?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objectives
The study's aim was to enhance awareness of acquired hemophagocytic syndrome (HPS) in adults by analyzing clinical features, and investigating the relationship between factors such as the Systemic Inflammation Index (SII) and the prognosis of HPS.
Methods
Clinical characteristics, survival data, and prognostic factors of 75 HPS patients admitted to our hospital between January 2012 and October 2022 were analyzed.
Results
In the high SII group, red blood cells, white blood cells, platelets, neutrophils, fibrinogen, and CD4 + cell activity were higher, and survival time was longer compared to the low SII group. Conversely, total bilirubin and direct bilirubin were higher in the low SII group (P ≤ 0.05). After applying the log-Rank or Breslow tests, HPS patients in the high SII group and those following the HLH-2004 protocol experienced a notably longer survival time. (χ2 = 4.291, P < 0.05; χ2 = 5.210, P < 0.05). Patients with poor prognosis showed higher age of onset, elevated levels of total, direct, and indirect bilirubin, and a greater rate of EBV infection, but reduced levels of red blood cells, platelets, hemoglobin, albumin, globulin, and HLH-2004 protocol usage rate(P < 0.05). Multivariate analysis and ROC curve results indicate that special attention is needed for patients with platelets < 42.5 × 109/L, albumin < 27.7 g/L, fibrinogen < 1.085 g/L, those not following the HLH-2004 protocol, and those who are EBV (+).
Discussion and Conclusion
Early diagnosis and following the HLH-2004 protocol are essential for patients with HPS clinical manifestations to improve prognosis. Additional research is necessary to examine the link between SII and HPS patients’ prognosis.
Introduction
Hemophagocytic syndrome (HPS), or hemophagocytic lymphohistiocytosis (HLH), is characterized by an excessive inflammatory response stemming from the abnormal activation of lymphocytes, monocytes, and macrophages. Symptoms include persistent fever, enlarged liver and spleen, reduced blood cell count, liver dysfunction, aberrant coagulation, and hemophagocytosis [Citation1]. HPS has two classifications: hereditary, resulting from genetic defects like PRF1 and UNC13D, and acquired, arising from infections, tumors, autoimmune diseases, and more. The condition is acute, advancing rapidly, and often leads to high mortality [Citation2].
The Systemic Inflammation Index (SII), defined as platelet count × neutrophil count/lymphocyte count, serves as a biological marker to measure inflammation and assess, diagnose, and track various diseases, including cancer [Citation3, Citation4]. Specific cancers show that increased neutrophils and peripheral blood platelets may foster cancer growth and spread, while lymphocytes contribute to tumor control [Citation5]. A heightened SII corresponds to an unfavorable cancer prognosis, manifesting in diminished survival rates and other negative outcomes [Citation5–7].
This study involved an analysis of data from 75 adult patients with acquired HPS, admitted to the Third Affiliated Hospital of Soochow University between January 2012 and October 2022. The evaluation covered their clinical features, laboratory indicators, treatment, and prognosis, aiming to increase understanding of acquired HPS and to examine prognostic determinants.
Patients and methods
Patients, diagnostic criteria and data collection
In this study, 75 adult patients with HPS (27 males, 48 females), aged 15–84 years with a median age of 58, were admitted to the Third Affiliated Hospital of Soochow University from January 2012 to October 2022. Diagnoses of HPS were made based on the Histiocytosis Society's 2004 (HLH-2004) standards and the latest Chinese guidelines (2022 edition), including eight criteria: (1) Fever for more than a week, with a peak of ≥38.5°C. (2) Splenomegaly. (3) Cytopenia in at least two lineages, with hemoglobin (Hb) < 90 g/L, platelet (PLT) < 100 × 109/L, or absolute neutrophil count (ANC) < 1 × 109/L. (4) Hypertriglyceridemia or hypofibrinogenemia, with serum triglyceride (TG) ≥ 3 mmol/L or fibrinogen (FIB) < 1.5 g/L after fasting. (5) Serum ferritin (SF) ≥ 500ug/L. (6) sCD25 ≥ 2400U/ml. (7) Decreased or absent NK cell activity. (8) Presence of hemophagocytic cells in the bone marrow, central nervous system, or lymph nodes. Follow-up was conducted until death or the end of the study period on October 31, 2022.
