ABSTRACT
Wilms’ tumor gene 1 (WT1) is a transcription and post-translational factor that has a crucial role in the biological and pathological processes of several human malignancies. For hematological malignancies, WT1 overexpression or mutation has been found in leukemia and myelodysplastic syndrome. About 70−90% of acute myeloid leukemia patients showed WT1 overexpression, and 6−15% of patients carried WT1 mutations. WT1 has been widely regarded as a marker for monitoring minimal residual disease in acute myeloid leukemia. Many researchers were interested in developing WT1 targeting therapy. In this review, we summarized biological and pathological functions, correlation with other genes and clinical features, prognosis value and targeting therapy of WT1 in hematological features.
Introduction
WT1 was originally considered as a tumor suppressor gene, first identified as Wilms’ tumor in 1990 [Citation1,Citation2]. Subsequent studies identified it as an oncogene in hematological malignancies and solid tumors [Citation2,Citation3]. For human hematologic malignancies, somatic mutations or overexpression of WT1 were frequently detected and had distinct molecular and clinical characterization. Somatic mutations contain insertion, deletion and base substitution mutations, and occur in approximately 6−15% of newly diagnosed Acute Myeloid Leukemia (AML) patients (excluding Acute Promyelocytic Leukemia (APL) patients) [Citation4–7], whereas WT1 was overexpressed in more than 70% AML patients [Citation8,Citation9]. In 38 cancer cell lines including in solid tumors and hematological malignancies, WT1 mRNA expressions were detected in 79% of cells [Citation10]. Wilms’ tumor protein 1 is a transcription factor and post-transcriptional regulator regulating cell growth, mitosis, differentiation and apoptosis [Citation11], and it participates in embryonic development and organ homeostasis [Citation12,Citation13]. WT1 has been regarded as a marker of monitoring minimal residual disease (MRD) in AML [Citation14]. Several studies demonstrated that its abnormality was correlated with the prognosis of AML patients. In this review, we summarized the research progress related to WT1 in hematologic malignancies, mainly focused on the roles of WT1 in different hematologic malignancies, the prognosis value, association with other genes and clinical features, as well as the recent findings concerning WT1 immune therapy. This may help further understand the roles and the mechanisms of WT1 in hematologic malignancies and design better anti-cancer treatment strategies.
WT1 structure and biological function
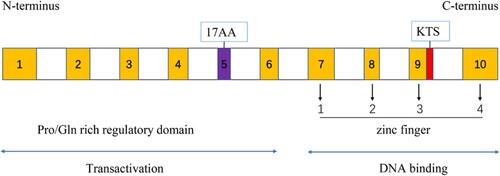
WT1 gene is located at chromosome 11p13, comprises 10 exons, and encodes an approximately 3-kb mRNA transcript [Citation1,Citation2,Citation15]. The WT1 protein, as shown in , comprises two functional domains: zinc fingers and a regulatory domain. Four canonical Cys2-His2 zinc fingers were encoded by exons 7–10 at the C-terminus, one zinc finger and four bind the 3’ part and the 5’ part of the DNA recognition sequence, respectively; N-terminal Pro/Gln-rich regulatory domain is responsible for the interaction with nucleic acids [Citation15,Citation16]. There are at least 36 isoforms in the WT1 protein, four major isoforms resulting from two distinct splices have been widely studied, inserting or deleting 17AA (17 amino acids) in the amino-terminal of the protein, and/or KTS (lysine (K) – threonine (T) – serine (S)) between the third and fourth zinc finger, four major isoforms are known as A(17AA-/KTS-), B(17AA+/KTS-), C(17AA-/KTS+) and D(17AA+/KTS+) [Citation16,Citation17].
Four major WT1 isoforms maintained a relative balanced ratio in the fetal kidney and Wilms’ tumor, referred to as 1.0:2.5:3.8:8.3 [Citation17]. Previous studies indicated the imbalanced ratio of the four isoforms could result in different phenotypes [Citation18], a typical example was that an altered ratio of WT1(+KTS) /WT1(-KTS) variant caused rare pre-malignant syndromes, namely, Frasier Syndrome, a kidney disease that begins in early childhood [Citation19], and different AML subtypes had different ratios of WT1(+KTS) /WT1(-KTS) [Citation20]. The WT1 protein is a transcription factor and essential in the development of multiple organs such as the kidney and gonads [Citation15,Citation21], heart [Citation22], spleen [Citation23], liver [Citation24], lung [Citation25], pancreas [Citation26] and retina [Citation27] via various signaling pathways.
In addition, different isoforms prefer to perform distinct functions. Ito et al. [Citation28] suggested that 17AA(+) WT1 inhibited cell apoptosis through the intrinsic apoptosis pathway in three leukemia cell lines that expressed WT1. Gu et al. [Citation29] indicated that 17AA(+) WT1 inhibited differentiation of NB4 cell. Bissanum et al. [Citation30] demonstrated that in triple-negative breast cancer cell line MDA-MB-231, WT1 isoform B and isoform C could increase cell migration and vasculogenic mimicry by activating the EphA2/β-catenin/vimentin pathway, whereas other isoforms do not. Oji et al. [Citation31] found that, in non-small-cell lung cancer and gastric cancer, WT1 isoform C upregulated homologous recombination genes to mediate DNA damage repair. In the AML cell line Kasumi-1, KTS(+) promoted cell growth, whereas KTS(-) had the opposite effect [Citation20]. These phenomena may explain why the WT1 gene has dual tumor suppressor/oncogene activity. The clinical findings of WT1 were shown in .
Table 1. The clinical findings of WT1.
Correlation between WT1 mutation/overexpression and clinical characteristics, molecular mechanism and clinical implication
WT1 in AML
The correlation between WT1 mutations/overexpression and clinical characteristics is shown in Table .
Table 2. Correlation between WT1 mutations/overexpression and clinical characteristics.
Currently, many research studies focus on exploring the underlying molecular mechanism of WT1. WT1 inhibited AML cell lines HL60 and KASUMI-1 proliferation via mutational p53 pathway [Citation32]. In AML cell line U937, miR-23b-3p inhibited WT1 to promote cell differentiation and reduce cell proliferation [Citation33]. When the WT1 gene was knockdown in AML cells, the cell counts and colony-forming ability were both reduced; in AML-mouse models, the spleen weight of the mouse was reduced and the lives of the mouse were prolonged than the controls, furtherly WT1 upregulated BCL2 expression to maintain the self-renewal of AML leukemia stem cells [Citation34]. Dysfunction of the WT1-MEG3 axis facilitated leukemogenesis in AML [Citation35]. WT1 mediated Adriamycin resistance through lncRNA HOTAIR/miR-20a-5p/WT1 axis in AML cells and the mouse model, and Curcumin could rescue the process by inhibiting this axis [Citation36]. As we all know, the PML-RARA fusion gene is the typical molecular feature of APL, Christopher et.al. [Citation37] analyzed 242 AML patients from the TCGA database, among all AML subtypes, WT1 expression was the highest in APL, and in vitro experiment, WT1 wild-type (WT1WT) function as a suppressor gene. To explain the paradoxical phenomenon that inactivating mutations of WT1 and overexpressed WT1WT were observed in AML, Christopher et al. [Citation38] imitated WT1 loss-of-function mutations in an inducible knockout mouse model, the result indicated the mutations can cooperate with PML-RARA fusion gene to drive APL, additionally, PML-RARA fusion gene can robustly induce WT1WT protein expression. Wagstaff. et al. [Citation39] first found that β-catenin regulated WT1 expression in AML.
