ABSTRACT
Background:
Cold agglutinin disease (CAD) is immune-mediated hemolytic anemia. The disease is caused by cold reactive autoantibodies that induce hemolysis through the activation of the complement pathway. Most patients with CAD are elderly, and half may have refractory CAD that may not respond to the first-line treatment option (i.e. rituximab). Some cases are refractory to multiple lines of therapy, including chemotherapeutic agents, which might be toxic, especially for elderly patients. Daratumumab is a human monoclonal antibody targeting CD 38 glycoprotein, a transmembrane protein highly expressed in lymphoid and plasma cells. Daratumumab is currently approved for treating multiple myeloma and is used mainly as a combination therapy with other agents.
Case presentation:
Our patient is a 69-year-old female diagnosed with CAD after presenting with severe anemia and significant circulatory symptoms. Rituximab was not effective in controlling her disease, and she refused other available chemotherapeutic agents due to their side effects profile. We used daratumumab combined with erythropoietin, which led to a dramatic response measured by stabilizing her hemoglobin levels and transfusion independence.
Conclusion:
Our case is the third reported case of refractory CAD successfully treated with daratumumab, which suggests that daratumumab might be a potential agent for the treatment and control of refractory Cold Agglutinin Disease.
Introduction
Cold agglutinin disease (CAD) is a rare cause of autoimmune hemolytic anemia characterized by the presence of cold antibodies that causes the red blood cells to agglutinate when the body is exposed to cold temperatures. This process can result in clinical symptoms of anemia, venous thromboembolism, and cold-induced symptoms such as acrocyanosis, livedo reticularis, and Raynaud phenomenon [Citation1]. The incidence of primary CAD is around 16 cases per million people per year, more in women, with a peak incidence in the sixth and seventh decades of life [Citation2].
The treatment modality depends on various factors, such as the patient’s age and overall condition, disease severity, and the presence of any underlying conditions. Avoiding cold environment is used to decrease hemolysis and cold-induced symptoms. Despite the absence of randomized trials and formal approval for any chemoimmunotherapy in CAD, various systematic studies have confirmed remission following B cell-targeted treatment. The use of an anti-CD20 antibody as a monotherapy is generally well-tolerated, and it has emerged as the most frequently reported treatment for CAD, especially in old and frail patients [Citation3,Citation4].
Other treatment options may include ibrutinib and bortezomib if rituximab-based treatment is ineffective or not recommended. Moreover, in recent years, daratumumab has rarely been reported as a potential treatment for refractory CAD [Citation5,Citation6]. Herein, we report a 69-year-old lady with primary cold agglutinin disease, refractory to rituximab and successfully managed with daratumumab.
Case presentation
A 69-year-old female who was previously healthy presented in May 2022 to our center for evaluation of severe anemia. She mentioned exertional shortness of breath, persistent dizziness, and fatigability over the last two months. Physical examination revealed pallor and Jaundice. No acrocyanosis, livedo reticularis, or features of Raynaud phenomenon, and no organomegaly or lymphadenopathy. The initial workup showed a hemoglobin (HB) level of 6.2 g/dL. Further lab works showed a high mean corpuscular volume (MCV) of 104, reticulocyte count of 11.5%, Indirect hyperbilirubinemia with a total bilirubin of 50.6 umol/L, indirect bilirubin of 38.5 mmol, lactate dehydrogenase (LDH) of 283 U/L and low haptoglobin (<10). Levels of iron profile, vitamin B12, and folate were normal. Peripheral smear revealed marked macrocytic anemia with polychromasia, rouleaux formation, agglutination, some spherocytes and ovalocytes, and few nucleated red blood cells (RBCs). Leukocyte smear reported mild lymphocytosis, a fair number of reactive lymphocytes, and some hypersegmented neutrophils with mild shift to the left and mild toxic features, and platelets smear showed mild thrombocytosis. Hemolytic anemia was suspected, and hemolysis markers were sent. HB electrophoresis and Glucose-6-phosphate dehydrogenase (G6PD) level were normal, and blood flow cytometry showed no features of paroxysmal nocturnal hemoglobinuria (PNH). Other investigations revealed low serum levels of compliments C3 and C4, high serum immunoglobulin M(IgM) level of 4.74 gm/L(reference range 0.4–2.3 gm/L) with normal Immunoglobulin G(IgG) and Immunoglobulin A(IgA) levels, and monoclonal IgM kappa of 3 g/L in serum protein electrophoresis. Abdominal ultrasonography showed no splenomegaly or hepatomegaly.
