ABSTRACT
Introduction:
There are conflicting results concerning the outcome of patients after an allogeneic hematopoietic stem cell transplantation (allo-HSCT) who required treatment in the intensive care unit (ICU). The aim of this study was to evaluate the outcome and prognostic parameters in terms of patient survival after allo-HSCT and admission to the ICU within the first 30 days after transplantation.
Methods:
Patients after allo-HSCT, who were ≥18 years and admitted to the ICU after the initiation of conditioning therapy and within the first 30 days after allo-HSCT at the University Hospital of Bonn between January 2017 and April 2021, were analysed retrospectively. Baseline data, laboratory parameters, established scoring systems, vital parameters, and outcome were collected.
Results:
44 patients (median age of 63 years) were analysed. The 90-day survival rate was 50% (N = 22) and the 1-year survival rate was 27% (N = 12). The 90-day and 1-year survival rates of patients who required MV were 38% (N = 13) and 18% (N = 6). There was a significant correlation between increased mortality and an APACHE-Score ≥20 (p = 0.03), a SAPS-II-Score ≥60 (p = 0.04) and a SOFA-Score ≥9 (p = 0.03). Invasive mechanical ventilation (p = 0.05) and vasopressor support (p = 0.03) showed a negative correlation with the outcome.
Conclusion:
This study found several parameters (APACHE-II-Score, SAPS-II-Score, SOFA-Score, MV and vasopressor support) associated with increased mortality after allo-HSCT and admission to the ICU. The outcome of allo-HSCT patients admitted to the ICU is not as poor as previously reported. Even older patients under long-term ventilation may benefit from intensive care therapy.
Introduction
The option of an allogeneic haematopoietic stem cell transplantation (allo-HSCT) extends the potentially curative treatment options for malignant and non-malignant haematologic diseases [Citation1]. Since 2000, various studies have shown that about 10–20% of all patients receiving an allo-HSCT require intensive care treatment during the peritransplant setting [Citation2,Citation3]. In the 1980s/1990s the mortality rate was up to 90%, especially for patients requiring invasive mechanical ventilation (MV) [Citation4–6]. In the last decades, the outcome of patients with allo-HSCT has improved significantly. This progress is based on enhanced transplant procedures (reduced-intensity conditioning regimes), better antimicrobial, antifungal therapies, and improved intensive care treatment (for example, non-invasive ventilation or lung protective ventilation strategies) [Citation7–10].
The most frequent cause of admission to the intensive care unit (ICU) in the peritransplant setting is sepsis, followed by respiratory failure in need of non-invasive respiratory supports such as high flow nasal cannula or continuous positive airway pressure (CPAP), MV, RRT, and circulatory instability requiring vasopressors [Citation11]. Overall, ICU admission after allo-HSCT is associated with a significant increase in mortality. In the current literature, the one-year survival of allogeneic stem cell-transplanted patients in the ICU varies between 5% and 33% [Citation12–15]. Most studies do not distinguish between very early ICU and late ICU in allo-HSCT. However, these collectives are different due to their chemotherapy side effects, immune suppression, blood count regeneration, and potential GvHD. To date, data on patients treated in the ICU in the peritransplant setting are scarce.
This study provides a retrospective analysis of patients after allo-HSCT who required treatment in the ICU after the initiation of conditioning therapy and within the first 30 days after allo-HSCT treatment at a single University Hospital in Germany. The study focusses on the patient characteristics admitted to the ICU in the peritransplant setting in correlation with the outcome. The aim of this study was to identify parameters that could serve as biomarkers that are associated with the outcome. This could ultimately support clinical decision-making.
Parts of this work were presented as a lecture at the 2022 annual meeting of the German Society of Hematology and Oncology in Vienna [Citation16].
Materials and methods
Study population
Data from all patients aged 18 years or older admitted to the ICU in the peritransplant period after allo-HSCT at the University Hospital of Bonn between January 1, 2017, and April 31, 2021, were retrospectively analysed. The peritransplant period was defined from the beginning of conditioning therapy until 30 days after allo-HSCT. For patients admitted to the ICU more than once, only the first stay was considered.