A variety of laboratory tests were performed, including blood routine tests, liver and lactate dehydrogenase functions, blood lipid and serum ferritin assessments, NK cell and soluble CD25 activity analyses, electrolyte and myocardial enzyme measurements, coagulation tests, etiology and immunology evaluations, peripheral blood smears, bone marrow cytology, biopsies, imaging studies, and other examinations to confirm diagnoses and ascertain the underlying causes.
Statistical analysis
SPSS 26.0 was employed for data analysis. Data with a normal distribution were denoted as mean ± standard deviation (±s), while non-normal distribution data were represented by the median and interquartile range [M(Q1,Q3)]. Count data were shown as a percentage (%). The analysis included various statistical methods such as independent sample t-tests, non-parametric rank sum tests, and chi-square tests for group comparisons. Survival curves were plotted using the Kaplan-Meier method, and the log-Rank or Breslow test was applied to analyze survival time and compare prognostic factors among groups. The ROC curve was used to determine the diagnostic ability of different factors. The significance level (α) was set at 0.05.
Results
Out of 75 patients, 20 (26.67%) had HPS caused by lymphoma, 2 (2.67%) by myelodysplastic syndrome (MDS-EB1), and 3 (4.00%) by leukemia. 34 (45.33%) had HPS caused by infection. The infections included viral cases such as EBV and cytomegalovirus, bacterial infections like Roseomonas, Staphylococcus hominis subsp, Corynebacterium striatum, Stenotrophomonas maltophilia, Streptococcus pneumoniae, and fungal infections such as Aspergillus. Autoimmune disorders caused HPS in 14 patients (18.67%), and the cause was unknown in 2 cases (2.67%).
The primary symptoms of HPS were persistent fever above 38.5°C unresponsive to antibiotics (81.33%), enlargement of the liver and spleen (62.66%), lymphadenopathy (56.00%), rash (18.67%), and multiple serous effusions (2.67%). Symptoms in the respiratory, digestive, and nervous systems could also occur. During the diagnosis of acquired HPS, the most frequent diagnostic indicators were SF [1500(1487.18, 1500)] ≥ 500 ug/L and persistent fever. Further details about the other indicators are provided in .
Table 1. HLH-2004 diagnostic indexs occurrence rate.
Other laboratory examinations commonly showed β2-microglobulin [5.18 (4.23, 7.10)] > 2.4 mg/L, gamma-glutamyl transferase (GGT) [115.50 (64, 265.10)] > 45 U/L, lactate dehydrogenase (LDH) [582 (354.50, 1111.25)] > 250 U/L, albumin [27.80 (24.40, 31.70)] < 35 g/L, apolipoprotein A1 [0.70 (0.50, 0.91)] < 1.20 g/L, fibrinogen degradation products [12.20 (5.45, 22.65)] > 5 ug/ml and D-dimer [4.35 (1.81, 9.17)] > 0.55 mg/L, comprising over 80% of the cases. Conversely, indirect bilirubin [7.85 (4.78, 12.68)] > 13.70 µmol/L, globulin (26.56 ± 6.24) < 20 g/L, apolipoprotein B [0.80 (0.64, 1.07)] > 1.1 g/L, low-density lipoprotein cholesterol [1.69 (1.18, 2.07)] > 3.36 mmol/L and CD8 + cell activity (30.73 ± 20.34) < 15% were less common, comprising less than 30% of the cases.
According to the median of the SII, the HPS patients were divided into the high SII group (n = 36, [679. 3282 (410. 4071, 1705. 4966)]) and the low SII group (n = 38, [37. 8062 (13. 4318, 129. 7439)]). The high SII group had statistically significantly higher red blood cells, white blood cells, platelets, neutrophils, fibrinogen, CD4 + cell activity, and low-density lipoprotein, along with a longer survival time compared to the low SII group. In contrast, total bilirubin and direct bilirubin were statistically significantly higher in the low SII group (P ≤ 0.05) ().
Table 2. Comparison of clinical characteristics and laboratory tests.
