ABSTRACT
Objective
Identify patient preference towards thrombopoietin-receptor agonists (TPO-RAs) and determine the clinical and social impact of immune thrombocytopenia (ITP) in Italy.
Methods
The Thrombopoietin-Receptor Agonist Patient experience (TRAPeze) survey collected responses from Italian residents from 17th January to 28th February 2022. TRAPeze utilized a discrete choice experiment (DCE) to elicit patient preferences towards TPO-RA attributes and a patient burden survey (PBS) to determine ITP disease characteristics and social impact.
Results
Seventy-six respondents completed the DCE, of which 69 completed both the DCE and PBS (mean [range] age 45 [18.0–73.0] years, 80% female). TPO-RA attributes with the greatest influence over respondent choice were method of administration (odds ratio [OR] 2.96; 95% confidence interval [CI] 2.16–4.06), drug-food interactions (OR 1.48; 95% CI 1.17–1.86) and frequency of dosing (OR 1.32; 95% CI 1.15–1.52). Respondents were more likely to prefer therapies administered orally over subcutaneous injection (OR 3.76; 95% CI 2.51–5.63), once weekly over once daily (OR 1.83; 95% CI 1.26–2.65), and therapies without food restrictions over with restrictions (OR 1.58; 95% CI 1.17–2.14).
The most frequently reported symptoms were bruising (82%), petechiae (65%) and fatigue (64%). Most respondents (84%) felt ITP impacted familial relationships and 71% of employed respondents reported fatigue influencing their ability to work, with 31% reducing working hours.
Conclusion
Although responses indicated a moderate perception of general health, ITP clearly impacted respondent work and social life. Our findings demonstrate respondents preferred TPO-RAs delivered orally, with less frequent dosing and without food restrictions.
1. Introduction
ITP is a rare autoimmune disorder characterized by impaired platelet production and increased platelet destruction, resulting in an overall reduction in platelet count [Citation1]. Patients typically present with symptoms consistent with bleeding, such as bruising, purpura and petechiae [Citation2], and have platelet counts <100× 109/L [Citation3].
Current recommended management focuses on prevention of bleeding events by raising platelet counts to >20–30 × 109/L [Citation2]. This has typically been achieved through immune suppression with corticosteroids [Citation2,Citation4]. Long-term corticosteroid use is, however, not preferable for patients due to high relapse rates and adverse effects [Citation5]. In addition, therapies which cause immune suppression have become increasingly unattractive since the COVID-19 pandemic, as immune suppression increases patient risk of contracting viral infection. Given the current landscape, ITP management has shifted away from long-term corticosteroid use. After initial treatment with corticosteroids, TPO-RAs now offer improved platelet levels while avoiding immune suppression.
Three TPO-RAs are currently approved for the treatment of ITP in Europe and the U.S.A.: eltrombopag (Revolade®), romiplostim (Nplate®) and avatrombopag (Doptelet®). TPO-RAs are considered to have comparable efficacy and safety profiles, and have prevented many ITP patients from prolonged exposure to repeated courses of corticosteroids or splenectomy [Citation6]. These drugs do, however, differ in characteristics such as mode of delivery, frequency of administration and dietary restrictions [Citation6–8]. Eltrombopag is administered as an oral tablet, once daily, two hours prior to and four hours after consumption of dairy, antacids or mineral supplements [Citation9]. Romiplostim is a subcutaneous injection, administered once weekly, by healthcare professionals in the clinic or by the patient at home [Citation10]. Avatrombopag is an oral tablet, taken once daily with food, without any dietary restrictions [Citation11].
TPO-RAs are generally considered a second-line treatment and their use is determined by regional reimbursement arrangements and patient priorities [Citation12]. Factors such as avoidance of splenectomy and achieving a durable platelet response contribute to clinicians’ decision to prescribe TPO-RAs [Citation4]. Given the differences in TPO-RA product attributes, there is growing consensus that the choice of TPO-RA could also be influenced by patients’ preferred route of administration [Citation2,Citation4,Citation12]. Moreover, should patient preference lead to switching between TPO-RAs for an alternative mode of delivery, it is becoming clearer that response to a second agent is often improved compared to the first [Citation13]. Therefore, switching TPO-RA due to patient preference may be an attractive option that should be considered prior to use of other treatments.