Data collection
Patient characteristics, laboratory parameters (such as sodium, potassium, creatinine, bilirubin, lactate dehydrogenase, C-reactive Protein, procalcitonin, albumin, urea, haematocrit, platelets, and lactate), different scoring systems such as SOFA-Score, qSofa-Score, EBMT, HCT-CI, PICAT-Score, SAPS-II-Score, APACHE-II-Score, allo-HSCT-related information, cause for ICU admission, the appearance of GvHD, and intensive care procedures (such as non-invasive respiratory support, MV, tracheostomy, RRT or the use of vasopressors) were collected ().
Table 1. Prognostic scores.
Table 2. Intensive care parameters (during ICU stay).
Table 3. Laboratory parameters (during the first 24 h after admission).
Concerning the diagnosis of sepsis, the definition from 2016 was used [Citation17].
Scoring systems
In the following, the scoring systems are briefly presented and explained.
EBMT: The European Group for Blood and Marrow Transplantation Score assesses the individual risks and opportunities of haemato-oncological stem cell transplantation for patients. The score is made up of 5 factors (age of the patient, stage of the disease, donor type and donor-recipient and time for diagnosis). The lowest risk is 0 point and the highest risk is 7 points [Citation18].
HCT-CI: The Haematopoietic Cell Transplantation-specific Comorbidity Index identifies comorbidities before allogeneic stem cell transplantation to evaluate the risk of the allogeneic transplantation. It is based on the CCI (Charlson Comorbidity Index) but includes organ function tests such as blood tests, echocardiography or body plethysmography [Citation19,Citation20].
SOFA: The Sepsis-Related Organ Failure Assessment Score (SOFA) assesses different organ systems (blood, blood pressure, liver, kidney and neurology) to evaluate the mortality risk of patients in the ICU [Citation21].
qSOFA-Score: The quick Sepsis-Related Organ Failure Assessment Score (qSOFA) is the simplified form of the SOFA score and is used for the initial assessment in the emergency room or ICU [Citation22].
PICAT-Score: The prognostic index for intensive care after allogeneic haematopoietic stem cell transplantation (PICAT) evaluates the outcome for these patients by different parameters (time to ICU, LDH, bilirubin, albumin, respiratory failure as the reason for ICU, PT-INR, myeloablative conditioning, age of the patient, HCT-CI) by adding coefficients to calculate the individual risk [Citation23].
SAPS-II-Score: The Simplified Acute Physiology Score is used to assess the physiological health status and general condition of the patient. The following parameters (neurology, blood pressure, age of the patient, chronic diseases, heart rate, body temperature, oxygenation index during ventilation PaO2/FiO2, urine volume and laboratory values (serum urea, leucocytes, potassium, sodium, bilirubin and bicarbonate)) are included in the calculation. The score is calculated from 0 to 8.63 as the highest risk [Citation24].
APACHE-II-Score: The Acute Physiology And Chronic Health Evaluation (APACHE) assesses the severity of the disease and the likelihood of the survival of the patients with different values (body temperature, arterial mean pressure, heart rate, breathing rate, oxygenation, arterial pH, sodium, potassium, bicarbonate, leucocytes, serum creatinine and haematocrit, neurology, chronic Health score, and the age of the patient) [Citation25].
Statistical analyses
Univariate analyses with the Kaplan–Meier method were performed. Factors with a p-value <0.05 were significant. A multivariate regression analysis was not possible due to the limited number of patients.
Patients who survived were censored in April 2022. If patients were lost to follow-up, they were censored on the last day of physician contact.
The baseline characteristics were reported by using median and range. The amount was used by N and percentage. OS analyses were done by Kaplan–Maier and log-rank tests.
Statistical analyses were performed by SPSS Statistics (IBM Corp., Armonk, NY) version 27 for MAC OS.
Results
Patient characteristics
Data of all patients after allo-HSCT, ≥ 18 years, admitted to the ICU, within the first 30 days after transplantation at the University Hospital of Bonn between January 2017 and April 2021, were analysed retrospectively. Baseline characteristics of all patients are summarized in . A total of 44 (26%) out of 170 patients who received an allo-HSCT between January 2017 and April 2021 required intensive care support in the peritransplant period.
Table 4. Patient characteristics admitted to the ICU.
