ABSTRACT
Thrombocytopenia is one of the most common hematological adverse reactions in chronic myeloid leukemia (CML) patients receiving tyrosine kinase inhibitors (TKI) therapy, causing life-threatening bleeding cases. However, there are fewer therapeutic drugs for TKI-induced thrombocytopenia. Eltrombopag is a non-peptide thrombopoietin receptor agonist used for the treatment of immune thrombocytopenia, aplastic anemia, and hepatitis C-associated thrombocytopenia. Nevertheless, studies of eltrombopag for TKI-induced thrombocytopenia are still lacking. This study retrospectively analyzed the clinical and test data of 21 CML patients with TKI-related thrombocytopenia. The results demonstrated that the median baseline value of thrombocytopenia in the 21 CML patients was 15.57 × 109/L [2–28 × 109/L]. Following treatment with eltrombopag, 16 patients had a significant increase in their platelet levels. The peak median for platelet increase in effective responders was 145.12 × 109/L (51–460 × 109/L). However, 5 patients failed to respond to eltrombopag. Moreover, 4 of the 21 patients enrolled had adverse reactions, including reversible liver function impairment, palpitation, headache, insomnia, and loss of appetite. Nonetheless, no cases of disease progression, thrombotic events, or myelofibrosis were observed. Hence, eltrombopag may be a useful adjunctive therapy for relieving TKI-related thrombocytopenia in patients with CML.
1. Introduction
Chronic myelogenous leukemia (CML) is a blood cancer with specific chromosomal abnormalities. The widespread availability of tyrosine kinase inhibitors (TKI) allows most patients to achieve a normal life expectancy. Currently, five TKIs have been approved for treatment by the FDA: imatinib, nilotinib, dasatinib, bosutinib, and ponatinib [Citation1]. Although TKI is well tolerated, there are still numerous adverse reactions. Among these, hematologic adverse reactions, especially ≥ grade 3 cytopenia, are the primary indicators for TKI reduction and discontinuation [Citation2]. However, frequent dose reduction and interruption may reduce the efficacy of TKI [Citation3]. Thrombocytopenia is one of the most common hematological adverse reactions in CML patients receiving TKI therapy, causing life-threatening bleeding cases. Reports of first-line TKI treatment revealed that the incidence of ≥ grade 3 thrombocytopenia was 4% to 14% with imatinib 400 mg/d, and it was 10% to 14% on nilotinib 600 mg/d, and 14% to 22% when dasatinib was used at 100 mg/d [Citation4–7].
Domestic and international guidelines recommend granulocyte colony-stimulating factor(G-CSF) as a clinical treatment for TKI-induced neutropenia. In addition, human erythropoietin injection is used clinically for TKI-induced anemia. However, there are fewer therapeutic drugs for TKI-induced thrombocytopenia. Eltrombopag is an oral, synthetic, non-peptide, small-molecule human thrombopoietin agonist used for the treatment of immune thrombocytopenia, aplastic anemia, and hepatitis C-associated thrombocytopenia [Citation8–10]. Recently, a non-rando-mized, phase II, single-arm study in America has established that eltrombopag can relieve TKI-induced thrombocytopenia in CML patients, thereby improving the efficacy of TKI [Citation11]. However, there is a lack of studies in China and the Asia-Pacific region. Therefore, we retrospectively analyzed the clinical data of 21 CML patients treated with eltrombopag for TKI-induced thrombocytopenia to provide a reference for its efficacy and safety in CML patients.
2. Objects and methods
2.1. Patients
21 CML patients treated with eltrombopag for TKI-related thrombocytopenia from January 2018 to August 2021 were included. The inclusion criteria were: (1) Patients who met the diagnostic criteria of the ELN2020 edition of the Chronic Myeloid Leukemia Diagnosis and Treatment Guidelines; (2) Patients with normal or increased platelet levels before TKI treatment and who had ≥ grade 3 thrombocytopenia after at least 2 weeks or more of TKI treatment; (3) Patients with concomitant comorbidities leading to thrombocytopenia were excluded; (4) Patients treated with eltrombopag. Dosage of Eltrombopag is 25–100mg. This study was approved by the Ethics Committee of Minda Hospital of Hubei Minzu University.
