ABSTRACT
Objective:
To explore the feasibility, safety and cost effectiveness of the use of peripherally inserted central catheter (PICC) in children with hemophilia A and inhibitors who underwent ITI treatment.
Method:
This retrospective cohort study evaluated the effect of PICC placement and ITI on bleeding rates, costs, and parents’ satisfaction before and within 6 months after PICC placement in children with hemophilia A and inhibitors.
Results:
A total of 20 children with hemophilia A and high-titer inhibitors were included, with a success rate for PICC placement of 100%, at a cost of ¥6730.50. Parents’ satisfaction with PICC was 100%, and the total length of catheter indwelling was 6055 days. In terms of curative effect, the success rate of ITI treatment was 75%, and the annualized bleeding rate was decreased from 10.90 ± 12.16 times before placement to 2.10 ± 3.32 times (p < 0.05). The transportation expense for children and their parents to the clinic decreased from ¥20,920 ± 32,274.57 before catheter placement to ¥2915 ± 2195.99 (p < 0.05). Time of children missed school and their parents missed work decreased from 10.85 ± 22.36 days to 1.90 ± 3.58 (p < 0.05) days and 40.33 ± 46.11 days to 3.83 ± 7.11 days (p < 0.05), respectively.
Conclusion:
The use of PICC for ITI treatment in children with hemophilia A and accompanying inhibitors in developing countries (e.g. China) can ensure the effect of ITI, reducing expense and improving the quality of life without obvious side effects.
1. Introduction
Hemophilia A (HA) is an X chromosome-linked inherited bleeding disorder caused by the deficiency of coagulation factor VIII (FVIII) [Citation1]. Its clinical manifestations are recurrent spontaneous bleeding, mainly joint and muscle bleeding, resulting in death or disability [Citation2,Citation3]. Intravenous supplementation of FVIII preparation is mainly used as an alternative therapy to control and prevent bleeding. However, in 20–30% of cases of severe HA the process of alternative therapy [Citation4] leads to a decline in curative effect or ineffectiveness of FVIII [Citation5,Citation6]. Inhibitors usually appear within 10–20 exposure days. Specifically, 90% of inhibitors occur in early childhood [Citation4], and these inhibitors must be actively removed.
Immune tolerance treatment (ITI), through frequent intravenous infusion of a large amount of FVIII for months to years, is the only method used to eliminate inhibitors at present, with an effectiveness rate of 70–80%, and should be implemented as soon as possible after the discovery of inhibitors [Citation7]. However, children in this age group have poor vascular condition and it is difficult to perform peripheral vein puncture, so it is necessary to establish an effective central venous access to ensure the implementation of ITI [Citation8]. Although it is reported that infusion ports are widely used in overseas countries [Citation9,Citation10], some difficulties have been apparent in developing in China [Citation11,Citation12]. PICC refers to a catheter that is inserted through peripheral vein puncture, with the tip of the catheter located in the superior vena cava or inferior vena cava, forming a central venous catheter like an infusion port. However, because PICC is used via peripheral vein puncture, it has the advantages of simple operation, less trauma, fewer coagulation factors, and lower expense. The indwelling time is 1 year, which is consistent with the general course of ITI treatment.
In our center, for children who have difficulty in establishing venous access for ITI treatment, their parents choose PICC insertion. Whether PICC is more suitable for children with hemophilia undergoing ITI treatment has not been reported. Therefore, this study aims to explore and summarize the real-world experience of PICC in ITI treatment so as to provide a reference for the choice of venous access in children with hemophilia treated by ITI in developing countries.
2. Materials and methods
2.1. Subjects
Retrospective cohort study design and cluster sampling were adopted. The subjects were children with hemophilia A and inhibitor who were treated in Beijing Children’s Hospital Affiliated to Capital Medical University from 1 January 2020 to 1 May 2022. The inclusion criteria were: (1) diagnosed with severe HA (FVIII: C < 1%); (2) planned for or being treated with ITI for high-titer inhibitor (inhibitor titer > 5 BU/mL); (3) first PICC placement; (4) catheter indwelling lasting for more than 6 months and maintained in our center. This study was approved by the Ethics Committee of Beijing Children’s Hospital Affiliated to Capital Medical University (Ethics No.: YHL201901), and informed consent forms were signed before PICC placement.