Twenty-six patients (59%) in the cohort were male with a median age of 63 years (range 22–75 years). Acute leukaemia (AML and ALL) were the most common indications for allo-HSCT (25 patients; 57%). Less frequent indications were myelodysplastic syndrome (8 patients; 18%), and chronic myelomonocytic leukaemia (3 patients; 7%). The median HCT-CI score at the initiation of conditioning therapy was 3 (range 0–12). The median EBMT score was 5 (range 2–6) ( and ).
Intensive care support
The most frequent symptoms leading to ICU admission were respiratory decompensation or failure (n = 27, 61%), followed by neurologic abnormalities (n = 8; 18%), and cardiac decompensation (n = 7, 16%). Cardiopulmonary resuscitation (CPR) was the reason for ICU admission for two patients (5%). A total of 36 patients (82%) were admitted due to septic symptoms. 22 patients had a pneumonia-induced sepsis. Three patients had a urinary tract infection (7%), four patients (9%) had a sepsis due to neutropenic enterocolitis, two patients (5%) were diagnosed with a systemic fungal sepsis, and five patients (11%) had a multifactorial sepsis. (). The median time between allo-HSCT and admission to the ICU was 5 days (range −1 days until +30 days). The median duration of stay in the ICU was 24 days (range 3–105 days). Considering the different supports a total of 34 patients (77%) required invasive MV, one patient required extracorporeal membrane oxygenation (ECMO) (2%), 13 patients (30%) received a tracheostomy, 33 patients (75%) required RRT, and 37 patients (84%) vasopressors.
Table 5. ICU characteristics.
Out of the 34 patients under invasive MV, a total of 24 patients (55%) had respiratory failure, eight patients (18%) had an impairment of vigilance, two patients (5%) were already intubated during CPR. 23 (52%) of all patients received non-invasive respiratory support (high flow nasal cannula or continuous positive airway pressure (CPAP) or both). Out of this group, 20 patients (87%) were switched to invasive mechanical ventilation because of NIV failure. Three of these patients (13%) did not need invasive MV after NIV ().
Out of the patients, who were treated with vasopressors, 20 patients (54%) had one single vasopressor (in this case noradrenalin), eleven patients (30%) had two different vasopressors (basically noradrenalin and vasopressin) and six patients (16%) had three different vasopressors (mainly noradrenaline, vasopressin and suprarenin or dobutamine).
Concerning the total dosage of vasopressors, eleven patients (30%) had a maximum dose of 30 µg/min noradrenaline, eight patients (22%) received 30–70 µg/min noradrenaline and 18 patients (48%) had more than 70 µg/min noradrenaline.
Parameters on admission to the intensive care unit
The following parameters were collected within the first 24 h after admission to ICU. The median GCS was 15 (range 3–15), the median qSOFA score was 2 (range 0–3), the median SOFA score was 10 (range 4–18), the median PICAT score was 3.1 (range 0.4–5.5), the median SAPS II score was 60 (range 28–114) and the median APACHE II was 20 (range 11–38). The following laboratory parameters were also obtained within the first 24 h after admission to ICU. The median lactate (mmol/l) was 1.4 (range 0.7–7.9 mmol), the median bilirubin (mg/dl) was 1.5 (range 0.2–30), the median creatinine (mg/dl) was 1.3 (range 0.4–3.4), the median albumin was 28 (range 9.5–58), the median c-reactive protein (CRP) (mg/dl) was 144 (range 21–466), and the median procalcitonin (PCT) (ng/ml) was 1.6 (range 0.2–163) ().
As this study exclusively focuses on the peritransplant period, all patients were aplastic and highly immunosuppressed. There was one patient with serious graft versus host reactions (GvHD) in our cohort. The most common GVHD was mild cutaneous GvHD I-II° (n = 10; 23%).
Survival and early mortality during intensive care
The median duration of treatment in the ICU was 24 days (range 3–105 days). Thirteen patients (30%) died during the first 14 days in the ICU. The 90-day survival rate was 50% (N = 22) and the 1-year survival rate was 27% (N = 12). The median survival of all patients was 88 days (range 4–1347 days).
In the following, attention is focused on the influence of intensive care measures on survival. A total of 34 patients (77%) received invasive MV. In this group, the 90-day survival rate was 38% (N = 13) and the 1-year survival rate was 18% (N = 6). The median survival was 58 days (range 4–1347). A total of 13 of 34 (38%) invasively ventilated patients underwent tracheostomy. Out of this group, 70% (N = 9) were still alive after 90 days and the 1-year survival rate was 23% (N = 3). Ten patients did not require MV. In this group, the 90-day survival rate was 90% (N = 9) and 60% (N = 6) were still alive after one year.