Despite therapeutic intervention, ITP is recognized to have a persistent impact on patient quality of life (QoL) [Citation14]. Previous patient burden studies in ITP have highlighted the role of fatigue as being detrimental to patient QoL [Citation14,Citation15]. As a symptom that continues through treatment, fatigue impacts patients’ social and work lives, often leading to a reduction in working hours [Citation14]. The social impact of ITP has further implications on the mental health of ITP patients, for whom depression and anxiety are a recognized risk [Citation14,Citation16]. Research on the patient experience and patient-oriented treatment priorities in ITP remains lacking. Thus, enriching our understanding of patients’ goals for therapy will benefit development of management strategies and future therapies for ITP treatment.
Currently, the European TRAPeze survey is the only study to have directly quantified the differential impact of product characteristic on TPO-RA choice [Citation15,Citation17]. The TRAPeze survey, previously conducted in the United Kingdom (UK) and the Netherlands (NL), explores patient preference regarding TPO-RAs and the burden of ITP in adult patients. In collaboration with clinical experts and ITP patient advocacy groups we fielded the TRAPeze discrete choice experiment (DCE) and a modified version of the patient burden survey (PBS) in Italy.
2. Methods
The European TRAPeze study is a cross-sectional, exploratory observational study of individuals with ITP. The survey was open to respondents in Italy from January 17th to 28th February 2022.
Respondents were recruited through dissemination of the survey by the Italian ITP patient advocacy group, Associazione Italiana Porpora Immune Trombocitopenica (AIPIT). The survey was disseminated via the AIPIT website and mailing list and was administered as an online questionnaire through the web platform SurveyEngine®.
The TRAPeze survey design is described in a previous publication [Citation15]. In summary, the survey consisted of two components: a DCE and PBS. The DCE was used to elicit respondents’ preference towards TPO-RA product characteristics based on their selection of preferred treatments from 10 pairs of hypothetical treatments. The PBS consisted of open and closed questions exploring respondents’ experiences with ITP. Some questions were removed from the Italian PBS (Appendix 1 – refer Supplementary material) to comply with regulation in Italy on the collection of patient data for market research. No changes were made to the DCE component of the Italian TRAPeze study. As TRAPeze was considered market research by the Italian regulator, ethical approval for the study was not required.
Processing and analysis of respondent data were performed as described previously [Citation15]. Responses were retrieved in Excel format from SurveyEngine®. Analysis of the DCE was performed in Stata (StataCorp LLC. 2023. Stata Statistical Software: Release 18). Not all respondents answered all questions; ‘I don’t know’ and ‘prefer not to say’ responses were also pragmatically excluded from analyses where appropriate. As a result, sample sizes reflect the total included responses for that question.
Inclusion criteria were as follows: ≥18 years of age, formal diagnosis of primary ITP from an ITP specialist.
3. Results
3.1. Demographics
A total of 76 respondents participated, of whom all completed the DCE and 69 completed both the PBS and DCE. A further 6 respondents provided incomplete DCE responses and were, therefore, excluded. All participants (100%, n = 76) were Italian residents. In the Italian cohort, respondents who completed the PBS were primarily female (80%, n = 55/69) and mean (standard deviation [SD]) age of respondents was 45 (14) years. The mean (SD) age of respondents at diagnosis was 32 (16) years and the mean (SD) time since diagnosis was 14 (15) years. Age distribution of respondents were as follows: 18–24; n = 6 (9%), 25–34; n = 11 (16%) 35–44; n = 18 (26%), 45–54; n = 15 (22%), 55–64; n = 13 (19%), ≥65; n = 6 (9%). The majority of respondents (87%, n = 60/69) reported living with a partner, family or friend. The remaining 13% of respondents (n = 9/69) reported living alone.
