ABSTRACT
Objective:
Identify patient experience and preference towards thrombopoietin-receptor agonists (TPO-RAs) in treatment of immune thrombocytopenia (ITP) in the Netherlands.
Methods:
The Thrombopoietin-Receptor Agonist Patient experience (TRAPeze) survey used a discrete choice experiment (DCE) to elicit patient preferences and a patient burden survey (PBS) to evaluate the clinical and social impact of ITP. TRAPeze collected responses from 6th October to 19th November 2021.
Results:
Seventy-six respondents completed the DCE: treatment preference appeared to be driven by method of administration (odds ratio [OR] 4.33; 95% confidence interval [CI] 2.88–6.52), frequency of dosing (OR 2.33; 95% CI 1.86–2.92) and drug–food interactions (OR 1.91; 95% CI 1.54–2.37). Respondents preferred therapies delivered orally over subcutaneous injection (OR 4.22; 95% CI 2.76–6.46), dosed once weekly over once daily (OR 2.37; 95% CI 1.58–3.54) and without food restrictions over with restrictions (OR 1.90; 95% CI 1.52–2.38). Sixty-nine respondents completed the DCE and PBS (mean [range] age 53 [19–83] years, 65% female). Seven incomplete PBS responses were excluded from analysis. Respondents were currently, or most recently, receiving eltrombopag (n = 43) or romiplostim (n = 26), of which 30% (n = 21/69) had previously received another TPO-RA. Loss (29%, n = 6/21) and lack (29%, n = 6/21) of response were the most common reasons for switching TPO-RA. Only 28% (n = 18/65) of respondents felt their TPO-RA increased energy levels.
Conclusion:
Patients preferred therapies delivered orally, dosed less frequently and without food restrictions. QoL of ITP patients on TPO-RAs can be improved; the burden analyses presented can inform future efforts towards this.
1. Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by reduced platelet counts, due to antibody-mediated platelet destruction and impaired platelet production. Patients usually present at diagnosis with a platelet count at least below 100 × 109/L and bleeding symptoms, such as bruising, petechiae, purpura and epistaxis [Citation1]. A definitive ITP diagnosis is achieved by exclusion of other causes of thrombocytopenia [Citation2].
Initial therapy is aimed at raising the platelet count to >20 × 109/L in newly diagnosed adult patients, and intervention should be individualized to best achieve the treatment goals of each patient [Citation3,Citation4]. Preferred first-line therapy is immune suppression with corticosteroids, or intravenous immunoglobulin, to provide rapid platelet increases [Citation4,Citation5]. By raising platelet counts and reducing bleeding risk, initial therapy can improve quality of life (QoL) [Citation6]. Extended use of corticosteroids is, however, problematic due to side effects yielding high relapse and low long-term remission rates [Citation7]. More recently, data have emerged suggesting shorter course high-dose corticosteroid regimens, such as dexamethasone, can be effective [Citation8]. Nonetheless, immune suppression became increasingly unattractive in the context of the SARS-CoV-2 (COVID-19) pandemic, where individuals on sustained regimens of immune suppressants are at higher risk of infection [Citation9].
Thrombopoietin-receptor agonists (TPO-RAs) that mimic endogenous thrombopoietin to increase platelet production without causing immune suppression are available for patients relapsing on or refractory to corticosteroids [Citation10–12]. Currently three TPO-RA agents are approved for second-line use in the United States and Europe: eltrombopag (Revolade®) [Citation13,Citation14], romiplostim (Nplate®) [Citation15,Citation16] and avatrombopag (Doptelet®) [Citation17,Citation18]. Overall response rates are ∼80% for all TPO-RAs and safety profiles are comparable [Citation19,Citation20]. The primary differences between these treatments relate to their product characteristics. Romiplostim, available in the European Union since 2009 [Citation16], is delivered by subcutaneous injection, either at the clinic or by the patient at home [Citation15,Citation16]. Eltrombopag, available in Europe from 2010 [Citation14], is an oral tablet, but must be taken 2 h before, or 4 h after, consuming food containing cations (e.g. iron and calcium from dairy) [Citation13,Citation14]. Avatrombopag was approved in 2021 for use in Europe [Citation18,Citation21], and is an oral tablet taken with food and without food type restrictions [Citation18]. Unsurprisingly some patients find adherence to dietary restrictions or subcutaneous delivery challenging, and newer therapies look to overcome these limitations [Citation20,Citation22]. For this reason, in addition to lack of response and adverse events, switching between TPO-RAs is common [Citation23].
While TPO-RA use is efficacious, only a small proportion of patients (approximately 30%) are able to discontinue treatment with a maintained response [Citation24,Citation25]. Many patients also experience persistent ITP symptoms throughout treatment [Citation26,Citation27]. QoL is typically lower in patients with ITP compared with healthy individuals [Citation28]. For many, persistent ITP-related fatigue also impacts their social functioning. Many patients demonstrate lower work productivity and require more sick leave due to their ITP [Citation29]. Clearly, an unmet need to improve patient QoL remains in this disease area. To understand this further, real-world evidence of the patient experience in ITP, and the impact of TPO-RA therapy, may be of value.
The European TRAPeze survey is currently the only study to investigate in tandem the patient experience in ITP and the impact of TPO-RA characteristics on therapy choice [Citation27,Citation30]. The original European survey was created with input from healthcare professionals (HCPs) and patient representatives. TRAPeze has previously been fielded in the UK and Italy [Citation27,Citation30], here results from the TRAPeze study in the Netherlands are reported.