The single patient, who received an ECMO, had a survival time of 1174 days and is still alive (ECMO for 16 days and mechanical ventilation for 71 days). 33 patients (75%) required RRT. Patients with RRT had a 90-day survival rate of 42% (N = 14) and a 1-year survival rate of 18% (N = 6). The median time of survival was 67 days (range 4–1347). The median duration of RRT was 17 days ().
Table 6. Outcome characteristics.
Thirty-seven patients (84%) needed vasopressor support. In this group, the 90-day survival rate was 41% (N = 15) and 19% (N = 7) were still alive after one year. The median survival was 62 days (range 4–1347). The median duration of vasopressor therapy was 16 days (range 1–84 days). Five patients (11%) required cardiopulmonary resuscitation (CPR). None of them survived in the ICU.
Survival functions
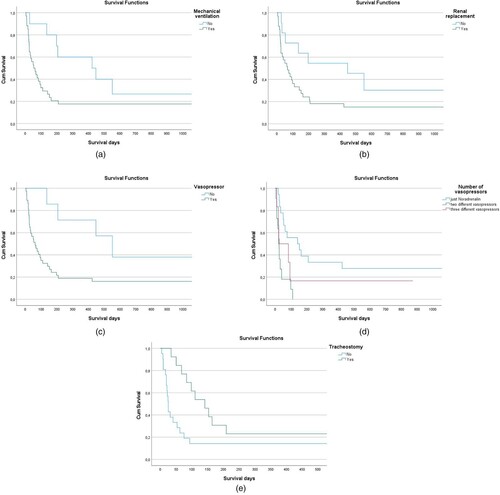
The need for mechanical ventilation was associated with a significantly decreased overall survival (p = 0.049) ((A)). Renal replacement had no impact on survival (p = 0.059) ((B)). Both administration of vasopressors and the number of vasopressors administered were associated with a significant decrease in survival (p = 0.029) ((C)) and (p = 0.002) ((D)). Furthermore, out of the patients requiring mechanical ventilation, there was a survival benefit in patients, who underwent tracheostomy (p = 0.013) ((E)).
Figure 1. (A). Overall survival according to the necessity of MV. The need for mechanical ventilation showed a marginally significant disadvantage in overall survival. (B). Overall survival according to the necessity of RRT. The use of renal replacement showed no significant disadvantage in survival. (C). Overall survival according to the necessity of vasopressors. The use of vasopressors showed a significant disadvantage in survival (p = 0.029). (D). Overall survival according to the number of vasopressors. The number of vasopressors also showed a significant disadvantage on OS. (E). Overall survival according to tracheostomy. The use of a tracheal cannula shows a higher overall survival.
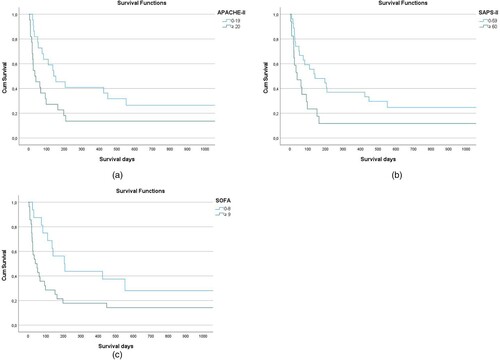
Furthermore, there was a significant correlation between increased mortality and an APACHE-II-Score ≥ 20 (p = 0.03) ((A)), a SAPS-II-Score ≥ 60 (p = 0.04) ((B)) and a SOFA-Score ≥ 9 (p = 0.03) ((C)). In contrast, there was no correlation between the risk scores prior to allogeneic stem cell transplantation (EBMT or HCT-CI) and survival in our study population (p = 0.3 or p = 0.4).
Figure 2. (A). Overall survival according to APACHE-Score. There was a significant correlation between an APACHE-Score ≥ 20 and mortality. (B). Overall survival according to SAPS-II-Score. There was a significant correlation between SAPS-II-Score ≥ 60 and mortality. (C). Overall survival according to SOFA-Score. There was a significant correlation between SOFA score ≥ 9 and mortality.