3.2. Disease characteristics
Of the respondents that completed the PBS (n = 69), when affected by ITP they reported experiencing a mean (SD) of 4 (2.4) symptoms. Bruising was reported as the most common symptom of ITP (82%, n = 54/66), followed by petechiae (65%, n = 43/66), fatigue (64%, n = 42/66), anxiety about platelet count (53%, n = 35/66) and haematoma (52%, n = 34/66). Of specific interest in patients taking TPO-RAs, thrombosis was reported by 4% (n = 3/66) of respondents. Heavy menstrual bleeding was also reported by 44% (n = 24/55) of the female cohort ((a)). Fatigue was the ITP symptom most frequently ranked as most negatively impactful (33%, n = 22/66) by respondents, followed by bruising (15%, n = 10/66), anxiety about platelet count (12%, n = 8/66), heavy menstrual bleeding (11%, n = 7/66) and petechiae (9%, n = 6/66) ((b)).
Figure 1. (A) ITP signs and symptoms experienced by respondents (n = 66, excluding responses ‘prefer not to say’ [n = 3]). (B) ITP signs and symptoms ranked by most negatively impactful on quality of life by respondents (n = 66, excluding respondents who experienced no symptoms [n = 3]).
![Figure 1. (A) ITP signs and symptoms experienced by respondents (n = 66, excluding responses ‘prefer not to say’ [n = 3]). (B) ITP signs and symptoms ranked by most negatively impactful on quality of life by respondents (n = 66, excluding respondents who experienced no symptoms [n = 3]).](/cms/asset/8bbfa94b-3a5b-445e-8b88-79d95e554e15/yhem_a_2253069_f0001_oc.jpg)
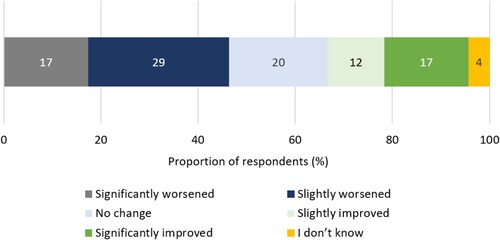
When asked to estimate their overall current health state on a scale of 1 (very poor) to 10 (excellent), the mean (SD) respondent score was 7.1 (1.7). Despite this, 67% (n = 46/69) of respondents reported their condition remaining static or worsening since diagnosis ().
The majority of respondents (91%, n = 63/69) were aware of their most recent platelet count value. The mean (SD) platelet count value was 98 (79) × 109/L.
3.3. Treatment preferences
A total of 76 DCE responses were analysed. The DCE demonstrated method of administration (OR 2.96; 95% CI 2.16–4.06), drug-food interactions (OR 1.48; 95% CI 1.17–1.86) and frequency of dosing (OR 1.32; 95% CI 1.15–1.52) were the most significant drivers of respondent preference identified among the attributes explored ((a)).
Figure 3. (A) Association between TPO-RA attributes and respondent preference towards TPO-RA treatments (n = 76). The red line indicates no effect (odds ratio = 1). The black lines indicate the lower to upper confidence intervals. (B) Association between TPO-RA attribute levels and respondent preference towards TPO-RA treatments (n = 76). The red line indicates no effect (odds ratio = 1). For each attribute category, the first attribute level plotted is the reference level. The black lines indicate lower to upper confidence intervals. *Two separate tests; one for measuring platelet count and one for liver function. **Must be taken 2 h before or 4 h after food containing dairy products or calcium; indigestion remedies (antacids); or mineral supplements.