All 75 patients received anti-infection therapies, along with cause-specific and symptomatic support treatments such as chemotherapy for tumor-related HPS, immunosuppression for autoimmune-related HPS, and anti-infection therapies for infection-related HPS. Additionally, 43 patients (57.33%) followed the HLH-2004 protocol, 17 (22.67%) received hormone therapy, 4 (5.33%) received hormone and primary disease-related chemotherapy, and 11 (14.67%) had no additional treatment. Of the 20 lymphoma patients (26.67%), 12 were treated with the HLH-2004 protocol, followed by R-CHOP, CHOPE, COEP-L, DDGP, and other chemotherapy regimens based on pathological typing. The remaining patients received only chemotherapy and hormonal treatment for primary lymphoma. Initial improvement rates showed slight differences among the three groups, but the chi-square test found no statistically significant differences (χ2 = 0.064, P = 0.968)().
Table 3. Outcomes of the initial hospitalization.
In this study, 75 HPS patients were followed until death or October 31, 2022, with three lost to follow-up. Of the remaining 72, 53 (73.61%) died, with survival times ranging from 1 to 4514 days and a median of 54 days. Survival curves were constructed using the Kaplan-Meier method, with rates at 1, 3, 5, 10, and 12 months of 54.2%, 41.7%, 30.3%, 27.2%, and 25.5%, respectively. The log-Rank test revealed no significant difference between the survival curves for three HPS cause groups (χ2 = 1.098, P = 0.578). The Breslow test showed a statistically significant longer survival time in patients treated with the HLH-2004 protocol (χ2 = 5.210, P < 0.05).
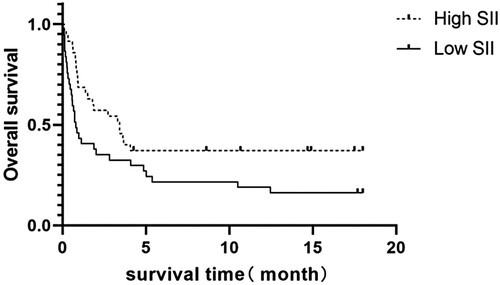
The patients were divided into two groups based on their median SII values: the high SII group (n = 35, [691. 1429(402. 3673, 1758. 5217)]) and the low SII group (n = 36, [37. 8062( 12. 0455, 123. 7923)]). In the high SII group, 22 patients (62.86%) died, with a median survival time of 3.43 months. In the low SII group, 30 patients (83.33%) died, with a median survival time of 0.8 months. The survival curves for the two groups were generated and analyzed using log-Rank tests (), which showed that the high SII group had a significantly longer survival time compared to the low SII group(χ2 = 4.291, P < 0.05).
To determine risk factors for adult-acquired HPS prognosis, patients were divided based on survival time into two groups: less than 30 days (poor prognosis) and more than 30 days (better prognosis), with the 30-day survival rate around 50% used for grouping. Surviving patients were included in the better prognosis group, and three lost-to-follow-up patients were excluded. Various tests were performed to compare age, gender, clinical features, and lab results between groups, with SF values above 1500 µg/L recorded as 1500 µg/L ( and ). The results revealed that the age of onset, total bilirubin, direct bilirubin, indirect bilirubin, and rate of EBV infection were significantly higher in the poor prognosis group. Conversely, the red blood cell count, platelet count, hemoglobin, albumin, globulin levels, and HLH-2004 protocol usage rate were higher in the better prognosis group (P < 0.05). Multivariate logistic regression identified the rate of EBV infection as a significant prognostic factor(OR = 67.854,95%CI 1.0096–4201.738, P < 0.05).
Table 4. Comparison of clinical characteristics.
Table 5. Comparison of laboratory tests.
In this study, univariate Cox regression analysis indicated that factors such as advanced age, low red blood cell count, low platelet count, low hemoglobin, low ALT, elevated total bilirubin, direct bilirubin, and indirect bilirubin, reduced albumin and globulin, low fibrinogen, high NK cell activity (b = 0.039, HR = 1.040, P = 0.037), and positive EB virus DNA status could contribute to a poor prognosis (P < 0.05). Subsequent multivariate Cox regression analysis further examined the variables identified by the univariate analysis. The results demonstrated that high albumin vs low albumin (HR < 0.001, 95%CI 0-0.623, P = 0.04), high fibrinogen vs low fibrinogen (HR < 0.001, 95%CI 0-0.290, P = 0.031), high platelets vs low platelets (HR = 0.131, 95%CI 0.018–0.963, P = 0.046), and the absence vs presence of the HLH-2004 protocol (HR = 1.491E + 27, 95%CI 397.912–5.584E + 51, P = 0.03) significantly influenced survival time.
