL [Citation35], but neurocognitive complications need to be carefully considered, especially for elderly patients [Citation36,Citation37]. Radiotherapy is not a priority, especially when other non-MTX regimens that can penetrate the blood–brain barrier have been developed. Cytarabine is a classic chemotherapeutic agent that can penetrate the blood–brain barrier in large doses. Cytarabine has proven efficacy in combination with high-dose MTX in patients with newly diagnosed PCNSL [Citation13]. However, severe infectious complications partly due to 4 doses of cytarabine occurred in up to 28% of patients in the study. In another retrospective study, cytarabine was administered as a single agent for 4 doses and resulted in severe toxicities such as thrombocytopenia and neutropenic fever, although granulocyte-monocyte colony-stimulating factor had been administered routinely [Citation18]. To reduce the toxicities, 2 doses of cytarabine were administered in our study. As a result, this dose was well tolerated even in elderly patients without routine administration of granulocyte colony-stimulating factor. Temozolomide, as an oral alkylating agent, has shown efficacy and safety in the treatment of newly diagnosed PCNSL when combined with high-dose MTX and radiotherapy [Citation38] or as consolidation treatment when combined with etoposide [Citation39]. For r/r PCNSL, temozolomide combined with Bruton’s kinase inhibitor ibrutinib achieved an ORR of 55% and a median PFS of 5.3 months [Citation40]. The cytarabine plus temozolomide regimen produced an ORR of 43.3% in our study, and the median PFS was not satisfactory. However, in the HLA-B low-affinity group, the combination achieved an ORR of 57.1% and a median PFS of 3 months. The results suggest that the combination is an option for patients with low-affinity KIR/HLA-B genotypes, especially for those with contraindications to ibrutinib.
In this study, patients with the KIR3DL1 and HLA-Bw6 or HLA-Bw4-80 T genotypes predictive of mismatch or low affinity benefited from the AT regimen with a higher response rate and longer median PFS than those of patients with KIR3DL1 and HLA-Bw4-80I genotypes predictive of high affinity. The prognostic effect of the KIR and HLA-I genotypes has been evaluated in other studies [Citation41,Citation42]. NK cells exert antitumor activity when the corresponding HLA-I ligand for KIR is missing. As found in hematopoietic stem cell transplantation (HSCT) settings, donor-recipient KIR3DL1/HLA-B combinations predictive of weak inhibition or mismatch were associated with lower relapse [Citation43]. To our knowledge, the impact of KIR and HLA genetic polymorphisms on nonimmune cytotoxic chemotherapy has not been evaluated in non-HCST settings before. Patients who harbor KIR3DL1/HLA-B pairs predictive of mismatch or low affinity would benefit from NK-cell activation. For those who had high-affinity KIR and HLA-B pairs, tumor cells could escape NK-cell killing by augmenting HLA-B expression. Deep structure involvement, which is predicted to be an unfavorable factor of PCNSL, was found to be associated with a slightly longer PFS, suggesting that the effect of the AT regimen, to some extent, was not dependent on cytotoxicity. We hypothesize that chemotherapy may, to some extent, trigger innate immune responses such as NK cell activation. If so, this phenomenon may not be restricted to PCNSL. The innate immune response during chemotherapy may be a universal phenomenon. Some studies have reported that chemotherapy reagents contribute to the initiation of tumor cell death by boosting natural killer cells [Citation44,Citation45].
KIR and HLA genotypes showed no significant impact on the OS of the cohort in this study. Patients whose tumors did not respond to the AT regimen continued to receive other chemotherapy or whole-brain radiation therapy. The results suggested that KIR3DL1/HLA-B pairs may not be associated with the prognosis of PCNSL. We did not observe that KIR3DL1/HLA-A or KIR2DL1/HLA-C2 pairs had any impact on the PFS or OS of this cohort. It could be inferred that KIR3DL1/HLA-B may have specific roles in the chemotherapy-induced immune response.
The study had some limitations. The sample size was small in this study, and the retrospective character of it made the conclusion that KIR3DL1/HLA-B was correlated with the outcome of chemotherapy not so convincing. The strength of the available data was undermined due to undersizing. The results need to be further confirmed in a larger prospective study with a comparative arm in the future. Interestingly, only four types of immune cells, B cells, T cells, macrophages, and dendritic cells, have been identified in the microenvironment of PCNSL to date [Citation46]. In our previous study, we evaluated CD56-positive NK cells in PCNSL tumor tissue, and immunohistochemical analysis showed few CD56-positive NK cells in the tumor microenvironment of PCNSL [Citation47]. If NK cells do not reside in the tumor microenvironment, how can they exert antitumor effects? Can NK cells migrate into the central nervous system from the peripheral blood after chemotherapy, which would mean that the AT regimen could boost NK activity and promote NK cells penetrating the blood-brain barrier? The answer remains to be elucidated.
