Clinical trials evaluate the intervention of treatments to find safer and more effective ways to prevent, detect, and treat diseases. Industry sponsorship in clinical trials saw its first cooperation with academic medicine in 1980, with the passage of the Bayh-Dole Act [Citation1]. With the advent of large corporations creating a source of funding to further research, this partnership saw the discovery and development of numerous drugs and devices that have improved the lives of countless individuals [Citation2]. However, most clinical trials are funded by pharmaceutical companies with financial stakes in the product being evaluated and the scientists conducting research during these trials receive monetary compensation [Citation3]. This poses several concerns about the biases that might play while conducting these trials. Unlike publicly funded studies, the industry-supported realm of clinical trials has led to numerous terminations for financial rather than scientific or ethical reasons [Citation4,Citation5]. The emphasis on monetary gain from these trials may in fact be the reason why these trials are not looking into the ethics of excluding or including certain populations.
Exclusion criteria in trials help ensure the safety of vulnerable patients from the adversity of trial products and increase the likelihood of reliable and reproducible results. Investigators are tasked with defining their criteria and explaining how the inclusion or exclusion of these criteria affects the external validity [Citation6]. Using broad inclusions and less-restrictive exclusion criteria helps studies provide more information on the product’s effects on the population it is intended for. On the other hand, restricted exclusion criteria may lead to less treatment option for the excluded populations. There needs to be a fine line between obtaining more information and the product’s safety and generalizability. Investigators in commercial products tend to use more restrictive exclusion criteria to yield favorable outcomes.
People that are infected with the Human Immunodeficiency Virus (HIV) are nowadays living longer due to effective antiviral therapy, however, they are at risk of not being included in clinical trials treatment, as novel cancer therapeutics have historically excluded patients with HIV [Citation7]. When accounting for treatments, effective treatment provides improvement to the general population as a whole [Citation8] not just specific subpopulations.
In a similar story, as for the scope of hepatitis, there is a significant burden in the impact of chronic infections in cancer patients [Citation9]. With 70% of cancer clinical trials investigating new drug applications excluding chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infections [Citation10], cancer patients with hepatitis infections are being prevented from receiving proper treatment. As a result, trial accrual is limited, results are not generalizable, and HCV patients’ access are hampered [Citation11].
Over- arbitrarily adding inclusion criteria restricts the cancer patients’ participation. This eventually causes the participation of relatively healthy individuals, who are not a valid representation of the real-world population. It was observed that during the pandemic there was little evidence of reduction in the enrollment in cancer clinical trials [Citation12]. Therefore, the exclusion criteria of cancer clinical trials were so robust before the pandemic that Coronavirus disease-19 (COVID-19) criteria in the exclusion criteria did not substantially affect the enrollments.
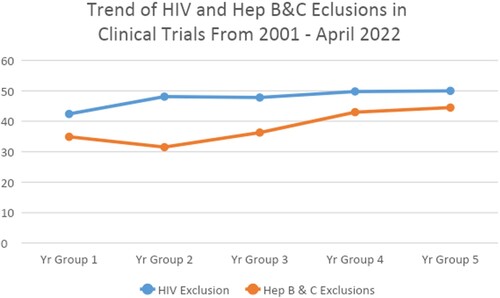
Our study pulled 4645 myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) trials from clinicaltrials.gov to investigate multiple cross-sectional relationships between variables. We used the NCT number, outcome measures, start date, status, phase of trials, sponsors, HIV exclusion, and hepatitis exclusion, to find out the relationships between trials and how the criteria are distributed across the MDS and AML clinical trials. We considered MDS and AML to optimize the number of data, making it effective and easy to manage, while also representing cancer patients. A descriptive analysis was carried out to explain the distribution of HIV and hepatitis exclusions across the cancer trials. To observe the trend of HIV and Hepatitis B & C (Hep B&C) exclusion criteria changes over time, we divided the total number of studies into five groups, Group 1 2001–2005; Group 2 2006–2010; Group 3 2011–2015; Group 4 2016–2020; and Group-5 2021–March 2022.