Direct antiglobulin test (DAT) reported strongly positive polyspecific antibodies (3+) and anti-complement C3D antibodies (2+), IgG negative, and eluate negative, suggesting cold agglutinin disease (CAD). Bone marrow aspiration showed hypercellularity, erythroid hyperplasia, and no plasma or blast cells were seen. Few scattered plasma cells with tiny clusters and a mixture of kappa and lambda light chain positive cells were noted in bone marrow biopsy. Chromosome analysis and karyotype of the bone marrow sample were normal. As the patient had a family history of pancreatic cancer in her mother and gastric cancer in her sister, we had to exclude other solid tumors as a possible secondary cause of CAD. A computerized tomography (CT) scan of the chest, abdomen, and pelvis and a whole-body positron emission tomography (PET) scan showed no evidence of malignancy. Breast mammography and ultrasonography were unremarkable. Workup for autoimmune diseases and viral screening for EBV, CMV, and hepatitis B and C were all negative, which led to a likely diagnosis of primary CAD.
In June 2022, the patient received two units of blood transfusion as she had severe circulatory symptoms of anemia and was advised to avoid cold exposure. A few weeks later, she had persistent dizziness and fatigue with a drop in HB to 6.8 g/dL requiring another blood transfusion. In July, she was started on a weekly dose of rituximab as a first line of treatment. Bendamustine was discussed with the patient, but she preferred not to receive a chemotherapeutic agent. Despite receiving six cycles of rituximab, she continued to be severely anemic during this period and continued to be transfusion dependent (Shown in ).
Figure 1. Hemoglobin values are shown as a gray line. The small red Icons represent the number of blood transfusion units given during each treatment period. Six units of blood were transfused before starting the treatment. A total of eight units were given during the six weeks treatment period with Rituximab. One unit was given during the six months treatment period with Daratumumab + EPO.
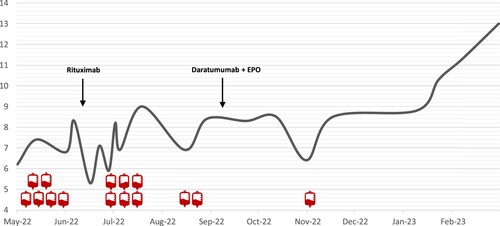
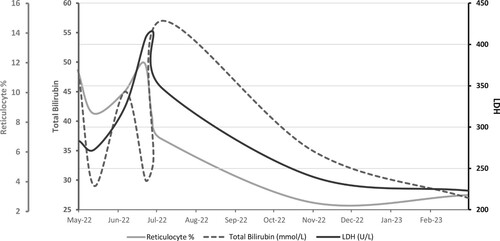
In September 2022, she was started on daratumumab as a second-line therapy. The dosing we used was 16 mg/kg weekly for eight weeks, then every two weeks for eight weeks, then monthly after that, which is the same dosing protocol approved for multiple myeloma. She also received a weekly dose of recombinant human erythropoietin (rhEPO) at 40,000 U/week. Over the next six months, the patient received 18 cycles of daratumumab, after which her HB levels stabilized, and she became asymptomatic. She was transfused once during the first two months of treatment with daratumumab, and she did not require any blood transfusion over the next four months (shown in ). Follow-up of hemolysis markers showed a marked reduction in LDH, total bilirubin, and reticulocyte counts after treatment with daratumumab (Shown in ). Repeated serum protein electrophoresis three months after treatment showed faint IgM kappa band seen on Immunofixation. The last HB reading in April 2023 was 13 g/dL, after which we stopped rhEPO. The patient is currently receiving monthly daratumumab therapy, and she is planned for 12–18 months of maintenance treatment.
Discussion
Cold agglutinin disease (CAD) is a form of autoimmune hemolytic anemia that is caused by cold agglutinins. The underlying cause of CAD is not well understood, but it is thought to be related to an overactive immune system. This condition could be primary without apparent underlying condition or secondary to other autoimmune disorders such as lupus, rheumatoid arthritis, or aggressive lymphoma. Cold agglutinin disease is more common in people over 50 and affects men and women equally [Citation7]. Treatment for CAD may involve nonpharmacologic management, for example, avoiding exposure to cold temperatures and using warm clothing, particularly during cold weather. Medications such as corticosteroids, rituximab, and immunosuppressants may also be used to suppress the immune system and reduce the production of cold agglutinins. In refractory and severe cases of CAD, which may be life-threatening, the treatment options are usually limited, and the introduction of daratumumab may be necessary and lifesaving [Citation1,Citation7].