Author contributions
Material preparation was performed by Zhiguang Lin and Huiwen Xu. Data collection was performed by Jingjing Ma, Yan Ma, and Qing Li. Data analysis was performed by Hui Kang, and Mengxue Zhang. The study design was performed by Zhiguang Lin and, Bobin Chen. All authors read and approved the final manuscript.
Statement of ethics
The study protocol was reviewed and approved by the Ethics Committee of Huashan Hospital, Fudan University (7 Dec 2021/2021-785). Informed consent was obtained from all individual participants included in the study.
Supplemental Material
Download MS Word (11.9 KB)Supplemental Material
Download MS Word (16.3 KB)Acknowledgments
We thank Dr Xiang Zhou for helping follow up with the patients.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Disclosure statement
The authors have no conflicts of interest to declare.
Additional information
Funding
References
- Shi Y, Han Y, Yang J, et al. Clinical features and outcomes of diffuse large B-cell lymphoma based on nodal or extranodal primary sites of origin: analysis of 1,085 WHO classified cases in a single institution in China. Chin J Cancer Res. 2019;31(1):152–161. doi:10.21147/j.issn.1000-9604.2019.01.10
- O’Neill BP, O’Fallon JR, Earle JD, et al. Primary central nervous system non-Hodgkin’s lymphoma: survival advantages with combined initial therapy? Int J Radiat Oncol Biol Phys. 1995;33(3):663–673. doi:10.1016/0360-3016(95)00207-F
- Mead GM, Bleehen NM, Gregor A, et al. A medical research council randomized trial in patients with primary cerebral non-Hodgkin lymphoma: cerebral radiotherapy with and without cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. Cancer. 2000;89(6):1359–1370. doi:10.1002/1097-0142(20000915)89:6<1359::AID-CNCR21>3.0.CO;2-9
- Biccler JL, Savage KJ, Brown PDN, et al. Risk of death, relapse or progression, and loss of life expectancy at different progression-free survival milestones in primary central nervous system lymphoma. Leuk Lymphoma. 2019;60(10):2516–2523. doi:10.1080/10428194.2019.1594219
- Eloranta S, Brånvall E, Celsing F, et al. Increasing incidence of primary central nervous system lymphoma but no improvement in survival in Sweden 2000-2013. Eur J Haematol. 2018;100(1):61–68. doi:10.1111/ejh.12980
- Sehn LH, Martelli M, Trněný M, et al. A randomized, open-label, Phase III study of obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-Cell lymphoma: final analysis of GOYA. J Hematol Oncol. 2020;13(1):71. doi:10.1186/s13045-020-00900-7
- Fritsch K, Kasenda B, Schorb E, et al. High-dose methotrexate-based immuno-chemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study). Leukemia. 2017;31(4):846–852. doi:10.1038/leu.2016.334
- Houillier C, Ghesquières H, Chabrot C, et al. Rituximab, methotrexate, procarbazine, vincristine and intensified cytarabine consolidation for primary central nervous system lymphoma (PCNSL) in the elderly: a LOC network study. J Neurooncol. 2017;133(2):315–320. doi:10.1007/s11060-017-2435-7
- Kobayashi H, Yamaguchi S, Motegi H, et al. Long-term evaluation of combination treatment of single agent HD-MTX chemotherapy up to three cycles and moderate dose whole brain irradiation for primary CNS lymphoma. Chemother. 2019;31(1):35–41. doi:10.1080/1120009X.2018.1546984
- Chen C, Sun P, Cui J, et al. High-dose Methotrexate plus temozolomide with or without rituximab in patients with untreated primary central nervous system lymphoma: a retrospective study from China. Cancer Med. 2019;8(4):1359–1367. doi:10.1002/cam4.1906
- Sun X, Liu J, Wang Y, et al. Methotrexate-cytarabine-dexamethasone combination chemotherapy with or without rituximab in patients with primary central nervous system lymphoma. Oncotarget. 2017;8(30):49156–49164. doi:10.18632/oncotarget.17101