In the model, h(t) represents the hazard function for HPS patients, ranging from [0,1], variables include onset age (x1, in years), red blood cell count (x2, × 1012/L), ALT level (x3, U/L), total bilirubin level (x4, umol/L), albumin concentration (x5, g/L), globulin concentration (x6, g/L), fibrinogen concentration (x7, g/L), NK cell activity percentage (x8, %), EB virus DNA status (x9, 1 for positive, 2 for negative), treatment plan (x10, 1 for using HLH-2004 protocol, 2 for not using), and platelet count (x11, × 109/L). The model's robustness is shown by a – 2 log-likelihood value of 1.048 and an Omnibus test's P < 0.001.
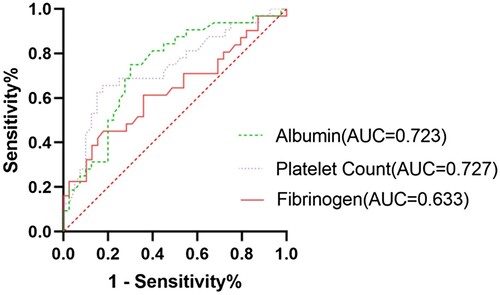
The study infers that survival time of HPS patients may be significantly impacted by albumin, fibrinogen, platelets, use of the HLH-2004 protocol, and EB virus infection status. ROC curves () identified poor prognosis with platelet count < 42.50 × 109/L (AUC = 0.727, Sensitivity = 0.6563, Specificity = 0.825), albumin < 27.7 g/L (AUC = 0.723, Sensitivity = 0.75, Specificity = 0.7), and fibrinogen < 1.085 g/L (AUC = 0.633, Sensitivity = 0.4516, Specificity = 0.8205). It should be noted that there was no statistically significant difference in prognosis based on SII levels, as determined by rank sum testing and Cox regression analysis (P > 0.05), and the ROC curve had no diagnostic value (AUC = 0.512).
Discussion
HPS is classified as hereditary (common in children under 6, often due to genetic defects) or acquired (frequent in adults, secondary to infections, tumors, autoimmune diseases, etc.). The study identified 25 tumor-related cases (mainly lymphoma), 34 non-specific secondary infections (with Epstein–Barr virus prevalent), and 14 autoimmune disease-related cases (mostly systemic lupus erythematosus), in line with other research [Citation8, Citation9]. In this investigation, all eight HLH-2004 diagnostic criteria were noted, with ferritin elevation and fever being more common. HPS might also exhibit multi-system non-specific symptoms. Typical lab findings include increased β2-microglobulin, fibrin degradation products, GGT, LDH, and D-dimer levels, along with reduced albumin and apolipoprotein A1, matching other studies [Citation10–12]. The swift progression and non-specific clinical signs of HPS (such as elevated ferritin, which cannot predict HPS occurrence [Citation13]) make early diagnosis challenging, often leading to detection at an acute and dangerous stage.
HPS and SII are closely connected. The SII serves as a biomarker to gauge inflammatory responses, reflecting both the immune system's functionality and inflammation intensity. A high SII value indicates an active immune response to inflammation. Conversely, HPS is an immune system disorder marked by its failure to effectively regulate cellular and tissue inflammation. As a result, we believe there's a tight connection between the SII and both HPS occurrence and prognosis. In patients with HPS and elevated SII, measurements such as red blood cell, white blood cell, platelet, and neutrophil counts exceed those in the low SII group, possibly mirroring the body's stress response to inflammation and augmented hematopoietic system activity. These observations hint that the inflammatory response is more obvious in the high SII group.