The initial GCS (p = 0.6) at the time of admission to the ICU, the qSOFA (p = 0.3) and the PICAT Score (p = 0.2) had no impact on OS.
Due to the high median age of the patients, different age groups were formed (<55 years and ≥ 55 years (p = 0,28), < 60 years and ≥ 60 years (p = 0,14), < 65 years and ≥ 65 years (p = 0,10), < 70 years and ≥ 70 years (p = 0,59). Age had no significant impact on the outcome.
In addition, remission status before allogeneic stem cell transplantation, donor type, and conditioning chemotherapy did not significantly affect survival.
Discussion
So far, there is only a little data on patients undergoing allo-HSCT admitted to the ICU during the peritransplant period. This study provides a retrospective analysis of a total of 44 patients admitted to the ICU in the peritransplant period in a German single centre. At the University Hospital of Bonn, intensive care procedures are not performed in the transplant unit.
The admission criteria of potential intensive care patients who have received allo-HSCT are difficult to define. Each admission is a case-by-case decision and should be discussed at an early stage with an experienced intensivist and haemato-oncologist.
Our patient population is of special interest due to its increased median age of 63 years and the outstanding number of serious comorbidities (median HCT-CI 3). Due to epidemiological developments, especially in the Western world, the interest concerning the outcome of older patients is currently coming to the fore. Due to the increasing number of older and fit patients, this patient group has become increasingly relevant. Despite considerable progress in the treatment and outcome of patients receiving allogeneic stem cell transplantation in recent decades, the choice of therapy for elderly patients remains controversial and outcomes still are unsatisfactory. Previous studies reported significantly younger patient populations. Lueck et al. reported a median age of 51 years and the oldest published population was by Borrega et al. with a median of 59 years [Citation12,Citation13].
Our data revealed a 90-day survival rate of 50% and a 1-year survival rate of 27%. In contrast, Borrega et al. reported a 90-day survival rate of only 37% and a 1-year survival rate of 16% [Citation23]. Lueck et al. reported a 1-year survival rate of 14–32%. Recent published studies reported 1-year survival rates between 16% and 33% [Citation12,Citation14,Citation15]. Therefore, our data showed a significantly longer 90 days and a longer 1-year survival than the population evaluated by Borrega et al. and is almost in common with Lueck et al.
Our favourable survival data could be because our ICU is part of the Department of Haemato-Oncology and is exclusively run by experienced haemato-oncologists specialized in ‘intensive care medicine’. Regarding patients in need of invasive MV, we found a 90-day survival of 38% and a 1-year survival of 18%. Compared to prior studies, the data are promising [Citation4,Citation5,Citation13]. Borrega et al. only reported a 1-year survival of ventilated patients at 5%. This is significantly lower than our 1-year survival of 18%. Unfortunately, the 90-day survival was not calculated in this study. In contrast, Lengliné et al. (median age of the collective 48 years) described a 90-day survival of ventilated post-allo-HSCT patients between 16% and 34%. The 1-year survival was not described [Citation26]. Lindgaard et al. (median age = 48 years) reported a 1-year survival of 11% [Citation27].
In our study, the performance of tracheostomy for better weaning or for expected long-term ventilation correlates with an improvement in overall survival (p = 0.013) compared to patients receiving invasive MV without tracheostomy. The data should be interpreted with great caution since a tracheostomy is only performed on a patient with an expected favourable outcome. Our findings are in line with previous data. Tanaka et al. showed better outcomes for patients with coronavirus, who underwent tracheostomy caused by prolonged MV [Citation28]. To date, there are no data on this topic in recipients of allo-HSCT admitted to ICU.
We found a significantly worse OS (p = 0.030) when two or more vasopressors were administered. Kew et al. have also shown that vasopressor use is associated with a decreased OS for patients after allo-HSCT admitted to the ICU. Our data are in line with Kew et al., who also reported a significant decrease in OS when vasopressors were administered.
One single patient in our study received extra-corporal membrane oxygenation (ECMO) support. This patient showed a survival of > 1000 days. In general allo-HSCT patients are high-risk ECMO patients; nevertheless, these should be discussed with the ECMO team in a timely manner.
In general, the outcome of patients with cardiac arrest after cardiopulmonary resuscitation is still poor.