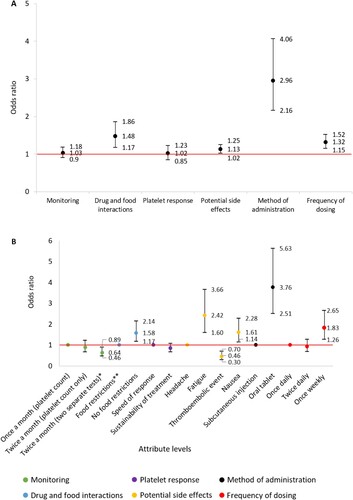
Analysis of treatment preference between attribute levels demonstrated respondents were 3.76 (95% CI 2.51–5.63) times more likely to select a TPO-RA administered orally over subcutaneous injection. Respondents were 1.83 (95% CI 1.26–2.65) and 1.58 (95% CI 1.17–2.14) times more likely to select a TPO-RA administered once weekly over once daily, or a therapy without food restrictions over with food restrictions, respectively. Interestingly, respondents were 2.42 (95% CI 1.60–3.66) and 1.61 (95% CI 1.14–2.28) times more likely to select a TPO-RA that had fatigue or nausea, respectively, and 0.46 (95% CI 0.30–0.70) times less likely to select thromboembolic event over headache as a potential side effect ((b)).
3.4. Healthcare resource utilization
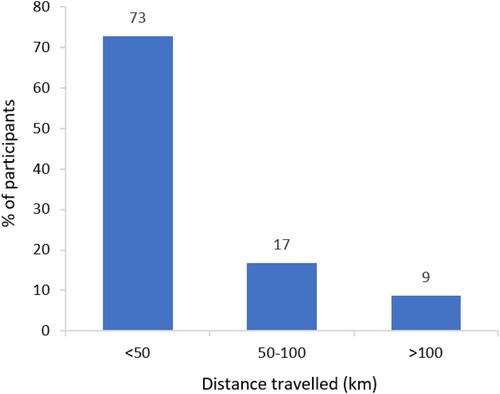
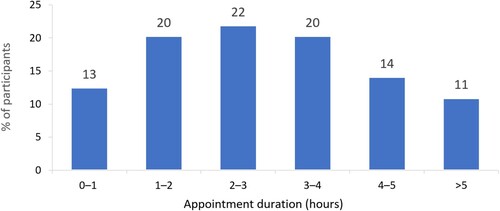
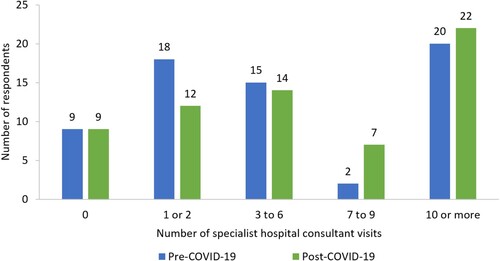
Frequency of specialist hospital consultant visits for ITP varied within the Italian cohort. The median (range) number of specialist visits in the period October 2019–March 2020 was 5 (‘0’–‘>10’), and in the period March 2020–February 2022 was 5 (‘0’–‘>10’), despite a difference of 15 months between timeframes (). These timeframes were selected to allow comparison between pre- and post-COVID-19. Respondents typically reported travelling <50 km to attend their specialist hospital consultant appointments (). However, the majority (67%, n = 43/64) of these appointments lasted >2 h; respondents were asked to consider travel and waiting time, in addition to appointment duration (). Over the 12 months preceding the study, 52% (n = 36/69) of respondents had seen a GP or family doctor, and 21% (n = 14/66) had used mental health services, due to ITP.
Figure 4. Number of specialist hospital consultant visits pre-COVID-19 (October 2019–March 2020) and post-COVID-19 (March 2020–February 2022) (n = 64).