The dramatic effect of daratumumab, which was observed by increasing the hemoglobin by about six g/dl, allowing transfusion independence over six months, and alleviating anemia symptoms, can be explained by its mechanism of action. The CD38 protein found on the surface of plasma cells is also expressed on the surface of cold agglutinin-producing cells, which are responsible for the autoimmune destruction of RBCs in CAD, which suggest that daratumumab may have an immunomodulatory effect [Citation6,Citation8,Citation9]. Daratumumab has been found to reduce the levels of certain cytokines, especially interleukin (IL)-6, which is the primary reason why the drug is effective for patients with multiple myeloma. Furthermore, it has a direct effect on other different cytokines IL-10, IL-17, interferon (IFN)-γ, tumor necrosis factor (TNF)-α that plays a key role in the pathogenesis of these autoimmune disorders [Citation6,Citation7,Citation10]. Several case series and case reports showed the successful use of daratumumab for conditions other than multiple myeloma and CAD. Pereda et al. [Citation11] reported three cases in which daratumumab resulted in the resolution of RBCs alloimmunization and donor-specific anti-HLA antibodies, which allowed for further curative treatment with hematopoietic stem cell transplantation [Citation11]. Additionally, two cases of refractory Immune thrombocytopenic purpura(ITP) with multiple relapses have also been treated successfully with daratumumab [Citation12].
Nooka et al. [Citation13] mentioned that Infusion-related reactions can occur in almost half of the patients receiving daratumumab. According to the authors, the most frequent reactions included fatigue, back pain, nausea, cough, upper respiratory tract infections, anemia, thrombocytopenia, and neutropenia. Pre- and post-infusion medications with antihistamines, corticosteroids, and acetaminophen can be used for the management of daratumumab infusion-related reactions [Citation13]. Our patient experienced grade 1 fatigue in three cycles of daratumumab with no other reported adverse effects. Daratumumab infusion can also interfere with pretransfusion testing and may result in a positive antibody screen in indirect antiglobulin tests [Citation14]. This phenomenon is explained by the presence of low levels of CD38 in the RBCs. Multiple strategies have been described by Werle et al. [Citation15] to overcome the interference of daratumumab in alloantibody screening. A common and widely used strategy is by treating the test cells with dithiothreitol (DTT). The authors have also introduced a novel method that involves the use of daratumumab Fab fragments to block CD38 epitopes on RBCs [Citation15].
A literature review of previous cases of primary CAD treated with daratumumab is summarized below. (Shown in ). The two patients responded well to treatment with daratumumab, evidenced by disease remission and transfusion independence. The dosing used in both cases was 16 mg/kg of daratumumab given weekly for eight weeks, then every two weeks for weeks 9–24, then a monthly dose. Although daratumumab showed promising efficacy in CAD patients, the initial response might take more than ten weeks. Our patient had a drop in HB after eight weeks of therapy and required 1 unit of blood transfusion, which raises the importance of close follow-up of HB for possible relapse, especially during the first 8–10 weeks of treatment. This is the third reported case of using daratumumab in primary cold agglutinin disease. It may be effective; however, it may take weeks to months to show a result. Further large-scale studies are recommended to confirm these findings.
Table 1. Literature review of two cases of cold agglutinin disease treated with daratumumab.
Consent to publish statement
Written informed consent was obtained from the patient for publication of the details of their medical case.
Ethics
This Case Report Proposal was reviewed and approved by the Medical Research Center, Hamad Medical Corporation, Qatar. The approval number is MRC-04-23-303.
Acknowledgment
I would like to thank the Internal Medicine Residency Program, Dr. Dabia Hamad Almohanadi, and the Medical Research Center for their scientific support.
Data availability statement
Data are available on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Abdelaziz Mohamed
Abdelaziz Mohamed, is a fourth-year internal medicine resident at Hamad Medical Corporation, Qatar.
Mohammed Alkhatib
Mohammed N. O. Alkhatib is a fourth-year internal medicine resident at Hamad Medical Corporation, Qatar.