- Han X, Ji Y, Ouyang M, et al. Efficacy and safety of HD-MTX based systemic chemotherapy regimens: retrospective study of induction therapy for primary central nervous system lymphoma in Chinese. Sci Rep. 2017;7(1):17053, doi:10.1038/s41598-017-17359-1
- Schorb E, Fox CP, Kasenda B, et al. Induction therapy with the MATRix regimen in patients with newly diagnosed primary diffuse large B-cell lymphoma of the central nervous system – an international study of feasibility and efficacy in routine clinical practice. Br J Haematol. 2020;189(5):879–887. doi:10.1111/bjh.16451
- Langner-Lemercier S, Houillier C, Soussain C, et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 2016;18(9):1297–1303. doi:10.1093/neuonc/now033
- Mao C, Chen F, Li Y, et al. Characteristics and outcomes of primary central nervous system lymphoma: a retrospective study of 91 cases in a Chinese population. World Neurosurg. 2019;123:e15–e24. doi:10.1016/j.wneu.2018.10.034
- Alnahhas I, Jawish M, Alsawas M, et al. Autologous stem-cell transplantation for primary central nervous system lymphoma: systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk. 2019;19(3):e129–e141. doi:10.1016/j.clml.2018.11.018
- Schenone L, Houillier C, Tanguy ML, et al. Intensive chemotherapy followed by autologous stem cell transplantation in primary central nervous system lymphomas (PCNSLs). Therapeutic outcomes in real life-experience of the French Network. Bone Marrow Transplant. 2022;57(6):966–974. doi:10.1038/s41409-022-01648-z
- Chamberlain MC. High-dose cytarabine salvage therapy for recurrent primary CNS lymphoma. J Neurooncol. 2016;126(3):545–550. doi:10.1007/s11060-015-1994-8
- Zhang JP, Lee EQ, Nayak L, et al. Retrospective study of pemetrexed as salvage therapy for central nervous system lymphoma. J Neurooncol. 2013;115(1):71–77. doi:10.1007/s11060-013-1196-1
- Ghesquieres H, Chevrier M, Laadhari M, et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective ‘proof of concept’ phase II study of the French Oculo-Cerebral lymphoma (LOC) Network and the Lymphoma Study Association (LYSA). Ann Oncol. 2019;30(4):621–628. doi:10.1093/annonc/mdz032
- Tun HW, Johnston PB, DeAngelis LM, et al. Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood. 2018;132(21):2240–2248. doi:10.1182/blood-2018-02-835496
- Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121–130. doi:10.1016/j.ejca.2019.05.024
- Terziev D, Hutter B, Klink B, et al. Nivolumab maintenance after salvage autologous stem cell transplantation results in long-term remission in multiple relapsed primary CNS lymphoma. Eur J Haematol. 2018;101(1):115–118. doi:10.1111/ejh.13072
- Frutoso M, Mortier E. NK cell hyporesponsiveness: more is not always better. Int J Mol Sci. 2019;20(18):4514, doi:10.3390/ijms20184514
- Shaffer BC, Le Luduec JB, Park S, et al. Prospective KIR genotype evaluation of hematopoietic cell donors is feasible with potential to benefit patients with AML. Blood Adv. 2021;5(7):2003–2011. doi:10.1182/bloodadvances.2020002701
- Li L, Kolk M, Fernandez-Vina M, et al. Interrogating the impact of KIR ligand mismatch in engraftment following HLA-disparate stem cell transplantation. Bone Marrow Transplant. 2020;55(12):2294–2297. doi:10.1038/s41409-020-0957-7
- Marra J, Greene J, Hwang J, et al. KIR and HLA genotypes predictive of low-affinity interactions are associated with lower relapse in autologous hematopoietic cell transplantation for acute myeloid leukemia. J Immunol. 2015;194(9):4222–4230. doi:10.4049/jimmunol.1402124
- Erbe AK, Wang W, Carmichael L, et al. Follicular lymphoma patients with KIR2DL2 and KIR3DL1 and their ligands (HLA-C1 and HLA-Bw4) show improved outcome when receiving rituximab. J Immunother Cancer. 2019;7(1):70. doi:10.1186/s40425-019-0538-8