WT1 has been regarded as a marker of prognosis and MRD in AML. Wang et al. [Citation40] analyzed the gene profile and clinical features of 870 pediatric AML patients, and found that WT1 mutations (WT1MT) were an independent poor prognostic factor for event-free survival (EFS) and overall survival (OS). Marceau-Renaut et al. [Citation41] detected gene profiling in 385 pediatric AML patients and found that WT1MT predicted poor outcomes and had a strong link with the NUP98-NSD1 fusion gene. Among normal karyotype AML patients, EFS was shorter in the WT1MT group than the WT1WT group, with no statistical difference in OS [Citation42]. For AML patients who underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT), higher WT1 mRNA expression levels at the newly diagnosed stage were found in patients who experienced relapse or no remission than in those without relapse after chemotherapy [Citation43]. A large number of studies indicated that higher expression of WT1 in pre-transplant and post-transplant was correlated with worse prognosis [Citation43–54]. No difference was observed in the mRNA expression levels of WT1 between pre-transplant and post-transplant for those patients who deceased within 2 years post-transplant [Citation43]. More studies were trying to improve the prognostic predictive value of WT1. Ikeno et al. [Citation55] revealed the predictive value of WT1 levels in different time points, patients with positive WT1 before day 180 post-HSCT had shorter progression-free survival (PFS) and OS than those with negative WT1 post-HSCT or those with positive WT1 after day 180 post-HSCT. In terms of MRD markers for AML, several studies have shown WT1 mRNA expression levels were superior to conventional methods such as short-tandem repeat-based chimerism analysis, XY-FISH, cytogenetic and molecular analyses [Citation44,Citation46,Citation56–58]. In 72 AML patients, Hao et al. [Citation59] identified that there was a significantly negative correlation between WT1 mRNA levels and donor chimerism; concerning the determination of MRD, the sensitivity of qRT – PCR analysis of WT1 mRNA was comparable to that of multiparameter flow cytometry (MFC); among 12 patients who experienced relapse, 11(91.7%) were both qRT – PCR and MFC positive. Another similar study [Citation60] also confirmed that the combination of qRT – PCR and MFC detection before transplantation increased the predictive value of relapse for AML patients, irrespective of induction therapy, conditioning regimen, risk group, disease status at HSCT, donor type or transplant year. Therefore, a combination of qRT – PCR and MFC methods to detect WT1 could improve the sensitivity of predicting relapse for AML patients who underwent allo-HSCT. Duléry et al. [Citation44] compared the ability to predict relapse between the WT1 mRNA expression levels in peripheral blood (PB) and that in bone marrow (BM) post-transplant, finally suggesting that PB was more predictive than in BM for AML who underwent allo-HSCT. A similar result was also found in AML patients who received chemotherapy [Citation8,Citation54,Citation61–68]. For elderly AML patients who received decitabine therapy, WT1 mRNA expression levels were significantly lower at the complete remission (CR) stage than that at diagnosis [Citation69]. For AML patients who received chemotherapy induction, no association between the cumulative incidence of relapse, OS and WT1 levels [Citation64]. The worse therapy responses in terms of overall response rate (ORR), median time to first relapse or risk of relapse as well as CR or resistance rate to therapy were found in the high-WT1 group [Citation8,Citation9,Citation47,Citation54,Citation63,Citation65]. Compared to normal BM samples, an interesting study demonstrated that there was strong correlation between WT1 and p53 protein expression rates in AML, and the p53/WT1 ratio could be a useful factor to predict the chemotherapeutic response [Citation70]. But for cases with mutant NPM1 (NPM1MT) or FLT3 internal tandem duplication mutations (FLT3ITD), no difference in ORR, OS and disease-free survival (DFS) was found between high-WT1 and low-WT1 groups [Citation63]. According to WT1 decreased levels after induction therapy (from baseline to post-induction), Du et al. [Citation63] found that lower ORR was observed in the < 1-log group than in the > 1-log group, but another study by Frairia [Citation66] illustrated that no difference in relapse risk and mortality risk was found between the < 2-log group andthe ≥ 2-log group, this may be attributed to different population and the cut-off value. Additionally, a study by Hidaka [Citation71] demonstrated that in every cytogenetic category AML group, WT1 expression did not have a significant prognostic effect. Mitrovic et al. [Citation72] evaluated the reduction value of WT1 expression in paired diagnosis/complete remission samples from APL patients and found that there was no relationship with relapse rate, DFS and OS. Thus, they demonstrated that WT1 expression level at different time points regarded as a marker monitoring MRD was not reliable. Contrary to the majority of studies, a study [Citation69] suggested that for elderly AML patients, a significantly higher OS was found in the high – WT1 group than the low-WT1 group.
Additionally, some studies explored the effect of co-recurrence of aberrant WT1 and other abnormal genes on the clinical characteristics and prognosis of AML patients. Among pediatric AML patients, in terms of mutated WT1, FLT3ITD and NUP98-NSD1 translocation, cases with double or triple mutations had worse prognoses than those that carried none of these mutations or one single-gene mutation [Citation73]. Also, some mutations, especially FLT3ITD, NPM1MT and NRAS mutation co-occurring with WT1MT in AML, were observed via single-cell DNA sequencing technology, an inferior prognosis was observed in AML patients carried WT1MT and FLT3ITD than those with only FLT3ITD [Citation7] or only WT1MT [Citation74]. Additionally, El Hussein et.al [Citation75] observed that among 15 NPM1MT AML patients, 27% relapsed with emerging WT1MT.
A study [Citation76] reported that among 69 AML patients with CEBPAdm, compared to AML patients with WTWT, those with co-mutations had significantly worse outcomes. Adnan-Awad et al. [Citation9] analyzed the relationship between two genes WT1 and Survivin expression and the clinical characteristics of AML patients. Patients in the double positive group had higher mean white blood cells (WBCs), BM blasts and total leukocytic counts than cases in the double negative group or single positive group, without exception, the OS was longer in the double negative group than the other group.
Single-nucleotide polymorphism rs16754 at exon 7 of WT1 was widely studied. The polymorphism contains two alleles: adenine (A) and guanine (G), which exhibit ethnic differences. As WT1MT, some studies indicated that mutant WT1 rs16754 GA/GG genotype predicted a favorable outcome compared with wild type WT1 rs16754 AA in AML including cytogenetically normal AML(CN-AML) [Citation77,Citation78], some studies described WT1 rs16754 genotype was not associated with the prognosis of CN-AML patients [Citation79,Citation80]. A meta-analysis clarified different outcomes of WT1 rs16754 genotype on prognosis were correlated with different population groups [Citation81].
WT1 in acute lymphoblastic leukemia (ALL)
The correlation between WT1 mutations/overexpression and clinical characteristics is also shown in .
Several studies clarified the molecular mechanism of WT1 in ALL. WT1MT and/or deletions occupied about 10% of T-ALL patients and were correlated with TLX3/HOX11L2 translocation [Citation82]. WT1MT conferred resistance to DNA damage via functional TP53 [Citation83]. In T-ALL, ETV6 mutation cooperates with WT1MT to abrogate DNA-binding activity, which can promote leukemia formation [Citation84]. Also, WT1 loss-of-function mutation cooperates with mutant IL-7Rα to drive leukemogenesis [Citation85].
To verify whether the WT1 gene was also of prognostic significance in ALL like in AML, the results of several studies were inconsistent. In terms of WT1MT, expectedly, neither adults nor children were associated with the prognosis of T-ALL patients [Citation82]. Additionally, contrary to the AML, high-WT1 predicted better outcomes in B-ALL patients (aged ≥ 14 years). Subtype analysis suggested that for patients who received chemotherapy only or those who received allo-HSCT, patients with positive WT1 had a favorable prognosis, but for the transplantation group the difference did not reach statistical significance [Citation86]. However, another study illustrated that children ALL patients in the high-WT1 group tended to have worse prognoses than those in the low-WT1 group [Citation87,Citation88].
