ABSTRACT
Background
The introduction of low titer group O whole blood (LTOWB) that contains potentially ABO-incompatible plasma and the increasing use of group A plasma, due to shortages of AB plasma, in trauma patients whose ABO group is unknown could put the recipients of incompatible plasma at risk of increased morbidity and mortality. This study evaluated civilian trauma patient outcomes following receipt of incompatible plasma.
Methods
One trauma center’s patient contributions to three multicenter studies of different trauma resuscitation strategies was analyzed; these patients were separated into two groups based on receipt of only compatible plasma versus receipt of any quantity of incompatible plasma. Multivariate analysis was performed to determine if receipt of incompatible plasma was associated with 24-hour or 30-day mortality.
Results
There were 347 patients eligible for this secondary analysis with 167 recipients of only compatible plasma and 180 recipients of incompatible plasma. The two groups were well matched demographically and on both prehospital and hospital arrival vital signs. The median (IQR) volume of incompatible plasma received by these patients was 684 ml (342, 1229). There was not a significant difference between the groups in 24-hour and 30-day mortality, nor in in-hospital or intensive care unit lengths of stay. In the Cox proportional-hazards regression model for both 24-hour and 30-day survival, receipt of incompatible plasma was not independently predictive of either mortality endpoint.
Conclusion
Receipt of incompatible plasma was not independently associated with increased mortality in trauma patients. Prospective studies are needed to confirm these findings.
Introduction
The practices of providing blood products for the resuscitation of civilian patients at risk of hemorrhagic shock, especially trauma patients, has evolved over time due in part to the re-introduction of blood products such as low titer group O whole blood (LTOWB) and the use of group A plasma during the initial resuscitation due to shortages of universally compatible group AB plasma [Citation1,Citation2]. For the former, the plasma will be incompatible with recipients who are not group O, hence whole blood units from donors with low titers of anti-A and -B are selected to reduce the risk of hemolysis [Citation3,Citation4]. For the latter, group A plasma will be compatible with recipients who are groups A and O, which constitute the majority of recipients in all ethnic groups [Citation5,Citation6], and the use of plasma from donors with low titers of anti-B reduces the risk of hemolysis for the B and AB recipients. Other sources of potentially incompatible plasma that could be transfused during a balanced ratio resuscitation strategy include the plasma in platelet units, cryoprecipitate units, and even in the red blood cell (RBC) units themselves. Thus, when considering the potential harm from transfusing incompatible plasma, all sources of plasma should be included.
Two studies have investigated the outcomes of trauma patients who received incompatible plasma from any source during their resuscitation [Citation7,Citation8]. These studies did not find increased mortality amongst the recipients of incompatible plasma compared to those who received only compatible plasma. However, in only one of those studies was plasma from all possible sources included [Citation8]; in the other study, only incompatible plasma from group A plasma units was considered [Citation7]. Thus, the current study is a secondary analysis of the patient contributions from one trauma center to two multicenter cluster randomized trials and one multicenter observational cohort study of different trauma resuscitation strategies and included all sources of plasma to investigate the safety of using incompatible plasma in trauma resuscitation.
Methods
One trauma center contributed data to two different cluster randomized clinical trials that investigated the effect of supplementing the standard of care for injured civilian patients at risk for hemorrhagic shock brought to hospital by helicopter with plasma (PreHospital Air Medical Plasma Trial, PAMPer) [Citation9] and with LTOWB (Pragmatic Prehospital Type O Whole blood Early Resuscitation, PPOWER) [Citation10], and to one prospective, multicenter, observational cohort study (Shock Whole blood and Assessment of Traumatic brain injury, SWAT) [Citation11]. Following ethics board approval, the individual patient clinical information that was supplied to these three trials from this one trauma center was abstracted from the trials’ databases. To be included in this secondary analysis, the patients had to have had an ABO group performed by this center’s transfusion service during their index trauma admission, and they had to have received at least one blood product in their first two calendar days after admission (see below) including prehospital transfusions. Patients were excluded from this secondary analysis if they did not have an ABO group performed during their trauma admission, and/or if they did not receive a transfusion within the first two calendar days of admission including prehospital transfusions. Furthermore, patients also had to have been fully enrolled in one of the three aforementioned trials to be included in this secondary analysis.