- Forlenza CJ, Boudreau JE, Zheng J, et al. KIR3DL1 allelic polymorphism and HLA-B epitopes modulate response to anti-GD2 monoclonal antibody in patients with neuroblastoma. J Clin Oncol. 2016;34(21):2443–2451. doi:10.1200/JCO.2015.64.9558
- Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–5043. doi:10.1200/JCO.2005.13.524
- Vilches C, Castaño J, Gómez-Lozano N, et al. Facilitation of KIR genotyping by a PCR-SSP method that amplifies short DNA fragments. Tissue Antigens. 2007;70(5):415–422. doi:10.1111/j.1399-0039.2007.00923.x
- Smith LK. HLA typing by direct DNA sequencing. Methods Mol Biol. 2012;882:67–86. doi:10.1007/978-1-61779-842-9_5
- Robinson J, Halliwell JA, McWilliam H, et al. IPD–the immuno polymorphism database. Nucleic Acids Res. 2013;41(Database issue):D1234–D1240. doi:10.1093/nar/gks1140
- Yuan XG, Huang YR, Yu T, et al. Primary central nervous system lymphoma in China: a single-center retrospective analysis of 167 cases. Ann Hematol. 2020;99(1):93–104. doi:10.1007/s00277-019-03821-9
- Kim N, Lim DH, Yoon SE, et al. Selective salvage radiotherapy could provide favorable outcomes in patients with refractory or relapsed primary central nervous system lymphoma. J Neurooncol. 2022;156(2):307–316. doi:10.1007/s11060-021-03909-1
- Ferreri AJM, Cwynarski K, Pulczynski E, et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017;4(11):e510–e523. doi:10.1016/S2352-3026(17)30174-6
- Ferreri AJM. Therapy of primary CNS lymphoma: role of intensity, radiation, and novel agents. Hematol Am Soc Hematol Educ Program. 2017;2017(1):565–577. doi:10.1182/asheducation-2017.1.565
- Chiesa S, Hohaus S, Falcinelli L, et al. Chemoradiotherapy with temozolomide after high-dose methotrexate for primary CNS lymphoma: a multicenter phase I study of a response-adapted strategy. Ann Hematol. 2020;99(10):2367–2375. doi:10.1007/s00277-020-04220-1
- Birsen R, Willems L, Pallud J, et al. Efficacy and safety of high-dose etoposide cytarabine as consolidation following rituximab methotrexate temozolomide induction in newly diagnosed primary central nervous system lymphoma in immunocompetent patients. Haematologica 2018;103(7):e296–e299. doi:10.3324/haematol.2017.185843
- Renaud L, Bossard JB, Carpentier B, et al. Treatment with temozolomide and ibrutinib in recurrent/refractory primary (PCNSL) and secondary CNS lymphoma (SCNSL). Eur J Haematol. 2021;107(3):370–373. doi:10.1111/ejh.13667
- Sekine T, Marin D, Cao K, et al. Specific combinations of donor and recipient KIR-HLA genotypes predict for large differences in outcome after cord blood transplantation. Blood. 2016;128(2):297–312. doi:10.1182/blood-2016-03-706317
- van der Ploeg K, Luduec L, Stevenson JB, et al. HLA-A alleles influencing NK cell function impact AML relapse following allogeneic hematopoietic cell transplantation. Blood Adv. 2020;4(19):4955–4964. doi:10.1182/bloodadvances.2020002086
- Boudreau JE, Giglio F, Gooley TA, et al. KIR3DL1/HLA-B subtypes govern acute myelogenous leukemia relapse after hematopoietic cell transplantation. J Clin Oncol. 2017;35(20):2268–2278. doi:10.1200/JCO.2016.70.7059
- Ruscetti M, Leibold J, Bott MJ, et al. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science. 2018;362(6421):1416–1422. doi:10.1126/science.aas9090
- Chang MC, Cheng HI, Hsu K, et al. NKG2A down-regulation by dasatinib enhances natural killer cytotoxicity and accelerates effective treatment responses in patients with chronic myeloid leukemia. Front Immunol. 2019;9:3152, doi:10.3389/fimmu.2018.03152
- Wei B, Liu Z, Fan Y, et al. Analysis of cellular heterogeneity in immune microenvironment of primary central nervous system lymphoma by single-cell sequencing. Front Oncol. 2021;11:683007, doi:10.3389/fonc.2021.683007
- Lin Z, Ma J, Ma Y, et al. Prognostic impact of peripheral natural killer cells in primary central nervous system lymphoma. Front Immunol Published Online. 2023;14. doi:10.3389/fimmu.2023.1191033