2.2. Information on PICC
2.2.1. PICC placement
PICC placement was carried out by trained and skilled specialized nurses in intravenous therapy. Before placement, children were sedated with chloral hydrate. Lidocaine was used for local anesthesia during placement. The catheter used was a three-way valve type PICC produced by U.S. C.R. Bard Inc. The catheter type was selected according to the blood vessel diameter judged by ultrasound, and the selection criterion was that the catheter diameter was less than 45% of the blood vessel diameter.
All of the participants in this group were children with high-titer inhibitors. Ten to 15 min before PICC placement, activated recombinant VIIa (rFVIIa, NovoSeven) was injected intravenously at a dose of 90 µg/kg. After the catheter was inserted, parents were instructed to apply local pressure and to give rFVIIa at the same dose every 2–4 h until the bleeding stopped.
2.2.2. Maintenance and use of PICC
Maintenance and use of PICC was carried out by trained nurses or parents qualified in its operation. Transparent dressing was maintained for at least every 7 days and the gauze dressing for at least every 48 h while the PICC was maintained together with changing the infusion connector. Before PICC was used, the blood was first pumped back, and the pulse-type tube (10 mL of normal saline) was used for drug administration. The pulse-type tube (10 mL of normal saline) was then deployed, with the remaining 2–3 mL of normal saline being used to seal the tube under positive pressure.
2.2.3. Process management of PICC
A professional management team composed of doctors in hemophilia, specialized nurses in intravenous therapy, and specialized nurses in hemophilia was established, and a WeChat group for the complete process management of PICC for children with hemophilia was set up. The children were followed up once a week for 1 month after placement. After parents fully mastered the home care of PICC, follow-up visits were planned once a month to emphasize the importance parents should attach to the catheter while remaining alert and avoiding complications. In the case of any problems with catheter use, they were advised to contact the intravenous therapy specialist nurse at any time for specific guidance.
2.3. Data collection
General information was obtained from the Hospital Electronic Medical Record, including information on age, gender, disease diagnosis, inhibitors, ITI treatment, and PICC placement. Outcome variables included indwelling time of PICC, incidence of complications, curative effect of ITI, bleeding rates, inpatient and outpatient expenses, number of days parents missed work and children missed school, and parents’ satisfaction with the catheter. The outcome indicators were obtained through the following. (1) Indwelling time of PICC and complications was obtained through follow-up of the specialized nurse in intravenous therapy and weekly catheter maintenance. (2) Curative effect of ITI was obtained through Hospital Electronic Medical Record. (3) Frequency of bleeding before and after placement, inpatient and outpatient expenses, and number of days parents missed work and children missed school were obtained by consulting the bleeding-related self-records of patients. Bleeding rates were evaluated before and within 6 months after PICC placement (ITI initiation), including annualized bleeding rate (ABR), annualized joint bleeding rate (AJBR), annualized spontaneous bleeding rate (ASBR). Bleeding rates were calculated with the formula (Number of bleeds) / (Number of monthes during efficacy period) × 12 months. (4) Success rate of self-injection was calculated by: Success rate of self-injection = Number of successful self-injections / total number of self-injections within the same period (6 months) × 100%. (5) Self-injection compliance score: a parent self-rating scale (0 to 10 points) was designed with 0 being the lowest score, indicating that the child has the lowest self-injection compliance, and 10 being the highest score, indicating that the child has the highest self-injection compliance – the higher the score, the better the compliance. There are 11 degrees in total: (1) 0 point: patient completely refuse and failure to carry out self-injection. Patient successfully carry out self-injection scores 1 to 10 points. (2) 1 point: under the compulsory restraint of more than 2 parents. (3) 2 points: under the compulsory restraint of 2 parents. (4) 3 points: under the compulsory restraint of 1 parent. (5) 4 points: under non-compulsory restraint of parents. (6) 5 points: under intensively urge, appease and encourage by parents. (7) 6 points: under intensively urge by parents. (8) 7 points: under the request of parents. (9) 8 points: under appease and encourage by parents. (10) 9 points: cooperate with self-injection under appease and encourage by parents. (11) 10 points: patient actively cooperate with self-injection. The same parent measured compliance before and within 6 months after PICC placement. (6) Execution degree of drug administration as prescribed = Times of drug administration as prescribed on time / Total times of drug administration as prescribed within the same period (6 months) × 100%. (7) Parents’ satisfaction with PICC: a parent self-evaluation questionnaire was designed with five options: very unsatisfied, unsatisfied, satisfied, quite satisfied, and very satisfied. When PICC had been used for 6 months, the questionnaire was filled out by the main caregivers of the children.