In our study, none of the patients in need of CPR survived. This finding is consistent with Borrega et al. They performed 17% CPR for patients after allo-HSCT in the ICU and none was alive after 1 year.
Patients during the peritransplant period are very vulnerable as most patients are aplastic and seriously immunocompromised. Thus, there is a risk of fulminant infection and septic shock. In our population, 36 patients (82%) developed sepsis or septic shock. In this group, the 30-day mortality was 36% and the 90-day mortality was 56%. Neumann et al. showed a mortality rate of 56% for septic shock patients after allo-HSCT during the ICU stay [Citation29]. Bauer et al. described a 30-day mortality of sepsis patients, who were not immunocompromised, of 27% and a 90-day mortality of 39% [Citation30].
It remains difficult to predict the survival of this particular patient population with scoring systems. The PICAT-Score was developed for this purpose [Citation31]. Our data did not show a significant correlation between PICAT-Score and mortality (p = 0.444). Several studies, such as Michel et al., also failed to show a significant benefit from the PICAT-Score [Citation32]. The HCT-CI Score is a generally established score to predict survival after HSCT. HCT-CI data for patients in the intensive care unit after allo-HSCT are inconsistent. Our data are in line with Borrega et al. and did not show any correlation between the HCT-CI Score and OS. Bayraktar et al. reported a significant decrease in OS in patients with HCT-CI ≥ 2 [Citation33]. In contrast, our data showed that the APACHE-II and SOFA-Scores are more predictive for OS. There was a significant correlation between an APACHE-II-Score ≥ 20 and mortality (p = 0.03) and SOFA score ≥ 9 and mortality (p = 0.03). The data of Michel et al. underline our data, which showed a strong correlation between APACHE-II-Score and mortality during the peri-transplant period [Citation32]. Our data also suggest a significant correlation between SAPS-II-Score ≥ 60 and mortality (p = 0.04). This is in line with Turki et al. who also showed a significant correlation between survival and SAPS-II-Score at admission [Citation34]. These outcomes underline that these scoring systems can be applied to allogeneic stem cell-transplanted patients in the ICU.
The current study has its limitations as it is a single-centre retrospective study and contains only a limited number of patients. Furthermore, the increased median age and comorbidities are a limitation in this study. All parameters were collected retrospectively. Further studies should be conducted preferably in a multicentre setting to confirm the results of our study. Patient characteristics, i.e. older age, and adapted transplantation procedures, such as reduced conditioning and alternative donor grafts continue to play an important role in outcomes. For patients who underwent allo-HSCT and who need intensive care support, highly qualified surveillance is required due to the persistently restricted prognosis. Therefore, these patients should only be treated in specialized centres with profound expertise in the field of allo-HSCT.
Conclusion
We found that the outcome of our allo-HSCT patients admitted to the ICU is not as poor as previously reported. Even older patients (median age of 63 years) under long-term ventilation may benefit from extensive intensive care therapy. Sepsis remains a major threat in this setting. SOFA, APACHE-II and SAPS-II have a significant impact on survival in this patient population (very early ICU in allo-HSCT) and can be also safely applied here.
Declarations
Availability of data and materials
The datasets used for the analysis in this study are available from the corresponding author upon reasonable request.
Ethical approval
All study investigators were staff of Department III of Internal Medicine at the University Hospital Bonn. Due to the retrospective manner, no interventions were carried out as part of the study. Patient care, data collection and analyses were performed by site personnel using current techniques of privacy assurance. Therefore, the Ethics Committee (University Bonn) approved this study under the following number: 346/22.
Acknowledgements
All authors contributed to the conception and design of the study. Material preparation, data collection and analysis were performed by Hannah Zenzen. The first draft of the manuscript was written by Hannah Zenzen and Michael Serries. All authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Gratwohl A, Pasquini MC, Aljurf M, et al. Worldwide network for blood and marrow transplantation (WBMT). One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol. 2015 Mar;2(3):e91–100. doi:10.1016/S2352-3026(15)00028-9. Epub 2015 Feb 27. Erratum in: Lancet Haematol. 2015 May;2(5):e184.
- Kew AK, Couban S, Patrick W, et al. Outcome of hematopoietic stem cell transplant recipients admitted to the intensive care unit. Biol Blood Marrow Transp. 2006 Mar;12(3):301–305. doi:10.1016/j.bbmt.2005.10.020.