WT1 in myelodysplastic syndromes (MDS)
The correlation between WT1 mutations/overexpression and clinical characteristics is shown in .
Similar to AML, WT1 was also regarded as a marker of prognosis in MDS. A few studies indicated that for MDS patients, high WT1 at diagnosis or elevated WT1 levels during stable disease often predicted worse outcomes [Citation61,Citation89–93]. A Chinese multicenter study [Citation89] enrolled 1042 MDS patients with thrombocytopenia, the result showed that cases with high-WT1 tended to have higher Revised International Prognostic Scoring System (IPSS-R) risk, worse cytogenetics prognosis and higher blast percentage. Additionally, for patients treated with azacytidine, lower WT1 mRNA expression levels were found in responders than non-responders, correspondingly, a higher responder rate was observed in the low-WT1 group than the high-WT1 group [Citation94]. According to IPSS-R risk categories, WT1 mRNA levels from PB at diagnosis could be used to predict PFS in MDS patients with very low/low risk and intermediate risk, similarly, also predicts OS in patients without allo-HSCT [Citation95]. An interesting study illustrated that for elderly MDS patients, shorter OS and PFS were observed in the high-WT1 group, additionally if divided patients into high-risk and low-risk groups according to World Health Organization Prognostic Scoring System or IPSS-R risk categories, no statistical difference in PFS and/or OS was observed in the subgroups except for the low IPSS-R risk group [Citation93].
WT1 in chronic myelogenous leukemia (CML)
Presently, there are relatively few studies regarding the roles of WT1 in CML.
Co-activation of WT1 and AP-1 proteins could result in WT1 upregulation and K562 cell proliferation [Citation96]. In Imatinib (IM)-treated K562 cell, activated HtrA2 down-regulated WT1 through the p38 MAPK signaling pathway [Citation97]. BCR/ABL1 activated the PI3 K/AKT signaling pathway then increased WT1 transcription [Citation98], overexpressed WT1-mediated IM resistance by directly regulating QPRT expression [Citation99].
El-Menoufy et al [Citation100] reported that in CML patients who received IM therapy, only at diagnosis and 3 and 6 months of therapy, a significant correlation between WT1 levels and BCR-ABL1 expression was found, during follow-up at 12 months and onward, the correlation was not shown (whether achieved remission or resistant to IM therapy for patients). More interestingly, two cases progressed to the advanced stage, WT1 expression increased while BCR-ABL1 decreased, this suggested WT1 may recognize clone evolution superior to BCR-ABL1 as a marker for monitoring disease progression.
Other
WT1MT were enriched in secondary AML compared to high-risk MDS, and this mutation was associated with disease progression and shorter OS [Citation101]. The frequency of WT1MT was found similar in mixed phenotype acute leukemia (41%) and in early T-cell precursor ALL (42%) [Citation102].
The optimal cut-off value of WT1
To provide independent prognostic information on WT1 in AML, in 2007, Cilloni et al. [Citation103] systematically evaluated nine qRT-PCR assays from 11 laboratories spread across eight countries. Exclusion criteria were lower sensitivity, lower efficiency, lower RNA specificity and inferior performance profile, finally the assay published previously by Van Dijk et al [Citation104]. was proposed as a standardized assay referred to as the European LeukemiaNet (ELN) WT1 assay, namely, forward primer: 5′-CGCTATTCGCAATCAGGGTTA-3′, reverse primer: 5′-GGGCGTGTGACCGTAGCT-3′; probe, 5′-FAM-AGCACGGTCACCTTCGACGGGA-TAMRA-3′, assay location was exon 1–2 of WT1 (less mutational than exon 7–9). Furthermore, to establish the threshold of distinguishing residual leukemia, this assay was used to evaluate the samples from normal controls and AML patients. The result showed that the value of WT1 copies/104 ABL1 copies ranged from 0 to 213 in BM and from 0.01 to 47.6 in PB; therefore, 250 copies/104ABL1 in BM and 50 copies/104ABL1 in PB were defined as the upper threshold of normal samples, and this cut-off value had the good efficiency of predicting prognosis in follow-up samples [Citation14]. However, one year later, the same author indicated approximately 50% of patients who reached normal WT1 levels still experienced relapse after induction chemotherapy [Citation105]. But, the majority of studies still followed the 2007 ELN standard [Citation9,Citation44,Citation49,Citation53,Citation61,Citation63,Citation86,Citation95]. Secondly, some studies determined the optimal cut-off value by receiver-operating characteristic curve analysis based on individual data [Citation8,Citation48,Citation50,Citation54,Citation72,Citation89]. Additionally, some studies use the percentile method or mean relative expression level in healthy controls to define the cut-off value of WT1 [Citation43,Citation64,Citation65,Citation87]. Sálek et al. [Citation62] defined the 10-fold and 100-fold values of the 2007 ELN reference value to discriminate patients’ prognoses. Qin et al. [Citation46] found that among 176 AML patients with t(8;21) after allo-HSCT, the cut-off value of WT1 in BM samples 1.8% was more accurate to predict the relapse than 0.6% of WT1 expression level in normal BM. Cho et al. [Citation106] retrospectively analyzed 425 AML patients who received allo-HSCT at CR, among three time points: before allo-HSCT and at 1 or 3 months after allo-HSCT, and different values of cut-off: median level, 100 copies, 25% top and 250 and 300 copies, finally before allo-HSCT and 250 copies were determined as the best predictive effective of post-transplant relapse. Nomdedéu et al. [Citation107] studied the relationship between WT1 mRNA expression levels at different time points and the prognosis of AML patients who underwent allo-HSCT, finally 100 copies were selected as a threshold, and the result illustrated that WT1 levels below the threshold before the allo-HSCT, after allo-HSCT and during the post-allo-HSCT follow-up all provided better prognostic information. However, contrary to the majority of studies, a study [Citation71] demonstrated that in each cytogenetic category AML group, WT1 expression did not have a significant prognostic effect based on the cut-off value of 1000/104 K562 RNA levels. Therefore, to date, the method of cut-off value defined WT1 overexpression or normal expression has not yet been completely unified, which varied among different published research studies maybe belong to different ethnicities, distinct treatment regimens and different disease stages, etc. In the future, more efforts are required to explore this threshold criterion.