3.5. Work and productivity
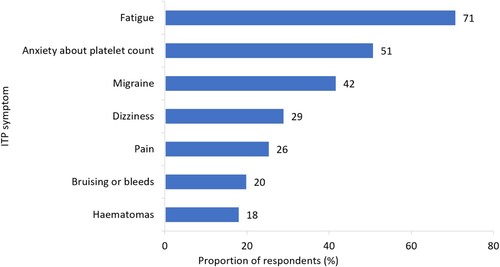
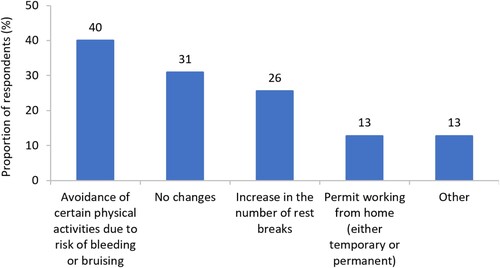
The majority of respondents (80%, n = 55/69) were employed at time of survey fielding and 64% (n = 35/55) of employed respondents reported being in full-time work. We found 29% (n = 16/55) of respondents had reduced the number of hours they worked per week. When asked which ITP symptoms had influenced respondent ability to fulfil their employment responsibilities, the most frequently reported symptoms were fatigue (71%, n = 39/55), anxiety about platelet count (51%, n = 28/55) and migraines (42%, n = 23/55) (). Many respondents also reported avoiding physical activities at work to reduce risk of bleeding or bruising (40%, n = 22/55), and increasing their number of rest breaks (26%, n = 14/55) ().
3.6. Wider social impact
When deciding to have children, female respondents were most concerned about bleeding during pregnancy or childbirth (36%, n = 18/50), and their children inheriting ITP (36%, n = 18/50) ().
Figure 9. ITP-related concerns that have impacted female respondents’ decision to have children (n = 50, excluding responses ‘I don’t know’ [n = 5]).
![Figure 9. ITP-related concerns that have impacted female respondents’ decision to have children (n = 50, excluding responses ‘I don’t know’ [n = 5]).](/cms/asset/a33d91d2-93c7-4e4e-b08c-ef6883d996e1/yhem_a_2253069_f0009_oc.jpg)
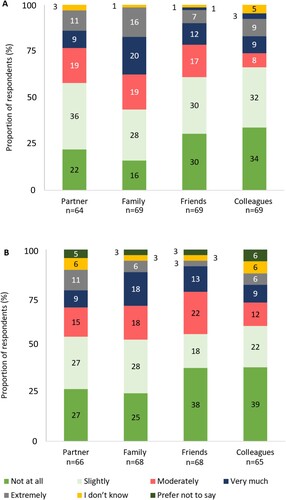
Most respondents felt that ITP negatively impacted their relationships, and that this impact had changed as their ITP had progressed since diagnosis ((a,b)) The most commonly impacted relationship was with their family (83%, n = 57/69) ((a)). Of those who provided free text responses on the impact of ITP on relationships, 13% (n = 4/32) of respondents mentioned adjusting parental responsibilities and 38% (n = 12/32) outlined the familial and social impacts of ITP-related anxiety.
Figure 10. (a) Degree of impact that respondents felt ITP has had on relationships (excluding responses: ‘not applicable’). (b) Change in the degree of impact that respondents felt ITP has had on relationships as disease has progressed since diagnosis (excluding responses: ‘not applicable’). Percentages may not sum to 100 due to rounding.
4. Discussion
Although the burden of ITP from the patient’s perspective has been previously reported, the TRAPeze study is the first to explore patient preferences towards TPO-RAs. The Italian cohort was mostly female, representative of the real-world ITP population [Citation18], with a mean age (45 years) slightly lower than is seen in clinical practice (48 years) [Citation19].
The Italian cohort DCE indicated method of administration, drug-food interactions and administration frequency were primary choice drivers of TPO-RA therapy. When comparing between attribute levels, this study found potential side effects were an important driver of choice amongst Italian respondents. Respondents were considerably more likely to select the DCE treatment scenario with fatigue as a potential side effect, rather than headaches. This is noteworthy, given that fatigue was identified as the most negatively impactful ITP symptom respondents experienced. We propose that preference for fatigue over headaches is due to ITP patients often already experiencing fatigue as a symptom of the disease.