However, after a sustained inflammatory response, HPS patients may see a decline in their body's ability to react to inflammation. At this stage, the SII may decrease, which can be accompanied by increases in total bilirubin, liver damage, and bone marrow suppression, as observed in the low SII group. This decrease may result from fatigue and malfunction in the body's inflammatory response system, preventing the body from creating a proper reaction to pathological stimuli. Survival analysis reveals that the high SII group's prognosis is more favorable than the low SII group's prognosis. According to the calculated formula, this conclusion is consistent with the poor prognostic results observed in HPS patients with a platelet count of < 42.50 × 109/L in the group comparison and Cox analysis. This situation might arise because the low SII group's bodily functions are more weakened: they demonstrate greater immune system suppression or dysfunction. Consequently, their reactions to infections or inflammation may be feeble or delayed, leading to quicker or more severe disease progression. This stands in opposition to the worse prognosis noticed in cancer patients with high SII.
Acquired HPS, a life-threatening condition, demands prompt intervention through three principal aspects: (1) dispensing drugs like corticosteroids, etoposide, cyclosporine A, and high-dose gamma globulin to manage severe hyperinflammatory cytokines; (2) offering symptomatic and supportive care, including anti-infective treatments for infections, red blood cell and platelet transfusions for pancytopenia, and coagulation factor supplements for abnormal coagulation function; (3) detecting and addressing the underlying primary disease as quickly as possible using examinations such as pathogenic tests, autoantibody tests, and cancer screenings. The definitive treatment for HPS is allogenic hematopoietic stem cell transplantation, necessitating HLA matching to locate a suitable donor [Citation14]. Most study patients were treated following the HLH-2004 protocol, and all received etiological and symptomatic care in the hospital.
The prognosis of HPS is generally poor. Jing He's team reported survival rates of 71.4%, 48.2%, 42.9%, 41.1%, 37.5%, and 28.6% at 1, 3, 6, 9, 12, and 18 months respectively [Citation15]. In our study, the corresponding survival rates at 1, 3, 5, 10, and 12 months were 54.2%, 41.7%, 30.3%, 27.2%, and 25.5%. These variations may be due to factors such as patient compliance and treatment methods. Overall, the outlook for HPS remains grim. In this study, the survival outcomes varied for patients with HPS resulting from different underlying causes. While previous research has indicated a better prognosis for HPS caused by autoimmune diseases compared to other factors [Citation15, Citation16], this study found no significant differences between the groups (χ2 = 1.220, P = 0.748). This lack of significance might be due to the limited patient numbers, resulting in smaller sample sizes and a reduced ability to detect variations. Differences in patient characteristics with the same underlying cause, as well as complications and treatments, could also notably influence survival outcomes.
Brastianos discovered that factors such as older age, thrombocytopenia, prolonged APTT, elevated triglycerides, raised lactate dehydrogenase, and tumor etiology predicted a poor prognosis for adult HPS patients [Citation17]. In this study, patients were divided into two groups based on whether their survival time was more than 30 days. Through comparison and analysis of the clinical features and laboratory test disparities between patients with different prognoses, it was revealed that the group with a poorer prognosis exhibited higher onset age, total bilirubin, direct bilirubin, indirect bilirubin, and EBV infection rate. Conversely, red blood cell count, platelet count, hemoglobin, albumin, globulin, and the usage rate of the HLH-2004 protocol were lower (P < 0.05). Multivariate analysis and ROC curve outcomes suggest that special attention should be given to patients with a platelet count < 42.5 × 109/L, albumin <27.7 g/L, fibrinogen <1.085 g/L, non-adherence to the HLH-2004 protocol, and EB virus infection. These findings hold indicative significance for forecasting the prognosis of acquired HPS patients.
In diagnosing HPS, reduced or absent NK cell activity is a standard criterion. However, in a paradox, patients with a poor prognosis have shown increased NK cell activity. This could be a compensatory mechanism, where the body depends on NK cells because of impairments or exhaustion in other immune cells from inflammation. Even with this increase, the overall immune dysfunction may still lead to undesirable outcomes. The limited sample size in this study suggests that this conclusion needs further confirmation through more exhaustive research.
Survival analysis using the log-Rank test did reveal statistical differences in prognosis among different SII groups. However, higher-performance univariate and multivariate Cox regression analyses failed to uncover a correlation between SII level and the prognosis of HPS patients. This lack of correlation might be attributed to the small sample size, or it may suggest that SII is only related to the onset of HPS, warranting further study.