Immune therapy: WT as a potential antileukemia target
WT1 overexpression in hematological malignancies raised the interest of focusing on vaccines targeting WT1. Elisseeva et al. [Citation108] reported that in 73 patients with hematopoietic malignancies, IgM, IgG and IgM + IgG WT1 antibodies were detected in 40, 40 (54.8%) and 24 (32.8%), respectively. Whereas, the values for 43 healthy volunteers were 7 (16.2%), 2 (4.7%) and none (0%). Furthermore, immunoglobulin isotype class switching of WT1 antibodies from IgM to IgG occurred along with the disease progression of MDS. These findings provide a reference for immunotherapy using the WT1. Plantinga et al. [Citation109] described cord blood-derived dendritic cells (DC) that were loaded with a WT1 peptivator that could lyse primary pediatric AML cells by stimulating WT1-specific T-cells. This appears to provide a novel option for refractory AML patients. Another pre-clinical study [Citation110] indicated thata combination of HAGE and WT1 vaccine was more effective than either one alone. Stimulating the production of CD8 + WT1-specific cytotoxic T lymphocytes (CTLs) was the main antileukemia effect of the vaccine. A few studies demonstrated that CTLs specifically kill WT1-expressing leukemia cells, WT1-specific CD8 CTLs followed by low-dose IL-2 showed higher persistence of anti-leukemia [Citation111,Citation112]. Spira et al. [Citation113] found that DSP-7888 could stimulate CTLs in patients with recurrent or advanced malignancies, and finally selected 10.5 mg intradermally as the further clinical evaluation administration by dose-escalation comparison. Galinpepimut-S(GPS), a multivalent WT1 peptide vaccine, is also investigated in AML [Citation114]. Anguille et al. [Citation115] performed a II phase clinical study, WT1 mRNA-electroporated DC vaccines resulted in antileukemic response in high-risk AML patients including elderly patients. In a model study of murine leukemia, on-target off-tumor toxicity or off-target was not observed [Citation116]. Kiguchi et al. [Citation117] described that in elderly AML patients, compared with placebo, OCV-501 (a vaccine WT1 peptide-based) therapy prolonged the OS of immune responders, and the vaccine was safe and well tolerated, the major drug adverse reactions were injection-site reactions, also, immunological responses were of high individual variability. Slight suppression of WT1 mRNA levels in the OCV-501 group compared to those of the control, unfortunately, an additional 4.2 years (median) of follow-up showed that there was no significant difference or even the opposite between the two groups [Citation118]. Currently, WT1 vaccines are mostly based on DCs or peptides, Shirakawa et al. [Citation119] indicated that an oral WT1 vaccine by recombinant Bifidobacterium, was superior to a peptide-based vaccine.
In addition to the above-mentioned vaccine treatment, other approaches were also explored. T cell receptor (TCR) gene therapy and DNA vaccine have been introduced as approaches targeting WT1 [Citation120–124]. Based on a TCR-like antibody, a novel WT1 T-cell bispecific antibody, compared with HLA-A*02 RMF-specific T-cell clone, exhibited higher cytotoxicity against primary AML cells, and interestingly, enhanced cytotoxicity against primary AML cells when combined with immunomodulatory drug lenalidomide [Citation10]. WT1-specific CTLs’ infusion after allogeneic sibling donor HSCT, 8-year EFS was higher than their previous studies (50% vs less than 30%) [Citation125]. Additionally, immune therapy is investigated in high-risk multiple myeloma patients who underwent allo-HSCT [Citation126].
Generally,the immune therapy of WT1 is expected to eliminate residual malignant cells after SCT or after chemotherapy to reduce the relapse. However, with weak and short persistence of immune response, immune tolerance still needs to be improved. Presently, many studies are mainly focusing on improving the following aspects: Immune adjuvant, CD4+ T cell help-enhanced immune responses, the frequency and tumor-infiltrating capability of WT1-specific CTLs, route of drug administration, dosage, timeline and interval time. Recent pre-clinical or clinical studies are listed in .
Table 3. Trials associated with immune therapy of WT1 in recent years.
Closing remarks and future perspectives
In this review, we summarized the research progress associated with WT1 in hematologic malignancies in recent years. This may contribute to developing more specific therapy strategies to improve the treatment effect of hematologic malignancies. However, there is still much room for understanding the role of WT1. How do novel isoforms influence the development of hematological malignancies? How transform WT1 immune therapy into clinical application? More studies are required to resolve these issues.
Author contributions
Chunli Xiang wrote the draft and revised it. Jie Wu and Hui Yan collected original draft and designed the tables and figures. All the authors read and approved the submitted version.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Call KM, Glaser T, Ito CY, et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell. 1990;60(3):509–520. doi:10.1016/0092-8674(90)90601-A
- Yang L, Han Y, Suarez Saiz F, et al. A tumor suppressor and oncogene: the WT1 story. Leukemia. 2007;21(5):868–876. doi:10.1038/sj.leu.2404624
- Qi XW, Zhang F, Wu H, et al. Wilms’ tumor 1 (WT1) expression and prognosis in solid cancer patients: a systematic review and meta-analysis. Sci Rep. 2015;5:8924. doi:10.1038/srep08924
- Rampal R, Figueroa ME. Wilms tumor 1 mutations in the pathogenesis of acute myeloid leukemia. Haematologica. 2016;101(6):672–679. doi:10.3324/haematol.2015.141796
- Hou HA, Huang TC, Lin LI, et al. WT1 mutation in 470 adult patients with acute myeloid leukemia: stability during disease evolution and implication of its incorporation into a survival scoring system. Blood. 2010;115(25):5222–5231. doi:10.1182/blood-2009-12-259390
- Luo P, Jing W, Yi K, et al. Wilms’ tumor 1 gene in hematopoietic malignancies: clinical implications and future directions. Leuk Lymphoma. 2020;61(9):2059–2067. doi:10.1080/10428194.2020.1762884
- Awada H, Durmaz A, Gurnari C, et al. The genomic landscape of Wilms’ tumor 1 (WT1) mutant acute myeloid leukemia. Blood. 2020;136(Supplement 1):28.
- Ahmad EI, El-Akad GM, Ismail WI, et al. Study of Wilms’ tumor 1 gene expression in patients with acute myeloid leukemia. Egypt J Haematol. 2019;44(4):195–203. doi:10.4103/ejh.ejh_26_19
- Adnan-Awad S, Meligui YME, Salem SE, et al. Prognostic impact of WT-1 and survivin gene expression in acute myeloid leukemia patients. Clin Lab. 2019;65(4):435–444.
- Augsberger C, Hänel G, Xu W, et al. Targeting intracellular WT1 in AML with a novel RMF-peptide-MHC specific T-cell bispecific antibody. Blood. 2021;138(25):2655–2669. doi:10.1182/blood.2020010477
- Shandilya J, Roberts SG. A role of WT1 in cell division and genomic stability. Cell Cycle. 2015;14(9):1358–1364. doi:10.1080/15384101.2015.1021525
- Chau YY, Hastie ND. The role of Wt1 in regulating mesenchyme in cancer, development, and tissue homeostasis. Trends Genet. 2012;28(10):515–524. doi:10.1016/j.tig.2012.04.004
- Wilm B, Muñoz-Chapuli R. The role of WT1 in embryonic development and normal organ homeostasis. Meth Mol Biol. 2016; 1467:23–39. doi:10.1007/978-1-4939-4023-3_3
- Cilloni D, Renneville A, Hermitte F, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27(31):5195–5201. doi:10.1200/JCO.2009.22.4865
- Pritchard-Jones K, Fleming S, Davidson D, et al. The candidate Wilms’ tumour gene is involved in genitourinary development. Nature. 1990;346(6280):194–197. doi:10.1038/346194a0
- Ullmark T, Montano G, Gullberg U. DNA and RNA binding by the Wilms’ tumour gene 1 (WT1) protein + KTS and -KTS isoforms-from initial observations to recent global genomic analyses. Eur J Haematol. 2018;100(3):229–240. doi:10.1111/ejh.13010
- Haber DA, Sohn RL, Buckler AJ, et al. Alternative splicing and genomic structure of the Wilms tumor gene WT1. Proc Natl Acad Sci USA. 1991;88(21):9618–9622. doi:10.1073/pnas.88.21.9618
- Kramarzova K, Stuchly J, Willasch A, et al. Real-time PCR quantification of major Wilms’ tumor gene 1 (WT1) isoforms in acute myeloid leukemia, their characteristic expression patterns and possible functional consequences. Leukemia. 2012;26(9):2086–2095. doi:10.1038/leu.2012.76
- Klamt B, Koziell A, Poulat F, et al. Frasier syndrome is caused by defective alternative splicing of WT1 leading to an altered ratio of WT1 +/−KTS splice isoforms. Hum Mol Genet. 1998;7(4):709–714. doi:10.1093/hmg/7.4.709
- Potluri S, Assi SA, Chin PS, et al. Isoform-specific and signaling-dependent propagation of acute myeloid leukemia by Wilms tumor 1. Cell Rep. 2021;35(3):109010. doi:10.1016/j.celrep.2021.109010
- Kreidberg JA, Sariola H, Loring JM, et al. WT-1 is required for early kidney development. Cell. 1993;74(4):679–691. doi:10.1016/0092-8674(93)90515-R
- Duim SN, Goumans MJ, Kruithof BPT. WT1 in cardiac development and disease. In: van den Heuvel-Eibrink MM, editor. Wilms tumor. Brisbane, AU: Codon; 2016.