- Pène F, Aubron C, Azoulay E, et al. Outcome of critically ill allogeneic hematopoietic stem-cell transplantation recipients: a reappraisal of indications for organ failure supports. J Clin Oncol. 2006 Feb 1;24(4):643–649. doi:10.1200/JCO.2005.03.9073. Epub 2005 Dec 27.
- Afessa B, Tefferi A, Hoagland HC, et al. Outcome of recipients of bone marrow transplants who require intensive-care unit support. Mayo Clin Proc. 1992 Feb;67(2):117–122. doi:10.1016/s0025-6196(12)61310-x.
- Faber-Langendoen K, Caplan AL, McGlave PB. Survival of adult bone marrow transplant patients receiving mechanical ventilation: a case for restricted use. Bone Marrow Transp. 1993 Nov;12(5):501–507.
- Torrecilla C, Cortés JL, Chamorro C, et al. Prognostic assessment of the acute complications of bone marrow transplantation requiring intensive therapy. Intens Care Med. 1988;14(4):393–398. doi:10.1007/BF00262895.
- Bayraktar UD, Nates JL. Intensive care outcomes in adult hematopoietic stem cell transplantation patients. World J Clin Oncol. 2016 Feb;7(1):98–105. doi:10.5306/wjco.v7.i1.98.
- Mayer S, Pastores SM, Riedel E, et al. Short- and long-term outcomes of adult allogeneic hematopoietic stem cell transplant patients admitted to the intensive care unit in the peritransplant period. Leuk Lymphoma. 2017 Feb;58(2):382–390. doi:10.1080/10428194.2016.1195499. Epub 2016 Jun 27.
- Azoulay E, Lemiale V, Mokart D, et al. Acute respiratory distress syndrome in patients with malignancies. Intens Care Med. 2014 Aug;40(8):1106–1114. doi:10.1007/s00134-014-3354-0. Epub 2014 Jun 5.
- Pène F, Percheron S, Lemiale V, et al. Temporal changes in management and outcome of septic shock in patients with malignancies in the intensive care unit. Crit Care Med. 2008 Mar;36(3):690–696. doi:10.1097/CCM.0B013E318165314B.
- Benz R, Schanz U, Maggiorini M, et al. Risk factors for ICU admission and ICU survival after allogeneic hematopoietic SCT. Bone Marrow Transp. 2014 Jan;49(1):62–65. doi:10.1038/bmt.2013.141. Epub 2013 Sep 23.
- Lueck C, Stadler M, Koenecke C, et al. Improved short- and long-term outcome of allogeneic stem cell recipients admitted to the intensive care unit: a retrospective longitudinal analysis of 942 patients. Intens Care Med. 2018 Sep;44(9):1483–1492. doi:10.1007/s00134-018-5347-x. Epub 2018 Aug 23.
- Borrega JG, Heger JM, Koehler P, et al. Allogeneic stem cell transplant recipients admitted to the intensive care unit during the peri-transplant period have unfavorable outcomes-results of a retrospective analysis from a German university hospital. Ann Hematol. 2022 Feb;101(2):389–395. doi:10.1007/s00277-021-04698-3 Epub 2021 Oct 20.
- Platon L, Amigues L, Ceballos P, et al. A reappraisal of ICU and long-term outcome of allogeneic hematopoietic stem cell transplantation patients and reassessment of prognosis factors: results of a 5-year cohort study (2009-2013). Bone Marrow Transp. 2016 Feb;51(2):256–261. doi:10.1038/bmt.2015.269. Epub 2015 Nov 16.
- Mayer S, Pastores SM, Riedel E, et al. Short- and long-term outcomes of adult allogeneic hematopoietic stem cell transplant patients admitted to the intensive care unit in the peritransplant period. Leuk Lymphoma. 2017 Feb;58(2):382–390. doi:10.1080/10428194.2016.1195499. Epub 2016 Jun 27.
- Mayer S. Jahrestagung der Deutschen, Österreichischen und Schweizerischen Gesellschaften für Hämatologie und Medizinische Onkologie, 7.–10. Oktober 2022, Wien: abstracts. Oncol Res Treat. 2022;45(suppl 2):152. doi:10.1159/000526456
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016 Feb;315(8):801–810. doi:10.1001/jama.2016.0287.