2.2. Response definitions
Effective response: PLT ≥ 50 × 109/L, or 30 × 109/L increase from the initial level for at least 3 months and no bleeding signs; Ineffective response: Sustained PLT < 50 × 109/L, or less than 30 × 109/L increase from the initial level for more than 3 months. CML responses were defined with regard to ELN recommendation.
2.3. Statistical analysis
The SPSS 19.0 statistical software was utilized to compile data. Student t-test was used for comparison before and after treatment. P-value < 0.05 was considered statistically significant.
3. Results
3.1. Patients’ characteristics
The 21 CML patients, composed of 15 males and 6 females, had a median disease duration of 20.6 months, and the median age was 44.19 years (18–74 years old). Patients’ CML disease stage: 20 cases in the chronic phase and 1 case in the blast phase. Before eltrombopag treatment, 12 patients achieved mCyR (minor cytogenetic remission), 7 patients had PCyR (partial cytogenetic remission), and 2 patients had noCyR. None of the 21 patients reached MMR (major molecular response). Three of them had comorbidities: 1 with secondary myelofibrosis, 1 with gout (receiving uric acid-lowering therapy), and 1 with tuberculosis (receiving anti-tuberculosis therapy) ().
Table 1. Characteristics of patients.
3.2. Response
All 21 CML patients experienced grade 4 hematological adverse reactions during the use of TKI-targeted therapy after diagnosis, accompanied by varying degrees of anemia and leukopenia. Before treatment with eltrombopag, the median baseline value of platelet was 15.57 × 109/L (2–28 × 109/L). 12 patients had switched TKI for thrombocytopenia and all patients discontinued TKI. Of the 21 patients, only 3 patients received eltrombopag at the first occurrence of TKI-related thrombocytopenia. Two patients’ platelet levels returned to normal without recurrence. Another patient’s platelet level remained 23–41 × 109/L after 40 days of treatment with 50 mg/day of eltrombopag. 18 patients underwent intractable TKI-related thrombocytopenia with a duration of a platelet count below 30 × 109/L for more than 60 days (). All these patients failed with platelet-raising therapies such as recombinant human thrombopoietin, interleukin-11, caffeic acid tablets, cortisol hormone, and gamma immunoglobulin. Additionally, 3 patients had ineffective platelet transfusions ().
Figure 1. The effect of Eltrombopag on blood count. PLT: Platelets; HGB: hemoglobin; WBC: White Blood Cells.
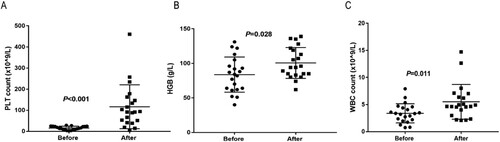
Table 2. TKI and Eltrombopag treatment.
After a median treatment time of 88 days (14 −360 days), 16 (76.2%) patients achieved an effective response with a median dosing time of 20.15 days (5 −60 days) of eltrombopag. The median peak platelet increase was 145.12 × 109/L (51–460 × 109/L) and the median time to optimal response was 45.7 days (14–210 days). 9 patients had platelet levels ≥100 × 109/L with a median time of 25.42 days (8–55 days). At the last follow-up, the median duration for sustained platelet response was 10 months (1–29 months). Given the risk of disease progression with prolonged frequent intermittent discontinuation, 16 patients with an effective response for more than 1 week restarted TKI at a reduced dose and continued on eltrombopag. At the last follow-up, 13 patients evaluated TKI response. 11 patients had decreased BCR-ABL IS and 6 patients achieved CCyR (complete cytogenetic remission) ().
Table 3. TKI response before and after treatment with Eltrombopag.