2.4. ITI regimen and outcome
2.4.1. ITI regimen
Low- dose ITI (50 FVIII IU/kg every other day) by itself (ITI-alone) or combined with immunosuppressants (rituximab and prednisone, ITI-IS) in children with HA with high-titer inhibitor according to prognostic risk factors [Citation20]. All children were treated with ITI after catheter placement.
2.4.2. ITI outcome
(1) Success: achieving inhibitor elimination (to inhibitor titer <0.6 BU at two consecutive visits >1 week apart). (2) Failure: Patients not achieve success after 1 year follow-up of ITI period.
2.5. Statistical analysis
We performed statistical analyses using SPSS version 20 (IBM, Armonk, NY). Categorical variables were expressed as number of cases/percentage (n/%), and Chi-square test was used for comparison of count data between groups. When n < 40 or expected count <1, we used the Fisher exact test. Continuous variables were expressed as mean ± standard deviation (mean ± SD). Based on the results of the normality tests, a paired Student’s t test was used to evaluate continuous variables. Two-tailed values of p < 0.05 were considered statistically significant.
3. Results
3.1. General information on subjects
A total of 20 children with severe HA and accompanying high-titer inhibitors were enrolled in this study. The average age of the children was 39.20 months (range, 13–132 months) at the time of catheter placement, the median inhibitor level was 102.4 BU/mL (range, 22–187 BU/mL), and 15 children (75%) were under 3 years old.
3.2. Situation of PICC placement
With the help of ultrasound, catheter placement was performed in all of the children. Catheters were inserted in 5 children by using the ultrasound-guided Seldinger technique and in 15 children by the Seldinger technique after preliminarily judging the insertion site with the help of ultrasound. The success rate of catheter placement was 100%. The average time for catheter placement was 40.10 min (range, 25–50 min), local bleeding at the puncture point completely stopped at 38.85 h (range, 10–96 h) after placement, and the children were discharged uneventfully within an average of 3 days (range, 2–5 days). Thirteen children were inserted with a 3Fr catheter and 7 children with a 4Fr catheter; catheter insertion was performed in the right upper arm in 15 children and the left upper arm in 5 children, and in the basilic vein in 12 children and the cephalic vein in 8 children. The tip of catheter was located at T4–T7 (the middle-lower one-third of superior vena cava).
3.3. Curative effect after PICC placement
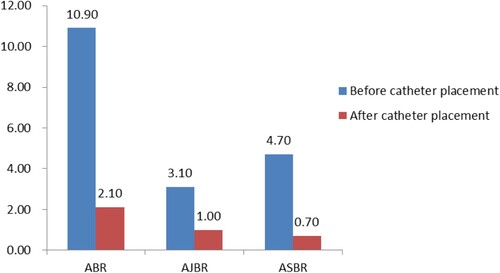
All children were treated with standardized ITI after catheter placement, and 15 children successfully achieved immune tolerance, a success rate of 75%. The inhibitor disappearance time was 8.2 (range, 1.8–18.8) months. Bleeding occurred in 15 children within 6 months before catheter placement, with an average frequency of 5.45 ± 6.08 (range, 2–20) times, ABR was 10.90 ± 12.16.Bleeding occurred in 8 children within 6 months after PICC placement, with an average frequency of 1.05 ± 1.66 (range, 1–6) times, ABR was 2.10 ± 3.32. The difference in bleeding frequency before and after placement was statistically significant (p < 0.05). The AJBR and ASBR also decreased significantly compared with that before placement, and the differences before and after placement were also statistically significant (p < 0.05) ().
3.4. Complications of PICC
PICC was used on the day of insertion, then daily or every other day during ITI treatment. Upon successful ITI, family preventive treatment was continued, i.e. coagulation factor was infused twice or three times a week. The total duration of catheter indwelling was 6055 days (185–365 days). During indwelling, there were 10 cases (50%) of rash under transparent film, 3 cases (15%) of leakage at the end of the catheter, 5 cases (25%) of catheter blockage, 4 cases (20%) of bleeding at a puncture point, 4 cases (20%) of redness, 2 cases (10%) of catheter slip, and 1 case (5%) of catheter-related bloodstream infection at different stages. After effective treatment, catheters in 2 patients (10%, 1 case with catheter-related bloodstream infection and 1 case with catheter slip of 5 cm) were removed, and ITI treatment has since been completed. Other complications were relieved after active treatment, and the use of the catheter continued.