- Herzer U, Crocoll A, Barton D, et al. The Wilms tumor suppressor gene wt1 is required for development of the spleen. Curr Biol. 1999;9(15):837–840. doi:10.1016/S0960-9822(99)80369-8
- Ijpenberg A, Pérez-Pomares JM, Guadix JA, et al. Wt1 and retinoic acid signaling are essential for stellate cell development and liver morphogenesis. Dev Biol. 2007;312(1):157–170. doi:10.1016/j.ydbio.2007.09.014
- Cano E, Carmona R, Muñoz-Chápuli R. Wt1-expressing progenitors contribute to multiple tissues in the developing lung. Am J Phys Lung Cell Mol Physiol. 2013;305(4):L322–L332. doi:10.1152/ajplung.00424.2012
- Ariza L, Cañete A, Rojas A, et al. Role of the Wilms’ tumor suppressor gene Wt1 in pancreatic development. Dev Dyn. 2018;247(7):924–933. doi:10.1002/dvdy.24636
- Wagner KD, Wagner N, Vidal VP, et al. The Wilms’ tumor gene Wt1 is required for normal development of the retina. EMBO J. 2002;21(6):1398–1405. doi:10.1093/emboj/21.6.1398
- Ito K, Oji Y, Tatsumi N, et al. Antiapoptotic function of 17AA(+)WT1 (Wilms’ tumor gene) isoforms on the intrinsic apoptosis pathway. Oncogene. 2006;25(30):4217–4229. doi:10.1038/sj.onc.1209455
- Gu WY, Chen ZX, Hu SY, et al. Changes in expression of WT1 isoforms during induced differentiation of the NB4 cell line. Haematologica. 2005;90(3):403–405.
- Bissanum R, Lirdprapamongkol K, Svasti J, et al. The role of WT1 isoforms in vasculogenic mimicry and metastatic potential of human triple negative breast cancer cells. Biochem Biophys Res Commun. 2017;494(1-2):256–262. doi:10.1016/j.bbrc.2017.10.043
- Oji Y, Tatsumi N, Kobayashi J, et al. Wilms’ tumor gene WT1 promotes homologous recombination-mediated DNA damage repair. Mol Carcinog. 2015;54(12):1758–1771. doi:10.1002/mc.22248
- Yao YY, Chai XX, Gong C, et al. WT1 inhibits AML cell proliferation in a p53-dependent manner. Cell Cycle. 2021;20(16):1552–1560. doi:10.1080/15384101.2021.1951938
- Cao LX, Zhang J, Ren HJ, et al. Effect of down-regulation of miR-23b-3p on the differentiation of acute myeloid leukemia via Wilms cancer gene 1. J Biomater Tissue Eng. 2021;11(7):1377–1382. doi:10.1166/jbt.2021.2696
- Zhou B, Jin XH, Jin WW, et al. WT1 facilitates the self-renewal of leukemia-initiating cells through the upregulation of BCL2L2: WT1-BCL2L2 axis as a new acute myeloid leukemia therapy target. J Transl Med. 2020;18(1).
- Lyu Y, Lou J, Yang Y, et al. Dysfunction of the WT1-MEG3 signaling promotes AML leukemogenesis via p53-dependent and -independent pathways. Leukemia. 2017;31(12):2543–2551. doi:10.1038/leu.2017.116
- Liu JM, Li M, Luo W, et al. Curcumin attenuates Adriamycin-resistance of acute myeloid leukemia by inhibiting the lncRNA HOTAIR/miR-20a-5p/WT1 axis. Lab Invest. 2021;101(10):1308–1317. doi:10.1038/s41374-021-00640-3
- Christopher MJ, Katerndahl CDS, LeBlanc HR, et al. Tumor suppressor function of WT1 in acute promyelocytic leukemia. Haematologica. 2021;107(1):342–346. doi:10.3324/haematol.2021.279601
- Christopher M, Menssen A, Gang M, et al. Expression of the tumor suppressor WT1 Is induced By PML-rara in acute promyelocytic leukemia. Â. Blood. 2017;130:2508.
- Wagstaff M, Tsaponina O, Caalim G, et al. Crosstalk between β-catenin and WT1 signaling activity in acute myeloid leukemia. Haematologica. 2023;108(1):283–289. doi:10.3324/haematol.2021.280294
- Wang Y, Weng WJ, Zhou DH, et al. Wilms tumor 1 mutations are independent poor prognostic factors in pediatric acute myeloid leukemia. Front Oncol. 2021;11:632094. doi:10.3389/fonc.2021.632094
- Marceau-Renaut A, Duployez N, Ducourneau B, et al. Molecular profiling defines distinct prognostic subgroups in childhood AML: a report from the French ELAM02 study group. Hemasphere. 2018;2(1):e31. doi:10.1097/HS9.0000000000000031
- Wang S, Zhang YX, Huang T, et al. Mutation profile and associated clinical features in Chinese patients with cytogenetically normal acute myeloid leukemia. Int J Lab Hematol. 2018;40(4):408–418. doi:10.1111/ijlh.12802
- Liu H, Wang X, Zhang H, et al. Dynamic changes in the level of WT1 as an MRD marker to predict the therapeutic outcome of patients with AML with and without allogeneic stem cell transplantation. Mol Med Rep. 2019;20(3):2426–2432.