Results from the TRAPeze study in Italy were highly aligned with cohorts from the UK and NL [Citation15,Citation17]. In all cohorts, the DCE identified the same three primary drivers of therapy choice: mode of administration, frequency of dosing and drug-food interactions. A marked difference between DCE results was observed in side effect preference of the Italian cohort. The potential side effects of fatigue, nausea and thromboembolic event were considerably stronger choice drivers for Italian respondents, compared with their UK and Dutch counterparts [Citation15,Citation17]. An important similarity between cohorts was seen in proportions of respondents reporting impact on social relationships. Further, themes of parenting pressures and platelet count anxiety were reported in free-text responses from all cohorts. Together, these commonalities point towards a shared patient experience of ITP. In future, more granular ITP patient QoL and treatment experience data in Italy will benefit geographical comparisons.
Consistent with existing literature [Citation14], bruising was the most common ITP symptom and fatigue was considered the most negatively impactful on QoL. Despite the reported negative impact of fatigue, the mean reported platelet count was above target levels for ITP patients [Citation20] and self-reported measures of health state indicated a moderate perception of general health. Respondents also demonstrated good perception of their disease status, as almost all were aware of their last recorded platelet count value.
This study found 44% of female respondents (35% of the whole cohort) experienced heavy menstrual bleeding, in line with previous research reporting the frequency in females with ITP to be in the range of 39–45% [Citation14,Citation21]. Heavy menstrual bleeding contributes to anaemia and fatigue observed in ITP, which is associated with a decreased QoL [Citation22]. Furthermore, over one-third of female respondents in this study reported concerns about bleeding during pregnancy or childbirth as impacting their decision to have children. This is of particular note, given ITP most commonly affects women of reproductive age [Citation23]. Moreover, anxiety about bleeding events, especially during menstruation and pregnancy, can affect some patients’ daily functioning [Citation14].
Mental health concerns also arise in the ITP population, driven by lifestyle changes imposed by the disease [Citation16]. While data on the psychological health of ITP patients are sparse, it is suspected that rates of depression and anxiety are higher in this population compared with healthy individuals [Citation24,Citation25]. We found one-fifth of respondents had used mental health services due to ITP in the 12 months preceding the study. This indicates the importance of factoring in mental health concerns in ITP management strategies.
Despite a difference of 15 months between timeframes, respondents reported similar frequencies of hospital visits between October 2019–March 2020 and March 2020–February 2022. From this, we can infer an overall reduction in specialist hospital visit frequency. Though a decline in frequency of hospital visits could correspond with the onset of the COVID-19 pandemic, due to the extended recall time, caution is recommended when examining the reduction in hospital visits. Hospital visits were also notably lengthy – with two-thirds of respondents reporting >2-hour duration of appointments. Committing extended periods of time to disease management can be challenging to integrate into patients’ lives and can indirectly contribute to the productivity loss seen in ITP [Citation26]. ITP had a clear social impact for respondents and the largest impact reported was on familial relationships. Parenting pressures and anxiety about platelet count were consistently mentioned as socially impactful. More than half of the female respondents have children, yet their decisions to have children were impacted by ITP – with worries about bleeding in childbirth and the passing of ITP to their children being their primary concerns.
The social impact of ITP also extended to the workplace. ITP symptoms affected respondents’ ability to fulfil employment responsibilities and impacted colleague relationships. This study found respondents avoided certain work activities due to bleeding and bruising risk and increased their number of rest breaks. When considered in conjunction with reduced working hours in one-third of respondents, the indirect societal cost of productivity loss in ITP is underscored.
Taken together with clinical parameters described here, we see ITP can have a significant impact on overall QoL. The TRAPeze studies continue to enrich our understanding of the impact of ITP on QoL and the importance of patient preference in ITP management.
5. Limitations
This study was limited in data collected on treatment patterns and experience. Study questions were adapted to local regulation, which did not permit collection of respondent-specific treatment information, preventing greater comparability of TPO-RA treatment experiences within the cohort and between TRAPeze cohorts from other countries. In addition, more granular clinical and patient experience data for the Italian cohort would be useful for informing the integration of patient choice in ITP management strategy in Italy.