3.5. Expense before and after PICC placement and its impact on life
Statistical analysis of the expense related to disease treatment before and after PICC placement showed that there was no significant difference with regard to total hospitalizations times, total length of hospitalization, total inpatient and outpatient expense, and accommodation expense due to medical treatment (p > 0.05), but the total hospitalization expense after placement was significantly lower than that before placement. The transportation expense for the children and their parents was reduced to a significant degree after placement (p < 0.05), Factor consumption for the children was increased significantly after PICC placement (p < 0.05), as shown in . After catheter placement, the number of days children missed school and parents missed work were significantly reduced (p < 0.05) ().
Table 1. Hospitalization and expense of children before and after catheter placement.
Table 2. Number of days parents missed work and children missed school.
3.6. Home therapy before and after PICC placement
Only 11 children received home therapy before catheter placement, whereas 20 children received home therapy after catheter placement. The success rate of self-injection before catheter placement was 34.50% but was 100% after catheter placement. Children’s acceptance score for self-injection was 4.65 ± 3.68 points and 9.85 ± 0.36 points before and after catheter placement, respectively. The execution degree of drug administration as prescribed was 81.50% and 100% before and after catheter placement, respectively (p < 0.05). These differences were statistically significant, as shown in . Regarding the self-designed questionnaire for parents, the reported satisfaction with PICC was 100%.
Table 3. Home therapy before and after PICC placement.
4. Discussion
4.1. Reasons for choosing PICC in China
In this study, children receiving ITI were all treated with PICC, which differs from previous reports from developed countries where central venous catheters for children with hemophilia were inserted mainly through infusion ports [Citation13,Citation14]. The main reason we chose PICC is that PICC placement is relatively simple. After peripheral vein puncture is successful, PICC can be inserted by using the Seldinger technique, especially following the development of ultrasound technology which makes the placement visible, greatly improving the success rate of puncture. The application of the Seldinger technique highlights the advantages of fine-needle puncture and minimal local trauma. Second, compared with infusion ports, the expense for PICC placement is lower, averaging ¥6730.50 (range, ¥2500–20,000) because the local trauma is slight, resulting in low risk of bleeding and low dosage of coagulation factor, a very important issue for developing countries where medical expense cannot be fully reimbursed. Third, the end of the PICC is exposed, which is more convenient for maintenance and use after placement, without additional puncture and any pain in children [Citation15]. Children can be injected with coagulation factor when they are relatively quiet or even asleep, which facilitates home therapy. Moreover, it is also very easy to remove the catheter after use, with a low rate of serious complications [Citation9,Citation16]. This suggests that children with hemophilia and their families have divergent preference in the choice of central venous catheter in countries with different economic status and development levels. As medical staff, we should master the insertion, maintenance, technology, and comprehensive management ability of each type of central venous catheter so as to establish the most suitable vascular access for individual children with hemophilia.
4.2. PICC placement ensures curative effect
In this study, the success rate of ITI treatment in children with hemophilia is 75%, and the success rate of ITI in HA (75%) is comparable with that of other large studies (60–80%) [Citation17–19], and our previous study (67.9%) [Citation20]. The inhibitor disappearance time is 8.2 (range, 1.8–18.8) months in this study, which is also consistent with our previous report of 9.4 (range, 2.1–25.1) months (None of the patients with central venous catheters) [Citation20]. Thus PICC placement provides effective venous access for regular and sufficient ITI treatment, and ensuring similar ITI outcome in those who have poor vascular condition with no chance to eradicate inhibitor without PICC. The evaluation of ITI outcome influenced by PICC would be analyzed in the future with large number of patients.
In addition, in this study the frequency of bleeding events after catheter placement decreased significantly, with an average number of bleeding events in children 6 months before and within 6 months after PICC placement of 5.45 ± 6.08 times and 1.05 ± 1.66 times, respectively (p < 0.05). This is mainly attributable to the success of ITI treatment, which ensures the effectiveness of coagulation factor infusion. This is also illustrated by the significantly increased consumption of clotting factors after PICC catheterization. In addition, PICC is easy use and does not bring any additional pain to the patient, thus making it highly acceptable to children, while the execution degree of drug administration as prescribed reaches 100%, which promotes the implementation of home therapy. Catheter insertion gives full play to the advantages of home therapy, further reduces the occurrence of bleeding events, and improves the satisfaction of parents.