For each eligible patient, their ABO group and the quantity and ABO group of all blood products that were transfused on the patients’ first two calendar days after admission were obtained from the center’s transfusion service. The transfusion service relies on the receipt of tags that are attached to the blood products that are kept in remote refrigerators in the emergency room and intensive care units to keep track of when patients receive emergency uncrossmatched transfusions from these remote locations. Although the time of transfusion is recorded by the clinicians on the tags attached to the units stored in remote refrigerators, the delivery of these tags to the blood bank can occur hours after the blood product has been transfused. This delay in returning the tags can be problematic if the patient is admitted and transfused close to midnight because the tags might not be returned until sometime during the next day at which time the transfusion service would become aware that the patient had received those units on the previous day. Furthermore, the transfusion service records the time that uncrossmatched units are issued from the blood bank as the time of transfusion, even if the units are not transfused instantaneously. Thus, the delayed returning of the tags from units taken from remote refrigerators and the assumption that uncrossmatched units issued from the blood bank are transfused at the time of issue can make accurately determining the number of blood products that a patient had received by a specific time of the day impossible. Thus, all transfusions that were administered over the first two calendar days following admission were recorded for this study. In addition, it has been demonstrated that the majority of hemorrhagic deaths in trauma occur well within the first 24 h after admission [Citation12–14]. Thus, the patient’s main exposure to blood products likely also occurs within this time frame, which would span a maximum of two calendar days.
The eligible patients were stratified into two groups: those who received only compatible (although not necessarily identical) plasma and those who received any quantity of incompatible plasma. Based on previously published values [Citation15], the volume of plasma in each blood product was considered to be as follows: RBC 40 ml, platelets 68 ml per whole blood platelet unit (multiplied by the number of units in the pool; an apheresis platelet was considered to contain 340 ml of plasma), cryoprecipitate 15 ml (multiplied by the number of units in the pool), LTOWB 342 ml, plasma 234 ml (apheresis plasma unit were considered to contain 468 ml of plasma). For the analysis of the total volume of blood products transfused, LTOWB was considered to have a total volume of 500 ml and an RBC unit had a total volume of 377 ml, while for the other blood products the total volume was equal to the plasma volume as they lack an RBC component.
In addition to the transfusion data, the demographics, prehospital and admission vital signs, hospital and intensive care unit (ICU) lengths of stay, and 24-hour and 30-day mortality outcomes were abstracted for each eligible patient from the information that had been collected for their initial study participation.
Descriptive statistics characterized the demographics, injuries of the patients, and outcomes of interest. Categorical variables were presented as frequencies and percentages and tested using the Chi-squared test. Continuous variables were expressed as medians with interquartile ranges (IQR) and were tested using Wilcoxon rank-sum. Twenty four-hour and 30-day mortality between compatible plasma and incompatible plasma recipients via log-rank test of Kaplan-Meier analysis were evaluated.
To further investigate these unadjusted findings, a multivariate analysis of survival was performed using a Cox proportional-hazards regression model. Two separate models were generated through backward-selection removing covariates with p ≥ 0.2 for 24-hour and 30-day mortality. The total number of blood product units transfused, nature of the plasma received (compatible vs. incompatible), sex, age, injury type (blunt vs. penetrating), traumatic brain injury, injury severity score, prehospital heart rate, prehospital respiratory rate, prehospital systolic blood pressure, prehospital Glasgow Coma Score, and prehospital intubation were initially included. The presence of traumatic brain injury (TBI) was determined by positive computerized tomography (CT) scan brain imaging. Sex was excluded through backward-selection and manually added to each of the final models. All regression models passed the proportional-hazards assumption on the basis of Schoenfeld residuals.