- Duléry R, Nibourel O, Gauthier J, et al. Impact of Wilms’ tumor 1 expression on outcome of patients undergoing allogeneic stem cell transplantation for AML. Bone Marrow Transplant. 2017;52(4):539–543. doi:10.1038/bmt.2016.318
- Deng DX, Wen JJ, Cheng YF, et al. Wilms’ tumor gene 1 is an independent prognostic factor for pediatric acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation. BMC Cancer. 2021;21(1):292. doi:10.1186/s12885-021-08022-0
- Qin YZ, Wang Y, Xu LP, et al. Subgroup analysis can optimize the relapse-prediction cutoff value for WT1 expression after allogeneic hematologic stem cell transplantation in acute myeloid leukemia. J Mol Diagn. 2020;22(2):188–195. doi:10.1016/j.jmoldx.2019.10.003
- Cho BS, Min GJ, Kim H, et al. Measurable residual disease assay with WT1 expression in acute myeloid leukemia Who underwent allogeneic hematopoietic stem cell transplantation; optimal threshold, time points, and candidates. Blood. 2018;132(Supplement 1):2759. doi:10.1182/blood-2018-99-110042
- Yoon JH, Kim HJ, Park SS, et al. Clinical outcome of autologous hematopoietic cell transplantation in adult patients with acute myeloid leukemia: who may benefit from autologous hematopoietic cell transplantation? Biol Blood Marrow Transpt. 2017;23(4):588–597. doi:10.1016/j.bbmt.2017.01.070
- Valkova V, Vydra J, Markova M, et al. WT1 gene expression in peripheral blood before and after allogeneic stem cell transplantation is a clinically relevant prognostic marker in AML – a single-center 14-year experience. Clin Lymphoma Myeloma Leuk. 2021;21(2):e145–e151. doi:10.1016/j.clml.2020.09.008
- Ido K, Nakamae M, Koh H, et al. The proportional relationship between pretransplant WT1 mRNA levels and risk of mortality after allogeneic hematopoietic cell transplantation in acute myeloid leukemia not in remission. Transplantation. 2019;103(10):2201–2210. doi:10.1097/TP.0000000000002662
- Candoni A, De Marchi F, Zannier ME, et al. High prognostic value of pre-allogeneic stem cell transplantation minimal residual disease detection by WT1 gene expression in AML transplanted in cytologic complete remission. Leuk Res. 2017;63:22–27. doi:10.1016/j.leukres.2017.10.010
- Candoni A, De Marchi F, Zanini F, et al. Predictive value of pretransplantation molecular minimal residual disease assessment by WT1 gene expression in FLT3-positive acute myeloid leukemia. Exp Hematol. 2017;49:25–33. doi:10.1016/j.exphem.2017.01.005
- Rautenberg C, Lauseker M, Kaivers J, et al. Prognostic impact of pretransplant measurable residual disease assessed by peripheral blood WT1-mRNA expression in patients with AML and MDS. Eur J Haematol. 2021;107(2):283–292. doi:10.1111/ejh.13664
- Mashima K, Oh I, Ikeda T, et al. Role of sequential monitoring of WT1 gene expression in patients with acute myeloid leukemia for the early detection of leukemia relapse. Clin Lymphoma Myeloma Leuk. 2018;18(12):e521–e527. doi:10.1016/j.clml.2018.07.298
- Ikeno S, Seto A, Sato T, et al. Retrospective analyses of wilms tumor gene-1 expression in acute myeloid leukemia and myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Tr. 2018;24(3):S234. doi:10.1016/j.bbmt.2017.12.218
- Rautenberg C, Pechtel S, Hildebrandt B, et al. Wilms’ tumor 1 gene expression using a standardized European LeukemiaNet-certified assay compared to other methods for detection of minimal residual disease in myelodysplastic syndrome and acute myelogenous leukemia after allogeneic blood stem cell transplantation. Biol Blood Marrow Transplant. 2018;24(11):2337–2343. doi:10.1016/j.bbmt.2018.05.011
- De Marchi F, Candoni A, Zannier ME, et al. Concomitant monitoring of WT1 and FLT3-ITD expression in FLT3-ITD acute myeloid leukemia patients: which should we trust as a minimal residual disease marker? Am J Hematol. 2017;92(5):E72–E74. doi:10.1002/ajh.24686
- Larisa G, Irina B, Rinat B, et al. Early reduction of WT1 transcript level during induction chemotherapy predicts for longer relapse-free and overall survival in de novo intermediate risk acute myeloid leukemia. Cl Lymph Myelom Leuk. 2018;18:S198.
- Hao Y, Cheng Y, Wu Q, et al. Combined usage of Wilms’ tumor gene quantitative analysis and multiparameter flow cytometry for minimal residual disease monitoring of acute myeloid leukemia patients after allogeneic hematopoietic stem cells transplantation. Exp Ther Med. 2018;15(2):1403–1409.
- Guolo F, Minetto P, Clavio M, et al. Combining flow cytometry and WT1 assessment improves the prognostic value of pre-transplant minimal residual disease in acute myeloid leukemia. Haematologica. 2017;102(9):e348–e351. doi:10.3324/haematol.2017.167254
- Giudice V, Gorrese M, Vitolo R, et al. WT1 expression levels combined with flow cytometry blast counts for risk stratification of acute myeloid leukemia and myelodysplastic syndromes. Biomedicines. 2021;9(4):387. doi:10.3390/biomedicines9040387
- Šálek C, Vydra J, Cerovská E, et al. WT1 expression in peripheral blood at diagnosis and during the course of early consolidation treatment correlates with survival in patients With intermediate and poor-risk acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2020;20(12):e998–e1009. doi:10.1016/j.clml.2020.07.014
- Du D, Zhu L, Wang Y, et al. Expression of WT1 gene and its prognostic value in patients with acute myeloid leukemia. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019;48(1):50–57.
- Martinez-Laperche C, Kwon M, Franco-Villegas AC, et al. Wilms tumor 1 gene expression levels improve risk stratification in AML patients. results of a multicentre study within the spanish group for molecular biology in haematology. Br J Haematol. 2018;181(4):542–546. doi:10.1111/bjh.14635
- Marjanovic I, Karan-Djurasevic T, Ugrin M, et al. Use of Wilms tumor 1 gene expression as a reliable marker for prognosis and minimal residual disease monitoring in acute myeloid leukemia With normal karyotype patients. Clin Lymphoma Myeloma Leuk. 2017;17(5):312–319. doi:10.1016/j.clml.2016.12.006
- Frairia C, Aydin S, Audisio E, et al. Post-remissional and pre-transplant role of minimal residual disease detected by WT1 in acute myeloid leukemia: a retrospective cohort study. Leuk Res. 2017;61:10–17. doi:10.1016/j.leukres.2017.08.008
- Lambert J, Lambert J, Thomas X, et al. Early detection of WT1 measurable residual disease identifies high-risk patients independently of transplantation in AML. Blood Adv. 2021;5(23):5258. doi:10.1182/bloodadvances.2021004322
- Yoon JH, Kim HJ, Kwak DH, et al. High WT1 expression is an early predictor for relapse in patients with acute promyelocytic leukemia in first remission with negative PML-RARa after anthracycline-based chemotherapy: a single-center cohort study. J Hematol Oncol. 2017;10(1):30. doi:10.1186/s13045-017-0404-4
- Cho BS, Shin SH, Lee SE, et al. Clinical outcomes of decitabine and the role of WT-1 expression as a surrogate prognostic marker for patients with elderly acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2018;18:S200–S201.
- Bani-Ahmad MA, Al-Sweedan SA, Al-Asseiri MA, et al. A proposed kinetic model for the diagnostic and prognostic value of WT1 and p53 in acute myeloid leukemia. Clin Lab. 2018;64(3):357–363.
- Hidaka D, Onozawa M, Hashiguchi J, et al. Wilms tumor 1 expression at diagnosis correlates With genetic abnormalities and polymorphism but is not independently prognostic in acute myelogenous leukemia: a hokkaido leukemia net study. Clin Lymphoma Myeloma Leuk. 2018;18(11):e469–e479. doi:10.1016/j.clml.2018.07.291
- Mitrovic M, Kostic T, Virijevic M, et al. The influence of Wilms’ tumor 1 gene expression level on prognosis and risk stratification of acute promyelocytic leukemia patients. Int J Lab Hematol. 2020;42(1):82–87. doi:10.1111/ijlh.13144
- Niktoreh N, Walter C, Zimmermann M, et al. Mutated WT1, FLT3-ITD, and NUP98-NSD1 fusion in various combinations define a poor prognostic group in pediatric acute myeloid leukemia. J Oncol. 2019;2019:1609128.