It is also noted that, due to dissemination of the survey through AIPIT, there is a potential selection bias for more engaged ITP patients. That is to say, only patients motivated to join the patient association were recruited. To account for this bias, future publications could look to disseminate surveys via haematology clinics in tandem with patient advocacy groups.
Recall time for specific questions may have introduced some unreliability in response accuracy. In some instances, respondents were required to provide information from over two years prior to taking the survey. Consequently, such data should be interpreted with caution.
6. Conclusion
Individuals with ITP experience a range of symptoms which have a significant burden on their long-term functioning and QoL. For instance, fatigue, which extends from being a symptom, to impacting their ability to fulfil employment responsibilities. The survey of the Italian cohort found patients preferred TPO-RA therapies that are orally administered, without food restrictions and are delivered less frequently. Until recently, such TPO-RA attributes were limited to eltrombopag and romiplostim, respectively. Newer TPO-RAs such as avatrombopag, which was approved for use in the EU in 2021, offer patients the ability to address these preferences. Further DCE analysis also found that respondents preferred fatigue and nausea over headache as potential TPO-RA side effects. A potential explanation for this may be that respondents preferred to experience the familiar ITP symptoms of fatigue and nausea over the less frequently experienced symptom of headache. Additionally, responses highlight the persistent impact of ITP on the work and social lives of patients. Socially, the impact of ITP centred on familial and colleague relationships, in addition to concerns regarding the decision to have children. Professionally, changes to workplace behaviour, such as reduction in working hours, provide evidence for the societal burden of ITP. Such evidence from the patient perspective on treatment preference and disease characteristics may facilitate improved ITP management in Italy.
Data accessibility
Data supporting the findings presented here are available from the corresponding author upon reasonable request.
Supplemental Material
Download MS Word (61.9 KB)Acknowledgements
The authors wish to thank all respondents for contributing to the survey. We are grateful for the contributions of Barbara Lovrencic from the Italian ITP patient association (Associazione Italiana Porpora Immune Thrombocitopenica) and thank the members of the association for their participation in this study. Sobi reviewed and provided feedback on the studies.
Disclosure statement
AL reports honoraria from Amgen, Novartis, Grifols and Sobi, and consultancy fees from Grifols. BL reports consultancy fees from Novartis, Ucb and Sobi. VM reports consultancy fees from Amgen, Bayer, Novartis and Sobi, and research funding from Grifols. AN reports honoraria from Amgen, Angle, Argenx, Dova, Novartis, Ono and Shionogi, and consultancy fees from Amgen, Angle, Argenx and Dova. MM reports consultancy fees from Novartis, Sobi and UCB. KW and DE are employees of Sobi. EG, ODP and SP are employees of Wickenstones and report funding from Sobi for this research.
Additional information
Funding
References
- Provan D, Newland AC. Current management of primary immune thrombocytopenia. Adv Ther. 2015;32(10):875–887. doi:10.1007/s12325-015-0251-z
- Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780–3817. doi:10.1182/bloodadvances.2019000812
- Khan AM, Mydra H, Nevarez A. Clinical practice updates in the management of immune thrombocytopenia. Pharm Therap. 2017;42(12):756.
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–3866. doi:10.1182/bloodadvances.2019000966
- Cuker A, Liebman HA. Corticosteroid overuse in adults with immune thrombocytopenia: cause for concern. Res Pract Thrombos Haemos. 2021;5(6):e12592, doi:10.1002/rth2.12592
- Al-Samkari H, Kuter DJ. Optimal use of thrombopoietin receptor agonists in immune thrombocytopenia. Ther Adv Hematol. 2019;10:2040620719841735. doi:10.1177/2040620719841735
- Deng J, Hu H, Huang F, et al. Comparative efficacy and safety of thrombopoietin receptor agonists in adults with thrombocytopenia: a systematic review and network meta-analysis of randomized controlled trial. Front Pharmacol; 2021:1951.
- Gilreath J, Lo M, Bubalo J. Thrombopoietin receptor agonists (TPO-RAs): drug class considerations for pharmacists. Drugs. 2021;81(11):1285–1305. doi:10.1007/s40265-021-01553-7
- EMC. Eltrombopag SmPC. 2022. Available from: https://www.medicines.org.uk/emc/product/567/smpc#gref.