Awni Alshurafa
Awni A. A. Alshurafa is a third-year fellow, department of hematology, Hamad Medical Corporation. Qatar.
Halima El Omri
Halima El Omri is a senior consultant physician, department of hematology, Hamad Medical Corporation. Qatar.
References
- Gabbard AP, Booth GS. Cold agglutinin disease. Clin Hematol Int. 2020 Sep;2(3):95–100. PMID: 34595449. doi:10.2991/chi.k.200706.001
- Berentsen S, Ulvestad E, Langholm R, et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica. 2006 Apr;91(4):460–466.
- Berentsen S. How I treat cold agglutinin disease. Blood. 2021 Mar;137(10):1295–1303. PMID: 33512410. doi:10.1182/blood.2019003809
- Berentsen S, Ulvestad E, Gjertsen BT, et al. Rituximab for primary chronic cold agglutinin disease: a prospective study of 37 courses of therapy in 27 patients. Blood. 2004 Apr;103(8):2925–2928. PMID: 15070665. doi:10.1182/blood-2003-10-3597
- Tomkins O, Berentsen S, Arulogun S, et al. Daratumumab for disabling cold agglutinin disease refractory to B-cell directed therapy. Am J Hematol. 2020 Jul;95. PMID: 32652632. doi:10.1002/ajh.25932
- Zaninoni A, Giannotta JA, Gallì A, et al. The immunomodulatory effect and clinical efficacy of daratumumab in a patient with cold agglutinin disease. Front Immunol 2021;12:649441. PMID: 33732266. doi:10.3389/fimmu.2021.649441
- Berentsen S. Cold agglutinin disease. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;2016(1):226–231. PMID: 27913484; PMCID: PMC6142439. doi:10.1182/asheducation-2016.1.226
- Tolbert VP, Goldsby R, Huang J, et al. Daratumumab is effective in the treatment of refractory post-transplant autoimmune hemolytic anemia: a pediatric case report. Blood. 2016;128:4819. PMID: 36524117. doi:10.1182/blood.V128.22.4819.4819
- Schuetz C, Hoenig M, Moshous D, et al. Daratumumab in life-threatening autoimmune hemolytic anemia following hematopoietic. stem cell transplantation. Blood Adv. 2018;2:2550–2553. PMID: 30291113. doi:10.1182/bloodadvances.2018020883
- Korver W, Carsillo M, Yuan J, et al. A reduction in B, T, and natural killer cells expressing CD38 by TAK-079 inhibits the induction and progession of collagen-induced arthritis in cynomolgus monkeys. Pharmacol Exp Ther. 2019;370:182–196. PMID: 31085699. doi:10.1124/jpet.119.256602
- Pereda MA, Hosahalli Vasanna S, Desai NJ, et al. Case report: daratumumab treatment in pre-transplant alloimmunization and severe hemolytic anemia. Front Immunol. 2022 Nov 29;13:1055473. PMID: 36524117; PMCID: PMC9744936. doi:10.3389/fimmu.2022.1055473
- Vernava I, Schmitt CA. Daratumumab as a novel treatment option in refractory ITP. Blood Cells Mol Dis. 2023 Mar;99:102724. Epub 2023 Jan 13. PMID: 36669360. doi:10.1016/j.bcmd.2023.102724
- Nooka AK, Gleason C, Sargeant MO, et al. Managing infusion reactions to new monoclonal antibodies in multiple myeloma: daratumumab and elotuzumab. J Oncol Pract. 2018 Jul;14(7):414–422. PMID: 29996069. doi:10.1200/JOP.18.00143
- Sullivan HC, Gerner-Smidt C, Nooka AK, et al. Daratumumab (anti-CD38) induces loss of CD38 on red blood cells. Blood. 2017 Jun 1;129(22):3033–3037. Epub 2017 Apr 3. PMID: 28373263; PMCID: PMC5454337. doi:10.1182/blood-2016-11-749432
- Werle E, Ziebart J, Wasmund E, et al. Daratumumab interference in pretransfusion testing Is overcome by addition of daratumumab Fab fragments to patients’ plasma. Transfus Med Hemother. 2019 Dec;46(6):423–430. Epub 2019 Mar 12. PMID: 31933572; PMCID: PMC6944924. doi:10.1159/000495773