Conclusion
In summary, acquired HPS has a range of causes, and its clinical features and laboratory tests are varied but non-specific, making early diagnosis difficult. The condition is dangerous, with generally poor prognosis. For patients showing clinical signs of HPS and having a platelet count of < 42.50 × 109/L, albumin <27.7 g/L, fibrinogen <1.085 g/L, and positive for EBV, early diagnosis and treatment are crucial. Upon confirmation of the diagnosis, it's advisable to promptly apply the HLH-2004 protocol to enhance the prognosis. The SII level seems to be somewhat correlated with the prognosis of HPS patients, indicating the need for further research.
Authors’ contributions
YL and WYG designed the study and revised the manuscript. HNY and YC collected and analyzed the data, conducted the research, and wrote the article. JL, YL, YJF, BY, and YL provided valuable suggestions. All authors read and approved the final manuscript and the submitted version. Each author agrees to take responsibility for their respective contributions and to ensure the accuracy and integrity of any part of the study.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of our hospital and was conducted in accordance with the Helsinki Declaration.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Al-Samkari H, Berliner N. Hemophagocytic lymphohistiocytosis. Annu Rev Pathol. 2018;13:27–49.
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–1516.
- Shibata S, Arima H, Asayama K, et al. Hypertension and related diseases in the era of COVID-19:a report from the Japanese society of hypertension task force on COVID-19. Hypertens Res. 2020;43(10):1028–1046.
- Chee YJ, Tan SK, Yeoh E. Dissecting the interaction between COVID-19 and diabetes mellitus. J Diabetes Investig. 2020;11(5):1104–1114.
- Meng L, Yang Y, Hu X, et al. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: a systematic review and meta-analysis. J Transl Med. 2023;21(1):79. doi:10.1186/s12967-023-03924-y
- Liu XC, Jiang YP, Sun XG, et al. Prognostic significance of the systemic immune-inflammation index in patients with cholangiocarcinoma: a meta-analysis. Front Oncol. 2022;12:938549.
- Zhou Y, Dai M, Zhang Z. Prognostic significance of the systemic immune-inflammation index (SII) in patients with small cell lung cancer: a meta-analysis. Front Oncol. 2022;12:814727.
- Lehmberg K, Nichols KE, Henter JI, et al. Consensus recommendations for the diagnosis and management of hemophagocytic lymphohistiocytosis associated with malignancies. Haematologica. 2015;100(8):997–1004.
- Yoon JH, Park SS, Jeon YW, et al. Treatment outcomes and prognostic factors in adult patients with secondary hemophagocytic lymphohistiocytosis not associated with malignancy. Haematologica. 2019;104(2):269–276. doi:10.3324/haematol.2018.198655
- Lin M, Park S, Hayden A, et al. Clinical utility of soluble interleukin-2 receptor in hemophagocytic syndromes: a systematic scoping review. Ann Hematol. 2017;96(8):1241–1251. doi:10.1007/s00277-017-2993-y
- Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood. 2015;125(19):2908–2914. doi:10.1182/blood-2015-01-551622
- Zhuo WB, Gao Y, Yang CY, et al. Clinical characteristics of hemophagocytic syndrome: analysis of 46 cases. Nan Fang Yi Ke Da Xue Xue Bao. 2018;38(6):769, -inside back cover.
- Schram AM, Campigotto F, Mullally A, et al. Marked hyperferritinemia does not predict for HLH in the adult population. Blood. 2015;125(10):1548–1552. doi:10.1182/blood-2014-10-602607
- Janka GE, Lehmberg K. Hemophagocytic syndromes–an update. Blood Rev. 2014;28(4):135–142.
- Jing H, Liqiong W, Xiaohua H, et al. Clinical etiology and prognosis of hemophagocyticlymphohistiocytosis: 56 cases and literature review. J Chongqing Med Univ. 2022;47(9):1104–1110.
- Brito-Zerón P, Kostov B, Moral-Moral P, et al. Prognostic factors of death in 151 adults with hemophagocytic syndrome: etiopathogenically driven analysis. Mayo Clin Proc Innov Qual Outcomes. 2018;2(3):267–276. doi:10.1016/j.mayocpiqo.2018.06.006
- Brastianos PK, Swanson JW, Torbenson M, et al. Tuberculosis-associated haemopha-gocytic syndrome. Lancet Infect Dis. 2006;6(7):447–454.