- Bhatnagar B, Kohlschmidt J, Orwick S, et al. Framework of clonal mutations concurrent with WT1 mutations in adults with acute myeloid leukemia: alliance for clinical trials in oncology study. Blood Adv. 2023;7(16):4671–4675. doi:10.1182/bloodadvances.2023010482
- El Hussein S, DiNardo CD, Takahashi K, et al. Acquired WT1 mutations contribute to relapse of NPM1-mutated acute myeloid leukemia following allogeneic hematopoietic stem cell transplant. Bone Marrow Transplant. 2022;57(3):370–376. doi:10.1038/s41409-021-01538-w
- Tien FM, Hou HA, Tang JL, et al. Concomitant WT1 mutations predict poor prognosis in acute myeloid leukemia patients with double mutant CEBPA. Haematologica. 2018;103(11):e510–e513. doi:10.3324/haematol.2018.189043
- Petiti J, Rosso V, Lo Iacono M, et al. Prognostic significance of The Wilms’ Tumor-1 (WT1) rs16754 polymorphism in acute myeloid leukemia. Leuk Res. 2018;67:6–11. doi:10.1016/j.leukres.2018.01.016
- Bedair HM, Attia MH, Gohar SF, et al. The prognostic impact of wilms tumor-1 polymorphism (rs16754) and human myeloid inhibitory C-type lectin-like receptor expression in cytogenetically normal-acute myeloid leukemia. Egyptian J. Med Human Genet. 2021;22(1).
- Ramzi M, Moghadam M, Cohan N. Wilms tumor-1 (WT1) rs16754 polymorphism and clinical outcome in acute myeloid leukemia. Turk J Haematol. 2019;36(1):67–68. doi:10.4274/tjh.galenos.2018.2018.0277
- Toogeh G, Ramzi M, Faranoush M, et al. Prevalence and prognostic impact of Wilms’ tumor 1 (WT1) gene, including SNP rs16754 in cytogenetically normal acute myeloblastic leukemia (CN-AML): an Iranian experience. Clin Lymphoma Myeloma Leuk. 2016;16(3):e21–e26. doi:10.1016/j.clml.2015.11.017
- Megías-Vericat JE, Herrero MJ, Rojas L, et al. A systematic review and meta-analysis of the impact of WT1 polymorphism rs16754 in the effectiveness of standard chemotherapy in patients with acute myeloid leukemia. Pharmacogenomics J. 2016;16(1):30–40. doi:10.1038/tpj.2015.80
- Tosello V, Mansour MR, Barnes K, et al. WT1 mutations in T-ALL. Blood. 2009;114(5):1038–1045. doi:10.1182/blood-2008-12-192039
- Bordin F, Piovan E, Masiero E, et al. WT1 loss attenuates the TP53-induced DNA damage response in T-cell acute lymphoblastic leukemia. Haematologica. 2018;103(2):266–277. doi:10.3324/haematol.2017.170431
- Roy U, Raghavan SC. Deleterious point mutations in T-cell acute lymphoblastic leukemia: mechanistic insights into leukemogenesis. Int J Cancer. 2021;149(6):1210–1220. doi:10.1002/ijc.33527
- Rodrigues GOL, Cramer SD, Winer HY, et al. Mutations that collaborate with IL-7Ra signaling pathways to drive ALL. Adv Biol Regul. 2021;80:100788. doi:10.1016/j.jbior.2021.100788
- Wang SJ, Wang C, Li T, et al. WT1 overexpression predicted good outcomes in adult B-cell acute lymphoblastic leukemia patients receiving chemotherapy. Hematology. 2020;25(1):118–124. doi:10.1080/16078454.2020.1735670
- Mikhael NL, Ibrahim AM, Helmy MA, et al. Wilms’ tumor gene (WT1) expression levels as prognostic marker in pediatric acute lymphoblastic leukemia. Egypt J Haematol. 2020;45(1):35–39. doi:10.4103/ejh.ejh_30_19
- Yu F, Shuang F, Jihong Z. Expression of WT1 gene and clinical significance in childhood acute B-cell lymphocytic leukemia of different immunophenotypes. Eur J Immunol. 2019;49:1537–1537.
- Huang QS, Wang JZ, Qin YZ, et al. Overexpression of WT1 and PRAME predicts poor outcomes of patients with myelodysplastic syndromes with thrombocytopenia. Blood Adv. 2019;3(21):3406–3418. doi:10.1182/bloodadvances.2019000564
- Du X, Geng S, Weng J, et al. WT1 mRNA expression is a good laboratory indicator for diagnosis of disease progression in MDS patients with stable disease. Leukemia Res. 2017;55:S133. doi:10.1016/S0145-2126(17)30342-9
- Zhang HY, Geng SX, Li MM, et al. Changes of WT1 mRNA expression level in patients with myelodysplastic syndromes after hypomethylating agents and its prognostic significance. Zhonghua Xue Ye Xue Za Zhi. 2019;40(5):417–421.
- Jiang Y, Liu L, Wang J, et al. The Wilms’ tumor gene-1 is a prognostic factor in myelodysplastic syndrome: a meta analysis. Oncotarget. 2018;9(22):16205–16212. doi:10.18632/oncotarget.23671
- Nagasaki J, Aoyama Y, Hino M, et al. Wilms tumor 1 (WT1) mRNA expression level at diagnosis Is a significant prognostic marker in elderly patients with myelodysplastic syndrome. Acta Haematol. 2017;137(1):32–39. doi:10.1159/000452732
- Maeda T, Matsuda A, Asou C, et al. Prognostic impact of peripheral blood Wilms’ tumour 1 mRNA expression levels in response to azacytidine in MDS: a single-centre analysis. Leuk Res Rep. 2021;15:100231.
- Rautenberg C, Germing U, Pechtel S, et al. Prognostic impact of peripheral blood WT1-mRNA expression in patients with MDS. Blood Cancer J. 2019;9(11):86. doi:10.1038/s41408-019-0248-y
- Anuchapreeda S, Rungrojsakul M, Tima S, et al. Co-activation of WT1 and AP-1 proteins on WT1 gene promoter to induce WT1 gene expression in K562 cells. Cell Signal. 2019;53:339–347. doi:10.1016/j.cellsig.2018.11.001
- Zhang L, Li Y, Li X, et al. Regulation of HtrA2 on WT1 gene expression under imatinib stimulation and its effects on the cell biology of K562 cells. Oncol Lett. 2017;14(3):3862–3868. doi:10.3892/ol.2017.6628
- Svensson E, Vidovic K, Lassen C, et al. Deregulation of the Wilms’ tumour gene 1 protein (WT1) by BCR/ABL1 mediates resistance to imatinib in human leukaemia cells. Leukemia. 2007;21(12):2485–2494. doi:10.1038/sj.leu.2404924
- Ullmark T, Montano G, Järvstråt L, et al. Anti-apoptotic quinolinate phosphoribosyltransferase (QPRT) is a target gene of Wilms’ tumor gene 1 (WT1) protein in leukemic cells. Biochem Biophys Res Commun. 2017;482(4):802–807. doi:10.1016/j.bbrc.2016.11.114
- El-Menoufy MAM, Ahmed MAR. Wilms’ tumor gene 1 expression can predict sudden disease progression to blast crisis in patients with chronic myeloid leukemia receiving imatinib therapy. Egypt J Haematol. 2018;43(1):38–43. doi:10.4103/ejh.ejh_5_18
- Makishima H, Yoshizato T, Yoshida K, et al. Dynamics of clonal evolution in myelodysplastic syndromes. Nat Genet. 2017;49(2):204–212. doi:10.1038/ng.3742
- Alexander TB, Gu Z, Iacobucci I, et al. The genetic basis and cell of origin of mixed phenotype acute leukaemia. Nature. 2018;562(7727):373–379. doi:10.1038/s41586-018-0436-0
- Cilloni D, Renneville A, Hermitte F, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27(31):5195–5201. doi:10.1200/JCO.2009.22.4865
- Dijk J, Knops G, Locht L, et al. Abnormal WT1 expression in the CD34-negative compartment in myelodysplastic bone marrow. Br J Haematol. 2002;118(4):1027–1033. doi:10.1046/j.1365-2141.2002.03728.x