To assess the association between receipt of incompatible blood plasma and the total number of blood product units transfused, the total volume of all blood products transfused, and the volume of plasma transfused between the incompatible and compatible plasma recipients, a generalized linear model (GLM) was built using the same method of backward-selection. Sex and age were both excluded through backward-selection and manually added to each of the final models. Variance inflation factors were evaluated to ensure that the variance of our regression coefficients was not due to multicollinearity. Statistical significance was determined at p < 0.05 level (2-sided). All data was analyzed using STATA version 17.0 (StataCorp, Texas).
Results
After meeting eligibility criteria for this study, there were 347 patients included in this secondary analysis (). Demographic and vital sign data were available for 115 of the 125 excluded patients and this cohort differed from the 347 included patients by having a significantly lower median injury severity score (ISS) and a lower proportion of patients with ISS ≥ 16 compared to the analyzed cohort, as well as a slightly but statistically significantly higher prehospital systolic blood pressure, which was no longer significantly higher upon arrival at the hospital (Supplementary Table 1).
Figure 1. Number of patients excluded from the analysis. There were 10 patients who were originally considered to be eligible for the PAMPer study and assigned a study number but who were ultimately found not to have satisfied the study’s inclusion criteria, therefore they were not included in PAMPer and were not available for this analysis.
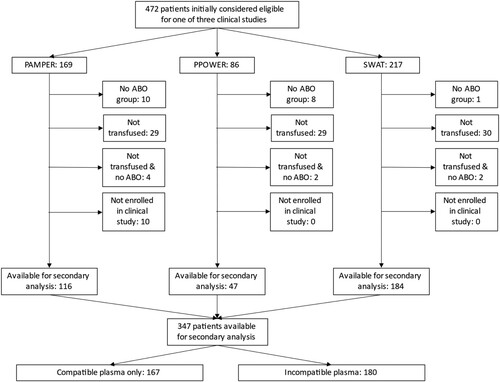
For the 347 patients included in this analysis, demonstrates the demographics of the 167 patients who received only compatible plasma and the 180 patients who received some incompatible plasma as well as their initial prehospital vital signs documented by the emergency medical services and the emergency department arrival vital signs. There were not any statistically significant differences in these parameters between the two groups of patients. demonstrates the ABO blood groups of the patients included in the analysis as well as the median number of blood products transfused. Because group O patients are the universal recipients of plasma, i.e. plasma from any ABO group will be compatible with group O recipients, it is not surprising that the majority (85.6%) of the patients in the compatible group were group O. Similarly, by design, the patients in the incompatible group received significantly more incompatible plasma than those in the compatible group. In this univariate analysis, the patients who received some incompatible plasma were also transfused with significantly more blood product units and a greater volume (in ml) of both total blood products and total plasma compared to the recipients of only compatible plasma. These relationships were also present in the GLM analysis except that the receipt of incompatible plasma was not associated with an increased number of blood product units transfused ().
Table 1. Patient demographics, initial prehospital vital signs, and emergency department arrival admission vital signs stratified by group. *Does not include patients from the SWAT study due to differences in the data collected between it and the other two studies.
Table 2. Quantity of blood products and volume of plasma transfused stratified by group in first 2 days after enrollment. A dose is prepared by pooling individual whole blood derived platelets or cryoprecipitate units together; typically 4–5 individual units are pooled to create a dose for an adult recipient.
Table 3. Generalized linear model for the total number of blood product units and the total volume of blood products transfused.
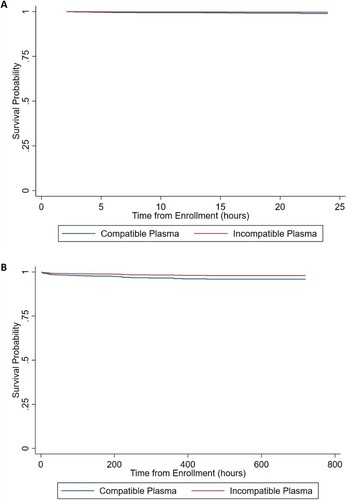
demonstrates the univariate analysis of 24 h and 30 d mortality as well as hospital and ICU lengths of stay. There was not a significant difference in these parameters between the two groups. Log-rank test via Kaplan-Meier analysis did not demonstrate significant differences between the two groups for 24-hour and 30-day survival (p = 0.672 and p = 0.808, respectively; data not shown). The Cox adjusted survival curves for 24-hour and 30-day survival are shown in (A) and (B), respectively; upon controlling for confounders, there was not a difference in survival between the two groups of patients. demonstrates the multivariate Cox proportional-hazards regression model for both 24-hour and 30-day survival; receipt of incompatible plasma was not significantly predictive of either mortality endpoint and in fact trended towards being protective from these endpoints.