4.3. Controllability of PICC catheter-related complications
PICC has its advantages in clinical application, but there is still an inevitable risk of complications [Citation10]. In this study, complications such as rash under transparent film, leakage at the end of the catheter, catheter blockage, catheter slip, and bleeding and redness at the puncture point occurred during the use of PICC. Catheters in 2 children (10%) were removed because of catheter-related bloodstream infection and catheter slip. Other complications were effectively treated, and the catheter was continuously used and removed until reaching its expiration date, which indicated that the complications of PICC were controllable. This is consistent with results from another study [Citation21]. Regarding the causes of complications, a rash under transparent film may be caused by children’s allergy to transparent dressing and disinfectant or local heating due to weather changes. The rash can be relieved after intervention by changing the disinfectant and dressing, fully drying the disinfected site, or instruction to avoid going out in hot weather to reduce local sweating. Because a PICC with a trimmable end was used in this study, leakage at the end of catheter was remedied by local trimming of the leaking part, after which the catheter could still be used. Bleeding at the puncture point is mainly related to the disease itself and can easily occur when the coagulation factor is insufficient and the child takes part in more activities. Redness at the puncture point, which is an early manifestation of infection is limited to local areas and is relieved after local intensive disinfection. Catheter blockage is mainly related to the operator’s method of flushing and sealing the catheter. The catheter can still used after thrombolysis with urokinase. Given the preceding analysis, therefore, we find that although there are many complications related to PICC in this study, the incidence of serious complications is evidently lower than that in previous studies [Citation1, Citation22].
4.4. PICC placement reduces the family financial burden
In this study, 6 months after PICC placement the transportation and accommodation expenses of children and their parents for medical treatment were reduced compared with those before catheter placement and to a statistically significant degree. Although there was no statistical difference between the total hospitalization expense and the total outpatient expense before and after catheter placement, the total hospitalization expense was significantly reduced compared with that before catheter placement while the reduced amount was more than the increased outpatient expense after catheter placement. Therefore, after PICC placement, parents’ financial burden was generally reduced. This conclusion differs from those of previous studies. It has been reported that complications after central venous catheter placement may lead to increased expense [Citation23]. Globe’s [Citation24] study found that the expenses of patients with central venous catheter insertion increased by 348% compared with those without catheter insertion. Buckley’s [Citation23] study also found that the length of hospitalization and hospitalization expense were increased after central venous catheter insertion. However, the central venous catheter used in these two studies was the infusion port, whereas PICC was used in this study. The significant reduction in transportation and accommodation expenses is due to the obvious reduction of bleeding after catheter placement, making it no longer necessary to frequently commute between the hospital and residence for medical treatment upon implementation of home therapy. Although the outpatient expense is increased after catheter placement, factor consumption is the main reason for the increased outpatient expense. This is mainly due to all children being treated with ITI after catheter placement, with preventive treatment beginning only after successful ITI treatment . The increase in factor consumption is the main reason for the increased outpatient expense after catheter placement. However, coagulation factor is within the scope of medical insurance reimbursement. Although the total expense is increased, the increase in personal payment is not so obvious. Meanwhile, this increased amount is far less than the reduced expenses for hospitalization, transportation, and accommodation after catheter placement. Therefore, PICC placement can reduce the financial burden of the family while meeting their treatment needs.
5. Conclusion
In this study, PICC is inserted in children with HA and accompanying inhibitors, which ensures the smooth implementation of ITI treatment; improves the success rate of self-injection, children’s compliance with self-injection, and execution degree of drug administration as prescribed; reduces the frequency of bleeding events, number of days parents missed work and children missed school, and transportation expense for parents taking their children for medical treatment; and improves the quality of life of the children. Parents’ satisfaction with the catheter is as high as 100%. This suggests that PICC placement is feasible and effective for Chinese children with HA scheduled for treatment with ITI and can be further popularized and utilized.
Author contributions
Chunli Wang, Yaguang Ding, Yingzi Zhen, Jie Cui prepared and carried out the PICC placement and maintenance during the study. Chunli Wang, Guoqing Liu, Runhui Wu and Zekun Li have been involved in drafting the manuscript or revising it critically for important intellectual content. Guoqing Liu, Wanru Yao, Ai Di, Ping Feng, Kun Huang Responsible for patient management during hospitalization. Chunli Wang and Guoqing Liu performed the statistical analysis. Runhui Wu is participated in design of the study. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Research data are not shared for this study.
Additional information
Funding
References
- Bedoya MA, Raffini L, Durand R, et al. Implantable venous access devices in children with severe hemophilia: a tertiary pediatric institutional experience. Pediatr Radiol. 2020;50(8):1148–1155.