We examined 3160 clinical studies found on the ‘clinicaltrials.gov.’ The database provided information, that was later used in Excel, ranging from NCT number (given it to specify a clinical trial) to the phase that the trial finished in or is currently in. Excel then categorized the industry that the trials fell under (academic, research, etc.) along with all the other parameters used in this study, specifically whether the clinical trial excluded patients with HIV, Hepatitis B, or Hepatitis C. Among them, 817 had academic collaborations, and academic institutions solely ran 373. Industry collaborations had 1322, and 683 were carried out solely by industries. Seven hundred nineteen had NCI/NIH collaborations and 99 had NCI/NIH only, 1675 had research organization collaborations and 702 were solely carried out by research organizations. The highest number of studies were found in phase 2, 1154 (40%), followed by phase 1, 813 (28%), phases 1 and 2 were 488 (17%), phase 3, 309 (11%), phases 2 and 3 were 60 (2%), and phase 4 was 51 (2%). Overall, a positive status in HIV was excluded by 1466 (51%) studies, 1164 (40%) studies excluded hepatitis (both B & C), and 1038 (36%) studies excluded both Hepatitis and HIV. Phase-wise, phase-1 studies excluded the highest (55%) HIV and phase-4 excluded the lowest, 37%. Phase-1 studies excluded the highest (45%) Hep B&C and phase-2&3 (studies which have both 2 and 3 together) excluded the lowest, 22%. Studies with only MDS excluded 53% HIV and 43% Hep B&C compared to 46% HIV and 46% Hep B&C of the studies with only AML. In sponsor wise exclusions, industry sponsored studies excluded HIV, Hep B&C, and both HIV and Hep B&C in a significant number compared to academic sponsored studies (50.9 vs 43.7, 41.9 vs 34.1, and 37.9 vs 31.4 respectively) (). In addition, industry-sponsored studies excluded a significant number of both HIV and Hep B&C combined compared to NCI/NIH-sponsored studies (37.5% vs 27.3% respectively, p = 0.0484).
Table 1. Two independent sample proportional comparison.
Over the years, there has been an observed higher trend of excluding HIV and Hep B&C patients from clinical trials, from 42% to 50%, and a similar trend is observed in Hep B&C exclusions ().
A study published in the Journal of Clinical Oncology in 2017 found that only five (1.7%) of 250 protocols allowed enrollment of HIV-positive patients with stable disease and/or adequate CD4+ T-cell counts [Citation13]. A review of HIV eligibility criteria in recent industry-supported studies leading to successful new drug applications conducted by the working group found that zero of 46 studies contained inclusion criteria for patients with HIV, 30 studies contained exclusion criteria, and nine studies discussed general exclusion of patients with active infection but did not specify HIV infection [Citation3]. People infected with HIV are experiencing average life expectancies due to the improvements in HIV treatment in the past 20 years [Citation14,Citation15]. Advancements in HIV and hepatitis treatments have significantly improved patient outcome, in the time periods analyzed, yet rather than this mirror these large gains, studies have rather become more conservative and exclusionary with time, creating an irrational and damaging response. Our study found that industry sponsored AML and MDS clinical trials excluded HIV, Hep B, and Hep C patients significantly more than their academic-sponsored counterparts. Furthermore, compared to NCI/NIH AML and MDS trials, industry-sponsored trials had higher amounts of criteria excluding all three (HIV, Hep B, and Hep C) conditions, entirely.
HIV recent advancements in anti-retroviral therapy (ART) have been seen with the advent of long-lasting ART (LA-ART). HIV infection rates and AIDS-related mortality have declined due to ART. In 2020, 680,000 people died due to an AIDS-related illness, a figure that has decreased 50% since 2010 [Citation16]. These treatments are made possible because of testing from clinical trials. The use of direct-acting antivirals (DAA’s) has led to many advancements in HCV antiviral therapy, to where 90% of HCV infections are cured by hepatitis treatment, as per the Center of Disease Control (CDC) [Citation17]. Excluding HIV patients from cancer clinical studies has often been justified by concerns over potential safety issues and interactions between HIV and study variables. However, such exclusions may not be justified given the advancements in HIV treatment and the potential benefits of including this population. Modern antiretroviral therapies, such LA-ART, have significantly improved the health and immune function of HIV patients, reducing the likelihood of complications. Rigorous medical supervision can effectively mitigate potential interactions between HIV medications and study interventions. Furthermore, excluding HIV patients may lead to a lack of representation of diverse patient populations, limiting the generalizability of study findings. Inclusive research practices, with proper informed consent and participant protections, can address these concerns while enabling a more comprehensive understanding of the effects of cancer treatments on individuals with HIV. Therefore, excluding HIV patients from cancer clinical studies based solely on safety concerns and potential interactions is not justified and may hinder the progress of medical research and the improvement of patient care.