The 5 patients displayed irresponsive to the eltrombopag with a median treatment of 42 days (30–120 days). Before taking eltrombopag, 3 of those patients experienced persistent platelet counts below 20 × 109/L during treatment. 1 patient had T315I-mutated in the BCR-ABL kinase region, 1 patient progressed to acute lymphoblastic leukemia, and 1 patient had an additional chromosomal mutation. The other 2 patients’ platelet levels could only be maintained at 20–41 × 109/L, and intermittent low-dose TKI therapy was administered under close monitoring for bleeding.
3.3. Safety evaluation
In this study, eltrombopag showed favorable safety in most CML patients. 1 patient developed reversible hepatic impairment, and recovered after discontinuing eltrombopag, 1 patient experienced palpitation, one case experienced a headache at a dose of 100 mg/qd of eltrombopag, and anorexia concomitant with insomnia occurred in 1 patient.
4. Discussion
Thrombocytopenia is one of the most common hematological adverse reactions in CML patients receiving TKI therapy. Severe thrombocytopenia can promote life-threatening bleeding. The latest NCCN guidelines state that TKI-induced cytopenia can be managed by temporarily interrupting TKI treatment and adjusting the dose [Citation12]. Early reports have shown some benefits of low-dose interleukin-11 in the treatment of TKI-related thrombocytopenia in CML patients [Citation13, Citation14]. Preliminary findings suggest that after an IL-11 treatment median duration of 7 months (1–16 Months later), 64% (9/14) of patients had improved platelet counts after treatment, and the median duration of response was 9 months (2.5–40 months) with a median peak platelet count of 110 × 109/L. This finding suggests a degree of efficacy with IL-11 for TKI-induced thrombocytopenia.
Eltrombopag is a second-generation thrombopoietic drug. It is an oral, synthetic non-peptide small molecule human thrombopoietin receptor agonist without endogenous thrombopoietin (TPO) molecular homology sequence. It does not produce TPO-neutralizing antibodies and cross-react and has no risk of persistent thrombocytopenia [Citation9]. In addition, eltrombopag selectively acts on the c-MPL transmembrane region away from endogenous TPO-binding sites, activating the downstream JAK/STAT, ATK, MAPK signaling pathways that promote megakaryocyte proliferation, differentiation, and maturation [Citation15, Citation16]. Recently, Mahran Shoukier et al. reported the effect of eltrombopag in managing thrombocytopenia associated with TKI in patients with CML and myelofibrosis in a prospective study. In their study, 80% (12/15) patients with CML achieved complete platelet response at doses of 50–300 mg/d after a median treatment duration of 18 months. Moreover, the median peak platelet count was 154 × 109/L (range, 74–893 × 109/L) and the median time to optimal response was 6 months (range, 2.1–13 months) [Citation11]. The above results indicated eltrombopag may help improve platelet counts in CML patients receiving TKI with recurrent thrombocytopenia. More evidence is still needed to prove the efficacy of eltrombopag. Here, we retrospectively investigated the efficacy and safety of thrombocytopenia in CML patients with TKI-induced thrombocytopenia. In our study, eltrombopag was effective in 16 of the 21 patients (76.2%), with a median peak platelet increase of 145.12 × 109/L (51–460 × 109/L). Consistent with the results of Mahran Shoukier et al, our results showed that eltrombopag is effective in managing thrombocytopenia associated with TKI. Early studies determined that the effective rate of IL-11 was 64%, the median peak platelet count was 110 × 109/L, and the sustained platelet reaction time was 9 months 14. Interestingly, our results and Mahran Shoukier et al showed that the effective rate of eltrombopag was 76.2% and 80%, the median peak platelet value was 145.12 × 109/L and 154 × 109/L, and the duration of persistent platelet response was 10 and 45 months, appeared to be better than interleukin-11 in managing TKI-induced thrombocytopenia.