- Gratwohl A. The EBMT risk score. Bone Marrow Transp. 2012 Jun;47(6):749–756. doi:10.1038/bmt.2011.110 Epub 2011 Jun 6.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8.
- Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005 Oct 15;106(8):2912–2919. doi:10.1182/blood-2005-05-2004 Epub 2005 Jun 30.
- Lambden S, Laterre PF, Levy MM, et al. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019 Nov 27;23(1):374, doi:10.1186/s13054-019-2663-7.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016 Feb 23;315(8):801–810. doi:10.1001/jama.2016.0287.
- Bayraktar UD, Milton DR, Shpall EJ, et al. Prognostic index for critically Ill allogeneic transplantation patients. Biol Blood Marrow Transp. 2017 Jun;23(6):991–996. doi:10.1016/j.bbmt.2017.03.003. Epub 2017 Mar 3.
- Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/north American multicenter study. JAMA. 1993 Dec 22-29;270(24):2957–2963. doi:10.1001/jama.270.24.2957. Erratum in: JAMA 1994 May 4;271(17):1321.
- Knaus WA, Zimmerman JE, Wagner DP, et al. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981 Aug;9(8):591–597. doi:10.1097/00003246-198108000-00008.
- Lengliné E, Chevret S, Moreau AS, et al. Changes in intensive care for allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transp. 2015 Jun;50(6):840–845. doi:10.1038/bmt.2015.55 Epub 2015 Mar 23.
- Lindgaard SC, Nielsen J, Lindmark A, et al. Prognosis of allogeneic haematopoietic stem cell recipients admitted to the intensive care unit: a retrospective, single-centre study. Acta Haematol. 2016;135(2):72–78. doi:10.1159/000440937 Epub 2015 Oct 20.
- Tanaka A, Uchiyama A, Kitamura T, et al. Association between tracheostomy and survival in patients with coronavirus disease 2019 who require prolonged mechanical ventilation for more than 14 days: a multicenter cohort study. Auris Nasus Larynx. 2022 Jun. S0385-8146(22)00164-X. doi: 10.1016/j.anl.2022.06.002. Epub ahead of print.
- Neumann F, Lobitz O, Fenk R, et al. The sepsis-related organ failure assessment (SOFA) score is predictive for survival of patients admitted to the intensive care unit following allogeneic blood stem cell transplantation. Ann Hematol. 2008 Apr;87(4):299–304. doi:10.1007/s00277-008-0440-9 Epub 2008 Jan 25.
- Bauer M, Groesdonk HV, Preissing F, et al. Sterblichkeit bei Sepsis und septischem Schock in Deutschland. Ergebnisse eines systematischen reviews mit Metaanalyse [Mortality in sepsis and septic shock in Germany. Results of a systematic review and meta-analysis]. Anaesthesist. 2021 Aug;70(8):673–680. doi:10.1007/s00101-021-00917-8. German Epub 2021 Feb 9. Erratum in: Anaesthesist. 2021 Jun 21.
- Bayraktar UD, Milton DR, Shpall EJ, et al. Prognostic index for critically ill allogeneic transplantation patients. Biol Blood Marrow Transp. 2017 Jun;23(6):991–996. doi:10.1016/j.bbmt.2017.03.003. Epub 2017 Mar 3.
- Michel CS, Teschner D, Schmidtmann I, et al. Prognostic factors and outcome of adult allogeneic hematopoietic stem cell transplantation patients admitted to intensive care unit during transplant hospitalization. Sci Rep. 2019 Dec;9(1):19911, doi:10.1038/s41598-019-56322-0.
- Bayraktar UD, Shpall EJ, Liu P, et al. Hematopoietic cell transplantation-specific comorbidity index predicts inpatient mortality and survival in patients who received allogeneic transplantation admitted to the intensive care unit. J Clin Oncol. 2013 Nov 20;31(33):4207–4214. doi:10.1200/JCO.2013.50.5867 Epub 2013 Oct 14.
- Turki AT, Lamm W, Schmitt C, et al. Platelet number and graft function predict intensive care survival in allogeneic stem cell transplantation patients. Ann Hematol. 2019;98:491–500. doi:10.1007/s00277-018-3538-8