- Cilloni D, Messa F, Arruga F, et al. Early prediction of treatment outcome in acute myeloid leukemia by measurement of WT1 transcript levels in peripheral blood samples collected after chemotherapy. Haematologica. 2008;93(6):921–924. doi:10.3324/haematol.12165
- Cho BS, Min GJ, Park SS, et al. WT1 measurable residual disease assay in patients With acute myeloid leukemia Who underwent allogeneic hematopoietic stem cell transplantation: optimal time points, thresholds, and candidates. Biol Blood Marrow Transp. 2019;25(10):1925–1932. doi:10.1016/j.bbmt.2019.05.033
- Nomdedéu JF, Esquirol A, Carricondo M, et al. Bone marrow WT1 levels in allogeneic hematopoietic stem cell transplantation for acute myelogenous leukemia and myelodysplasia: clinically relevant time points and 100 copies threshold value. Biol Blood Marrow Transplant. 2018;24(1):55–63. doi:10.1016/j.bbmt.2017.09.001
- Elisseeva OA, Oka Y, Tsuboi A, et al. Humoral immune responses against Wilms tumor gene WT1 product in patients with hematopoietic malignancies. Blood. 2002;99(9):3272–3279. doi:10.1182/blood.V99.9.3272
- Plantinga M, Presti L, de Haar V, et al. Clinical grade production of wilms’ tumor-1 loaded cord blood-derived dendritic cells to prevent relapse in pediatric AML after cord blood transplantation. Front Immunol. 2020;11:559152. doi:10.3389/fimmu.2020.559152
- Almshayakhchi R, Nagarajan D, Vadakekolathu J, et al. A novel HAGE/WT1-ImmunoBody(R) vaccine combination enhances anti-tumour responses when compared to either vaccine alone. Front Oncol. 2021;11:636977. doi:10.3389/fonc.2021.636977
- Gao LQ, Bellantuono I, Elsasser A, et al. Selective elimination of leukemic CD34 + progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 2000;95(7):2198–2203. doi:10.1182/blood.V95.7.2198
- Chapuis AG, Ragnarsson GB, Nguyen HN, et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med. 2013;5(174):174ra127. doi:10.1126/scitranslmed.3004916
- Spira A, Hansen AR, Harb WA, et al. Multicenter, open-label, phase I study of DSP-7888 dosing emulsion in patients with advanced malignancies. Target Oncol. 2021;16(4):461–469. doi:10.1007/s11523-021-00813-6
- Maslak PG, Dao T, Bernal Y, et al. Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia. Blood Adv. 2018;2(3):224–234. doi:10.1182/bloodadvances.2017014175
- Anguille S, Van de Velde AL, Smits EL, et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood. 2017;130(15):1713–1721. doi:10.1182/blood-2017-04-780155
- Minagawa H, Hashii Y, Nakajima H, et al. Enhanced antitumor activity of a novel, oral, helper epitope-containing WT1 protein vaccine in a model of murine leukemia. BMC Cancer. 2023;23(1):167. doi:10.1186/s12885-023-10547-5
- Kiguchi T, Yamaguchi M, Takezako N, et al. Efficacy and safety of Wilms’ tumor 1 helper peptide OCV-501 in elderly patients with acute myeloid leukemia: a multicenter, randomized, double-blind, placebo-controlled phase 2 trial. Cancer Immun Immunr. 2022;71(6):1419–1430. doi:10.1007/s00262-021-03074-4
- Naoe T, Saito A, Hosono N, et al. Immunoreactivity to WT1 peptide vaccine is associated with prognosis in elderly patients with acute myeloid leukemia: follow-up study of randomized phase II trial of OCV-501, an HLA class II-binding WT1 polypeptide. Cancer Immunol Immunother. 2023;72(8):2865–2871. doi:10.1007/s00262-023-03432-4
- Shirakawa T, Kitagawa K. Antitumor effect of oral cancer vaccine with Bifidobacterium delivering WT1 protein to gut immune system is superior to WT1 peptide vaccine. Hum Vaccin Immunother. 2018;14(1):159–162. doi:10.1080/21645515.2017.1382787
- Tawara I, Kageyama S, Miyahara Y, et al. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood. 2017;130(18):1985–1994. doi:10.1182/blood-2017-06-791202
- Chapuis AG, Egan DN, Bar M, et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med. 2019;25(7):1064–1072. doi:10.1038/s41591-019-0472-9
- van Amerongen RA, Hagedoorn RS, Remst DFG, et al. WT1-specific TCRs directed against newly identified peptides install antitumor reactivity against acute myeloid leukemia and ovarian carcinoma. J Immunother Cancer. 2022;10(6):e004409. doi:10.1136/jitc-2021-004409
- Walters JN, Ferraro B, Duperret EK, et al. A novel DNA vaccine platform enhances neo-antigen-like T cell responses against WT1 to break tolerance and induce anti-tumor immunity. Mol Ther. 2017;25(4):976–988. doi:10.1016/j.ymthe.2017.01.022
- Chaise C, Buchan SL, Rice J, et al. DNA vaccination induces WT1-specific T-cell responses with potential clinical relevance. Blood. 2008;112(7):2956–2964. doi:10.1182/blood-2008-02-137695
- Kim HJ, Sohn HJ, Hong JA, et al. Post-transplant immunotherapy with WT1-specific CTLs for high-risk acute myelogenous leukemia: a prospective clinical phase I/II trial. Bone Marrow Transplant. 2019;54(6):903–906. doi:10.1038/s41409-018-0383-2
- Koehne G, Devlin S, Landau H, et al. WT1 heteroclitic epitope immunization following autologous stem cell transplantation induces WT1-specific immune responses and improves survival in patients with high-risk multiple myeloma. J Clin Oncol. 2017;35:8016. doi:10.1200/JCO.2017.35.15_suppl.8016
- Miwa H, Beran M, Saunders GF. Expression of the Wilms’ tumor gene (WT1) in human leukemias. Leukemia. 1992;6(5):405–409.
- Oji Y, Ogawa H, Tamaki H, et al. Expression of the Wilms’ tumor gene WT1 in solid tumors and its involvement in tumor cell growth. Jpn J Cancer Res. 1999;90(2):194–204. doi:10.1111/j.1349-7006.1999.tb00733.x
- Oka Y, Udaka K, Tsuboi A, et al. Cancer immunotherapy targeting Wilms’ tumor gene WT1 product. J Immunol. 2000;164(4):1873–1880. doi:10.4049/jimmunol.164.4.1873
- Oka Y. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci USA. 2004;101(38):13885–13890. doi:10.1073/pnas.0405884101
- Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–5337. doi:10.1158/1078-0432.CCR-09-0737
- Wang BH, Guan W, Lv N, et al. Genetic features and efficacy of decitabine-based chemotherapy in elderly patients with acute myeloid leukemia. Hematology. 2021;26(1):371–379. doi:10.1080/16078454.2021.1921434
- Wang XR, Chang Y, Yuan XY, et al. Overexpressed WT1 exhibits a specific immunophenotype in intermediate and poor cytogenetic risk acute myeloid leukemia. Ann Hematol. 2020;99(2):215–221. doi:10.1007/s00277-019-03808-6
- Ueda Y, Ogura M, Miyakoshi S, et al. Phase 1/2 study of the WT1 peptide cancer vaccine WT4869 in patients with myelodysplastic syndrome. Cancer Sci. 2017;108(12):2445–2453. doi:10.1111/cas.13409