Table 4. Univariate analysis of mortality and lengths of stay analyses stratified by group.
Table 5. Multivariate Cox proportional-hazards regression model for 24-hour and 30-day mortality. ISS = injury severity score, GCS = Glasgow coma scale.
Discussion
Consistent with other reports, this secondary analysis of one center’s patient contributions to three multicenter studies did not find an increase in mortality or morbidity following the transfusion of ABO-incompatible plasma products to injured civilian patients despite receipt of a greater number and volume of blood products and more plasma compared to recipients of only compatible plasma.
In one of the previous reports, only the plasma from group A plasma units that were transfused to groups A, B, and AB recipients was considered in the analysis. Group O patients were excluded from the study [Citation7]. Nevertheless, the mean quantity of group A plasma transfused to the groups B and AB patients was 4 units, which is approximately one liter of incompatible plasma. The current study did not exclude group O recipients and as such is more representative of the trauma population as a whole. The second previous study included trauma patients from all ABO groups, but the median (5th–95th percentile) volume of incompatible plasma transfused was 342 ml (40-2003) [Citation8]. The median volume of incompatible plasma transfused in this study was actually double that at 684 ml, or approximately 3 units of plasma.
Recently, a third study that also addressed this question of mortality following incompatible plasma transfusion was published [Citation16]. In that study, the survival of non-group O trauma patients who received ABO-specific RBCs following uncrossmatched group O RBC/LTOWB transfusion was compared to group O trauma patients who also received group O RBC/LTOWB during their resuscitation. The patients in the former group would have been at risk of experiencing hemolysis-related adverse events from the destruction of both their autologous RBCs and the ABO group-specific RBCs that were transfused following their initial resuscitation with a median (5th–95th percentile) of 4 (1–18) units of LTOWB, which included a volume of incompatible plasma similar to the median of 684 ml of incompatible plasma transfused in the current study. In the fixed marginal effects plot of 6-, 24-hour mortality as well as 30-day mortality, the rate of death amongst the non-group O recipients was not significantly different from group O patients despite receiving a significant amount of incompatible plasma. Several other studies have also compared the clinical and laboratory safety of transfusing LTOWB to both group O and non-group O recipients and none have shown an increased incidence of adverse events amongst the non-group O recipients who could be susceptible to hemolysis compared to the group O patients [Citation17–19]. Similarly, a recent analysis of the Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) database revealed that transfusion of single or multiple doses of ABO non-identical platelets to trauma patients did not result in increased mortality compared to recipients of ABO-identical platelets [Citation20]. Thus, the concept that the transfusion of incompatible plasma leads to increased mortality in trauma patients has not been proven.
It was interesting that receipt of incompatible plasma was associated with receipt of higher volumes of all blood products transfused and also the total volume of plasma transfused. Perhaps this was due in part to the transfusion of significantly more LTOWB units to the patients who received incompatible plasma compared to those who only received compatible plasma (2 vs. 0 units, respectively, p < 0.001) because a LTOWB unit had the largest volume of any of the blood products transfused. Nevertheless, despite receiving more blood product units and a greater volume of blood products and plasma compared to the only compatible plasma group (), receipt of incompatible plasma was not an independent predictor of 24-hour or 30-day mortality (). In fact, despite being associated with higher transfusion volumes, the receipt of incompatible plasma-containing products appeared to be nearly protective from these mortality time points because the Cox-hazard ratios were <1.0 with p-values close to significance (). While it is unlikely that incompatible plasma is actually protective in trauma, these results suggest that if compatible plasma-containing products are not available, potentially incompatible plasma-containing products, including LTOWB, should be used during trauma resuscitation.