In a previous study using different doses of eltrombopag for ITP in the Caucasian population, 28%, 70% and 81% of patients treated with 30, 50, and 75 mg per day for 6 weeks met the primary endpoint, respectively, indicating that the increase in platelet count occurs in a dose-dependent way [Citation17]. In a series of studies in Asian populations, 25 mg/d was mostly used as the initial therapeutic dose for ITP patients, with a maximum dose of 75 mg/d [Citation18–20]. In our study, the dose range of eltrombopag was 25–75 mg/d, except for 1 patient with CML-AP who was given a dose of 50–100 mg/d. Four patients treated with 25 mg/day eltrombopag achieved an effective response. 5 patients taking 50–100 mg//day eltrombopag did not obtain an effective response. The dose-dependent effect in this study for TKI-induced thrombocytopenia was not significant due to inconsistent treatment duration and limited sample. In Mahran Shoukier’s study [Citation11], the dose of eltrombopag was 50–300 mg/d, which may be related to the different metabolism of eltrombopag in different ethnicities [Citation21, Citation22]. In Mahran Shoukier et al’ study, the median time to best response was 6 months (range, 2.1–13 months), ten patients had sustained platelet recovery after stopping eltrombopag with a median duration of sustained platelet response of 45 months (range, 3–69 months) [Citation11]. In our study, patients achieved the best response with a shorter duration of 45.7 days (14–210 days). 4 patients maintained normal platelet levels after stopping eltrombopag for more than 3 months.
Previous concerns about the use of eltrombopag is that stimulate leukemia cell growth as some leukemia blast cells express thrombopoietin receptor. However, an in vitro study reported that eltrombopag could inhibit the cell cycle of leukemia cells from the G1 phase to the S phase and slow down cell division [Citation23]. In the present study, 13 patients evaluated TKI response, of which 11 patients had decreased BCR-ABL IS and 6 patients achieved CCyR at the last follow-up. Similarly, nine patients with CML experienced an improvement in the cytogenetic response during the observation period of eltrombopag treatment. These results showed a minimal risk of disease progression in CML patients treated with eltrombopag. Still, further investigation is warranted to determine whether eltrombopag can indeed inhibit the growth of leukemia cells in CML patients.
In this study, eltrombopag was quite safe for TKI-induced thrombocytopenia. One patient developed reversible hepatic impairment and recovered after discontinuing eltrombopag. 3 patients developed grade 1–2 adverse reactions, such as palpitation, headache, loss of appetite, and insomnia. However, eltrombopag was discontinued in 1 patient due to abnormal liver function in Mahran Shoukier's study and 2 ponatinib-treated patients experienced thrombotic events following eltrombopag treatment. In addition, some grade 3–4 unexpected adverse events irrespective of attribution including fatigue, infection, diarrhea, insomnia, rash, headache, edema, and metabolic abnormalities were reported [Citation11]. This could be related to the lower dose of eltrombopag in our study.
Overall, our study showed that eltrombopag is effective and has a favorable safety in the treatment of TKI-induced thrombocytopenia. Our study has several limitations, it is a retrospective study with a small sample size of 21 patients. Due to the small sample size, the risk factors influencing the efficacy of eltrombopag were not analyzed.