- Liesner RJ, Khair K, Hann IM. The impact of prophylactic treatment on children with severe haemophilia. Br J Haematol. 1996;92:973–978.
- Soucie JM, Evatt B, Jackson D. Occurrence of hemophilia in the United States. Am J Hematol. 1998;59:288–294.
- Escuriola C, Kreuz W. The immune tolerance induction(ITI) dose debate: does the international ITI study provide a clearer picture? Haemophilia. 2013;19(1):12–17.
- Marijke van den Berg H, et al. Different impact of factor VIII products on inhibitor development. Thromb J. 2016;14(1):31–31.
- Baojun S, Shiwei Y, Pingchong L, et al. Clinical study on factor VIII inhibitor in children with hemophilia A. Chinese J Hematol. 2020;41(2):138–142.
- Thrombosis and hemostasis group of hematology branch of Chinese medical association, Chinese hemophilia cooperation group. Chinese guideline to hemophilia treatment (2020 edition). Chinese J Hematol. 2020;41(4):265–271.
- Rocino A, Franchini M, Coppola A. Treatment and prevention of bleeds in haemophilia patients with inhibitors to factor VIII/IX. J Clin Med. 2017;6:46–52.
- Kulkarni R, Presley RJ, Lusher JM, et al. Complications of haemophilia in babies (first two years of life): a report from the centers for disease control and prevention universal data collection system. Haemophilia. 2016;23:207–214.
- Ewenstein BM, Valentino LA, Journeycake JM, et al. Consensus recommendations for use of central venous access devices in haemophilia. Haemophilia. 2004;10:629–648.
- Veps LK, Lassila R, Arola M, et al. Complications associated with central venous access device in children with haemophilia: a nationwide multicentre study in Finland. Haemophilia Official J World Feder Hemophilia. 2015;21(6):747–753.
- Langley AR, Stain AM, Chan A, et al. Experience with central venous access devices (CVADs) in the Canadian hemophilia primary prophylaxis study (CHPS). Haemophilia. 2015;21(4):469–476.
- Adriana F, Kim N, Kay D, et al. Central venous access device insertion and perioperative management of patients with severe haemophilia A: a local experience. Blood Coagul Fibrinol. 2016;27:156–159.
- Hedi B, Maud M, Alexandre T, et al. Outpatient central venous access device insertion in very young children with severe haemophilia. Blood Coagulation and Fibrinolysis. 2020;31:490–499.
- Santagostino E, Mancuso ME. Venous access in haemophilic children: choice and management. Haemophilia. 2010;16(1):20–24.
- Titapiwatanakun R, Moir C, Pruthi RK, et al. Central venous access devices for paediatric patients with haemophilia: a singleinstitution experience. Haemophilia. 2009;15:168–174.
- Nogami K, Taki M, Matsusuhita T, et al. The Japanese immune tolerance induction(J-ITI) study in haemophilia patients with inhibitor: outcomes and successful predictors of ITI treatment. Haemophilia. 2018;24:328–337.
- Lenk H, Group ITTS. The German registry of immune tolerance treatment in hemophilia–1999 update. Haematologica. 2000;85(Suppl. 10):45–47.
- Dimichele D. Inhibitors: resolving diagnostic and therapeutic dilemmas. Haemophilia. 2002;8(3):280–287.
- Zekun L, Zhenping C, Guoqing L, et al. Low-dose immune tolerance induction alone or with immunosuppressants according to prognostic risk factors in Chinese children with hemophilia A inhibitors. Res Pract Thromb Haemost. 2021;5:125–162.
- Delarbre B, Dabadie A, Stremler N, et al. Introduction of the of a pediatric PICC line in a French university hospital: review of the first 91 procedures. Diagn Interv Imaging. 2014;95:277–281.
- Khair K, Ranta S, Thomas A, et al. The impact of clinical practice on the outcome of central venous access devices in children with haemophilia. Haemophilia. 2017;23:e276–e281.
- Buckley B, Dreyfus J, Prasad M, et al. Burden of illness and costs among paediatric haemophilia patients with and without central venous access devices treated in US hospitals. Haemophilia Official J World Feder Hemophilia. 2018;24(3):e93–e102.
- Globe DR, Curtis RG, Koerper MA, et al. Utilization of care in haemophilia: a resource- based method for cost analysis from the Haemophilia Utilization Group Study (HUGS). Haemophilia. 2004;10(Suppl. 1):63–70.