The main limitation of the current study is its relatively small sample size, which might have been too small to detect adverse events from hemolysis. Based on the data from the previous studies, hemolysis is expected to be an uncommon event. In fact, almost two-thirds of the cases of hemolysis following the transfusion of ABO incompatible plasma-containing products reported in a recent scoping review occurred following the transfusion of a product that had an incompatible antibody titer >256 [Citation21]; since all of the LTOWB plasma transfused in the US is likely to have a titer <256 [Citation22–24], and some of the group A plasma that is transfused to recipients of unknown ABO group is also tittered [Citation1], hemolysis, and its downstream adverse events, is unlikely to occur following the transfusion of these products. The lack of a signal of harm from the transfusion of incompatible plasma to trauma patients in this and the previous studies suggests that this practice is indeed safe in this population. Another limitation of this study is that, as described above, it is possible that not all of the patient’s transfusions in their first 24-hours were recorded if the tags from uncrossmatched units taken from remote refrigerators were returned to the blood bank in a delayed manner, or that transfusions administered after 24-hours might have been documented as being administered within the first 24-hours after admission. However, as the majority of deaths from hemorrhage occur within the first 6-hours from admission [Citation14], it is likely that the actual window in which the patients received the majority of their blood products was much shorter than 24 h and probably occurred closer to the time of admission, if not before admission, than to the end of 24 h. Furthermore, this study did not specifically evaluate biochemical or clinical markers of hemolysis amongst the recipients of incompatible plasma. Therefore, these data cannot be used to indicate that hemolysis did not occur amongst these patients, but only to indicate that if it was occurring, increased morbidity and mortality did not ensue. The patient demographics, analytes, and outcomes presented in this study were limited by the data collection in the primary study in which the patient was enrolled; the intention of this secondary analysis was to investigate the effect of incompatible plasma on mortality, which was an endpoint captured in each of the three primary studies. Lastly, the potential for immunomodulatory effects following the exposure to incompatible plasma, even at a low titer, were not investigated in this study; this cohort of patients who were exposed to incompatible plasma might be an ideal cohort in which to investigate the possible effects of immunomodulation from receipt of incompatible plasma.
The results of this study support the previous findings that the administration of incompatible plasma does not lead to increased morbidity and mortality compared to the administration of compatible plasma in trauma patients even though incompatible plasma transfusion was associated with greater quantities of blood products transfused. If compatible products are available, they should be the first choice of products selected because they do not pose a risk of hemolysis. However, given the trends towards the increasing use of LTOWB early in the resuscitation and the use of group A plasma due to group AB plasma shortages in the US, a life-saving ABO-incompatible plasma transfusion should not be denied to a massively bleeding patient for fear of increasing mortality.
Supplemental Material
Download MS Word (18.1 KB)Disclosure statement
The authors have no conflicts to declare except for PCS who consults for Hemanext, Cerus, CSL Behring, and is on the scientific advisory board for Haima and is a co-founder and chief medical officer for Kalocyte.
References
- Yazer MH, Spinella PC, Anto V, et al. Survey of group A plasma and low-titer group O whole blood use in trauma resuscitation at adult civilian level 1 trauma centers in the US. Transfusion. 2021;61:1757–1763. doi: 10.1111/trf.16394
- Yazer MH, Diaz-Valdes JR, Triulzi DJ, et al. Wider perspectives: it’s a changing world-the use of ABO-incompatible plasma for resuscitating massively bleeding patients. Br J Haematol. 2023;201(6):1245–1247.
- Yazer MH, Cap AP, Spinella PC. Raising the standards on whole blood. J Trauma Acute Care Surg. 2018;84:S14–S17. doi: 10.1097/TA.0000000000001778
- AABB Press. AABB technical manual. Baltimore (MD): AABB Press; 2020.
- Haspel RL, Bakhtary S, Miller Y, et al. Considering equity in transfusion medicine practice. Br J Haematol. in press.