Data sharing statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966–984. doi:10.1038/s41375-020-0776-2
- Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30(8):1648–1671. doi:10.1038/leu.2016.104
- Sneed TB, Kantarjian HM, Talpaz M, et al. The significance of myelosuppression during therapy with imatinib mesylate in patients with chronic myelogenous leukemia in chronic phase. Cancer. 2004;100(1):116–121. doi:10.1002/cncr.11863
- Kantarjian HM, Hughes TP, Larson RA, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. 2021;35(2):440–453. doi:10.1038/s41375-020-01111-2
- Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: The dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34(20):2333–2U54. doi:10.1200/JCO.2015.64.8899
- Hjorth-Hansen H, Stenke L, Soderlund S, et al. Dasatinib induces fast and deep responses in newly diagnosed chronic myeloid leukaemia patients in chronic phase: clinical results from a randomised phase-2 study (NordCML006). Eur J Haematol. 2015;94(3):243–250. doi:10.1111/ejh.12423
- Hwang WL, Chen TC, Lin HY, et al. NOVEL-1st: an observational study to assess the safety and efficacy of nilotinib in newly diagnosed patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase in Taiwan. Int J Hematol. 2022;115(5):704–712. doi:10.1007/s12185-022-03311-1
- Visco C, Rodeghiero F, Romano A, et al. Eltrombopag for immune thrombocytopenia secondary to chronic lymphoproliferative disorders: a phase 2 multicenter study. Blood. 2019;134(20):1708–1711. doi:10.1182/blood.2019001617
- Desmond R, Townsley DM, Dumitriu B, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014;123(12):1818–1825. doi:10.1182/blood-2013-10-534743
- McHutchison JG, Dusheiko G, Shiffman ML, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357(22):2227–2236. doi:10.1056/NEJMoa073255
- Shoukier M, Borthakur G, Jabbour E, et al. The effect of eltrombopag in managing thrombocytopenia associated with tyrosine kinase therapy in patients with chronic myeloid leukemia and myelofibrosis. Haematologica. 2021;106(11):2853–2858.
- Deininger MW, Shah NP, Altman JK, et al. Chronic myeloid leukemia, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(10):1385–1415. doi:10.6004/jnccn.2020.0047
- Ault P, Kantarjian H, Welch MA, et al. Interleukin 11 May improve thrombocytopenia associated with Imatinib mesylate therapy in chronic Myelogenous leukemia. Leuk Res 2004;28(6):613–618. doi:10.1016/j.leukres.2003.11.003
- Aribi A, Kantarjian H, Koller C, et al. The effect of interleukin 11 on thrombocytopenia associated with tyrosine kinase inhibitor therapy in patients with chronic myeloid leukemia. Cancer. 2008;113(6):1338–1343. doi:10.1002/cncr.23718
- Sun H, Tsai Y, Nowak I, Liesveld J, Chen Y. Eltrombopag, a thrombopoietin receptor agonist, enhances human umbilical cord blood hematopoietic stem/primitive progenitor cell expansion and promotes multi-lineage hematopoiesis. Stem Cell Res. 2012; 9(2): 77-86.
- [Di] Buduo CA, Currao M, Pecci A, et al. Revealing eltrombopag's promotion of human megakaryopoiesis through AKT/ERK-dependent pathway activation. Haematologica. 2016;101(12):1479–1488. doi:10.3324/haematol.2016.146746
- Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237–2247. doi:10.1056/NEJMoa073275
- Tomiyama Y, Miyakawa Y, Okamoto S, et al. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J Thromb Haemost. 2012;10(5):799–806. doi:10.1111/j.1538-7836.2012.04695.x
- Kim YK, Lee SS, Jeong SH, et al. Efficacy and safety of eltrombopag in adult refractory immune thrombocytopenia. Blood Res. 2015;50(1):19–25. doi:10.5045/br.2015.50.1.19
- Tripathi AK, Shukla A, Mishra S, et al. Eltrombopag therapy in newly diagnosed steroid non-responsive ITP patients. Int J Hematol. 2014;99(4):413–417. doi:10.1007/s12185-014-1533-y
- Gibiansky E, Zhang J, Williams D, et al. Population pharmacokinetics of eltrombopag in healthy subjects and patients with chronic idiopathic thrombocytopenic purpura. J Clin Pharmacol. 2011;51(6):842–856. doi:10.1177/0091270010375427
- Wu K, Thapar M, Farrell C, et al. Population pharmacokinetic and pharmacodynamic modeling and effects on platelet counts of different dosages of eltrombopag in Chinese patients With chronic primary immune thrombocytopenia. Clin Ther. 2015;37(7):1382–1395. doi:10.1016/j.clinthera.2015.03.024
- Roth M, Will B, Simkin G, et al. Eltrombopag inhibits the proliferation of leukemia cells via reduction of intracellular iron and induction of differentiation. Blood. 2012;120(2):386–394. doi:10.1182/blood-2011-12-399667