Constantly adding many criteria such as age, diabetes, HIV, heart disease, hepatitis, or even relatively unhealthy physical conditions, without scientific or clinical reason, is keeping many patients out of trials which are ultimately costing the trial time, money, data integrity, and consistency with the real world. Reducing these arbitrary barriers are important to increase enrollment in clinical trials.
References
- Bayh-Dole Act. 1980: United States of America. p. 3018.
- Okike K, Kocher MS, Mehlman CT, et al. Industry-sponsored research. Injury. 2008;39(6):666–680. doi: 10.1016/j.injury.2008.02.013
- Chopra SS. Industry funding of clinical trials: benefit or bias? JAMA. 2003;290(1):113–114. doi: 10.1001/jama.290.1.113
- Lièvre M, Ménard J, Bruckert E, et al. Premature discontinuation of clinical trial for reasons not related to efficacy, safety, or feasibility commentary: early discontinuation violates Helsinki principles. BMJ. 2001;322(7286):603–606. doi: 10.1136/bmj.322.7286.603
- Psaty BM, Rennie D. Stopping medical research to save money: a broken pact with researchers and patients. JAMA. 2003;289(16):2128–2131.
- Patino CM, Ferreira JC. Inclusion and exclusion criteria in research studies: definitions and why they matter. J Bras Pneumol. 2018;44(2):84.
- Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint research statement. J Clin Oncol. 2017;35(33):3737. doi: 10.1200/JCO.2017.73.7916
- National Research Council. The prevention and treatment of missing data in clinical trials. Washington (DC): National Academies Press; 2010.
- Torres HA, Shigle TL, Hammoudi N, et al. The oncologic burden of hepatitis C virus infection: a clinical perspective. CA Cancer J Clin. 2017;67(5):411–431.
- Jin S, Pazdur R, Sridhara R. Re-evaluating eligibility criteria for oncology clinical trials: analysis of investigational new drug applications in 2015. J Clin Oncol. 2017;35(33):3745–3752. doi: 10.1200/JCO.2017.73.4186
- Torres HA, Pundhir P, Mallet V. Hepatitis C virus infection in patients with cancer: impact on clinical trial enrollment, selection of therapy, and prognosis. Gastroenterology. 2019;157(4):909–916. doi: 10.1053/j.gastro.2019.01.271
- Bakouny Z, Labaki C, Bhalla S, et al. Oncology clinical trial disruption during the COVID-19 pandemic: a COVID-19 and cancer outcomes study. Ann Oncol. 2022;33(8):836–844. doi: 10.1016/j.annonc.2022.04.071
- Negri F, Missale G, Antoni AD, et al. Hepatocellular cancer therapy in patients with HIV infection: disparities in cancer care, trials enrolment, and cancer-related research. Transl Oncol. 2021;14(9):101153. doi: 10.1016/j.tranon.2021.101153
- Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355
- Lewden C, Bouteloup V, De Wit S, et al. All-cause mortality in treated HIV-infected adults with CD4 ≥ 500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol. 2012;41(2):433–445. doi: 10.1093/ije/dyr164
- Mahy MI, Sabin KM, Feizzadeh A, et al. Progress towards 2020 global HIV impact and treatment targets. J Int AIDS Soc. 2021;24:e25779. doi: 10.1002/jia2.25779
- Zhang X, Direct anti-HCV agents. Acta Pharm Sin B. 2016;6(1):26–31. doi: 10.1016/j.apsb.2015.09.008