- Yazer MH, Diaz-Valdes JR, Triulzi DJ, et al. Considering equality in transfusion medicine practice. Br J Haematol. 2023;201:1245–1247. doi: 10.1111/bjh.18830
- Dunbar NM, Yazer MH, Biomedical Excellence for Safer Transfusion (BEST) Collaborative and the STAT Study Investigators. Safety of the use of group A plasma in trauma: The STAT study. Transfusion. 2017;57:1879–1884.
- Seheult JN, Dunbar NM, Hess JR, et al. Transfusion of blood components containing ABO-incompatible plasma does not lead to higher mortality in civilian trauma patients. Transfusion. 2020;60:2517–2528. doi: 10.1111/trf.15710
- Sperry JL, Guyette FX, Brown JB, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379:315–326. doi: 10.1056/NEJMoa1802345
- Guyette FX, Sperry JL. Prehosptial low titer group O whole blood is feasible and safe: results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2022;93:e175–e176.
- Sperry JL, Cotton BA, Luther JF, et al. Whole blood resuscitation and association with survival in injured patients with an elevated probability of mortality. J Am Coll Surg. 2023;237(2):206–219.
- Oyeniyi BT, Fox EE, Scerbo M, et al. Trends in 1029 trauma deaths at a level 1 trauma center: impact of a bleeding control bundle of care. Injury. 2017;48:5–12. doi: 10.1016/j.injury.2016.10.037
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11.
- Holcomb JB, Moore EE, Sperry JL, et al. Evidence-based and clinically relevant outcomes for hemorrhage control trauma trials. Ann Surg. 2021;273:395–401. doi: 10.1097/SLA.0000000000004563
- Seheult JN, Anto V, Alarcon LH, et al. Clinical outcomes among low-titer group O whole blood recipients compared to recipients of conventional components in civilian trauma resuscitation. Transfusion. 2018;58:1838–1845. doi: 10.1111/trf.14779
- Yazer MH, Dunbar NM, Hess JR, et al. Transfusion of ABO-group identical red blood cells following uncrossmatched transfusion does not lead to higher mortality in civilian trauma patients. Transfusion. 2023;63(Suppl. 3):S46–S53.
- Brill JB, Mueck KM, Tang B, et al. Is low-titer group O whole blood truly a universal blood product? J Am Coll Surg. 2023;236:506–513. doi: 10.1097/XCS.0000000000000489
- Yazer MH, Corcos A, LS J, et al. Receipt of at least 4 units of low titer group O whole blood with titer<100 does not lead to hemolysis in adult trauma patients. Transfusion. 2022;62(Suppl. 1):S72–S79. doi: 10.1111/trf.16724
- Ruby KN, Dzik WH, Collins JJ, et al. Emergency transfusion with whole blood versus packed red blood cells: a study of 1400 patients. Transfusion. 2023;63(4):745–754.
- Bougie DW, Reese SE, Birch RJ, et al. Associations between ABO non-identical platelet transfusions and patient outcomes—a multicenter retrospective analysis. Transfusion. 2023;63:960–972. doi: 10.1111/trf.17319
- McCullagh J, Cardigan R, Brunskill SJ, et al. Assessing the risks of haemolysis as an adverse reaction following the transfusion of ABO incompatible plasma-containing components - a scoping review. Blood Rev. 2022;56:100989. doi: 10.1016/j.blre.2022.100989
- Yazer MH, Spinella PC. An international survey on the use of low titer group O whole blood for the resuscitation of civilian trauma patients in 2020. Transfusion. 2020;60(Suppl. 3):S176–S179.
- Yazer MH, Spinella PC. Review of low titre group O whole blood use for massively bleeding patients around the world in 2019. ISBT Sci Ser. 2019;14:276–281. doi: 10.1111/voxs.12495
- Yazer MH, Spinella PC. The use of low-titer group O whole blood for the resuscitation of civilian trauma patients in 2018. Transfusion. 2018;58:2744–2746. doi: 10.1111/trf.14869