- EMC. Romiplostim SmPC. 2021. Available from: https://www.medicines.org.uk/emc/product/567/smpc#gref.
- EMC. Avatrombopag SmPC. 2021. Available from: https://www.medicines.org.uk/emc/product/11837/smpc#gref.
- Thachil J, Bagot C, Bradbury C, et al. A United Kingdom immune thrombocytopenia (ITP) forum review of practice: thrombopoietin receptor agonists. Br J Haematol 2018;180(4):591–594. doi:10.1111/bjh.14395
- González-Porras JR, Godeau B, Carpenedo M. Switching thrombopoietin receptor agonist treatments in patients with primary immune thrombocytopenia. Ther Adv Hematol. 2019;10:2040620719837906. doi:10.1177/2040620719837906
- Cooper N, Kruse A, Kruse C, et al. Immune Thrombocytopenia (ITP) World Impact Survey (iWISh): patient and physician perceptions of diagnosis, signs and symptoms, and treatment. Am J Hematol 2021;96(2):188–198. doi:10.1002/ajh.26045
- McDonald V, Newland A, Morgan M, et al. Patient preferences and experiences regarding thrombopoietin-receptor agonists for immune thrombocytopenia in the United Kingdom and Ireland (TRAPeze UK & IE study). Hematology. 2021;26(1):799–808. doi:10.1080/16078454.2021.1978689
- Alesci S, Schwan V, Miesbach W, et al. Rare bleeding disorders are associated with depression and anxiety. Hamostaseologie. 2013;33:S64–S68.
- Jansen A, McDonald V, Newland A, et al. P1636: patient preferences and experiences regarding thrombopoietin-receptor agonists for immune thrombocytopenia in the Netherlands (trapeze nl study). HemaSphere. 2022;6:1517–1518. doi:10.1097/01.HS9.0000849400.99937.5f
- Bennett D, Hodgson ME, Shukla A, et al. Prevalence of diagnosed adult immune thrombocytopenia in the United Kingdom. Adv Ther. 2011;28(12):1096–1104. doi:10.1007/s12325-011-0084-3
- Grimaldi-Bensouda L, Nordon C, Michel M, et al. Immune thrombocytopenia in adults: a prospective cohort study of clinical features and predictors of outcome. Haematologica. 2016;101(9):1039, doi:10.3324/haematol.2016.146373
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood, J American Soc Hemat. 2009;113(11):2386–2393.
- [van] Dijk WE, Punt MC, van Galen KP, et al. Menstrual problems in chronic immune thrombocytopenia: a monthly challenge – a cohort study and review. Br J Haematol 2022;198(4):753–764. doi:10.1111/bjh.18291
- Mansour D, Hofmann A, Gemzell-Danielsson K. A review of clinical guidelines on the management of iron deficiency and iron-deficiency anemia in women with heavy menstrual bleeding. Adv Ther. 2021;38(1):201–225. doi:10.1007/s12325-020-01564-y
- Fogarty PF. Chronic immune thrombocytopenia in adults: epidemiology and clinical presentation. Hematology/Oncology Clinics. 2009;23(6):1213–1221. doi:10.1016/j.hoc.2009.08.004
- Terrell DR, Reese J, Branesky D, et al. Depression in adult patients with primary immune thrombocytopenia. Am J Hematol 2016;91(10):E462–E4E3. doi:10.1002/ajh.24484
- Kruse A, Kruse C, Potthast N, et al. Mental health and treatment in patients with immune thrombocytopenia (ITP); data from the platelet disorder support association (PDSA) patient registry. Blood. 2019;134:2362, doi:10.1182/blood-2019-122278
- Tarantino MD, Mathias SD, Snyder CF, et al. Impact of ITP on physician visits and workplace productivity. Curr Med Res Opin. 2010;26(2):319–328. doi:10.1185/03007990903451298