2. Methods
The European TRAPeze study is a cross-sectional, exploratory observational study of individuals with ITP. The survey was open to respondents in the Netherlands from 6th October to 19th November 2021.
Respondents were recruited by the Dutch patient advocacy group, ITP Patiëntenvereniging Nederland, who circulated the survey to members. ITP Patiëntenvereniging Nederland comprises approximately 700 members as of April 2023 [Citation31]. The survey was shared via email and publicized on the website and social media (Facebook) of ITP Patiëntenvereniging Nederland – these communications contained links to the survey. However, due to the public nature of ITP Patiëntenvereniging Nederland’s Facebook page, we are unable to precisely estimate the number of individuals who were made aware of the study. The survey was professionally translated from English into Dutch and administered as an online questionnaire through the web platform SurveyEngine®.
Clinical experts in ITP were represented on the study steering committee and provided input during survey question development.
The TRAPeze survey design has been described previously [Citation27]. In short, the survey comprised two sections: a discrete choice experiment (DCE) and patient burden survey (PBS). The DCE elicited respondent preference to product attributes based on their selection of preferred treatments from 10 pairs of hypothetical treatments. The PBS presented respondents with open and closed questions regarding their experiences with ITP. The Dutch survey was developed according to Dutch regulations and the survey was reviewed and approved by the Dutch Clinical Research Foundation. Changes were made to the PBS component of the Dutch version of the survey, compared with the original English version, to ensure relevance and cultural sensitivity of questions; none were made to the DCE component (Appendix 1).
Respondent’s data were processed and analyzed as described previously [Citation27]. Responses were retrieved in Excel format from SurveyEngine®. Analysis of the DCE was performed in Stata (StataCorp LLC. 2023. Stata Statistical Software: Release 18). The responses from the treatment preference section were analyzed using a mixed logit model to estimate the odds of participant preference towards TPO-RA product characteristics, with the OR and 95% CI reported in this paper. Not all respondents answered all questions and ‘I don’t know’ and ‘prefer not to say’ responses were pragmatically excluded where appropriate. As a result, all n numbers have been provided with the total included responses for that question.
Inclusion criteria were as follows: ≥18 years of age, formal diagnosis of primary ITP according to the American Society of Haematology (ASH) and International Consensus Report (ICR) guidelines [Citation3,Citation4], currently receiving or previously received a TPO-RA for a minimum of 3 months, with at least some of the treatment received in the last 12 months.
Avatrombopag was not included in this study as no patients in the Netherlands had been taking the drug for >3 months at time of survey fielding [Citation32].
3. Results
3.1. Demographics
A total of 76 respondents participated, all of whom completed the DCE and 69 of whom also provided a complete PBS. Demographic data were derived from the latter 69 patient cohort, who provided complete PBS responses. The cohort was 65% (n = 45/69) female, and the age of respondents was 19–83 years. The mean (standard deviation [SD]) age of respondents was 53 (14) years, the mean (SD) age at time of ITP diagnosis was 44 (15) years and the mean (SD) time since diagnosis was 9 (7) years. Age distribution of respondents were as follows (n = 67, excluding two responses ‘prefer not to say’): 18–24; n = 3 (5%), 25–34; n = 4 (6%) 35–44; n = 10 (15%), 45–54; n = 14 (21%), 55–64; n = 24 (36%), ≥65; n = 12 (17%).
3.2. Disease characteristics
Respondents (n = 69) reported experiencing a mean (SD) of 4 (2) symptoms when affected by their ITP. Fatigue was the most commonly reported symptom of ITP (83%, n = 57/69), followed by bruising (81%, n = 56/69), petechiae (71%, n = 49/69), anxiety regarding platelet counts (48%, n = 33/69) and unexplained nose bleeds (29%, n = 20/69) ((A)). Fatigue was also the most negatively impactful symptom on respondent QoL (56%, n = 38/68), followed by anxiety about platelet count (10%, n = 7/68), bruising (10%, n = 7/68), petechiae (7%, n = 5/68) and unexplained nose bleeds (4%, n = 3/68) ((B)).
Figure 1. (A) ITP signs and symptoms experienced by respondents (n = 69). (B) ITP signs and symptoms ranked by most negatively impactful on quality of life by respondents (n = 68) (excluding responses ‘no symptoms’ [n = 1]). Percentages may not sum to 100 due to rounding. *Heavy menstrual bleeding calculated as % of female cohort (n = 45).
![Figure 1. (A) ITP signs and symptoms experienced by respondents (n = 69). (B) ITP signs and symptoms ranked by most negatively impactful on quality of life by respondents (n = 68) (excluding responses ‘no symptoms’ [n = 1]). Percentages may not sum to 100 due to rounding. *Heavy menstrual bleeding calculated as % of female cohort (n = 45).](/cms/asset/0cba9734-76e0-4cec-8743-8a5a661ebba4/yhem_a_2267942_f0001_oc.jpg)
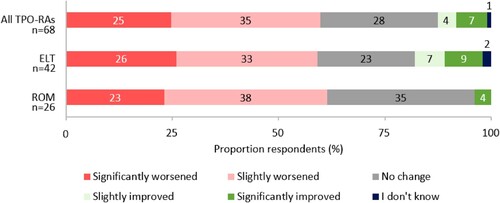
When asked to estimate their current health state, on a scale of 1 (very poor) to 10 (excellent), the mean (SD) respondent score was 6.8 (1.6), which was the same for respondents most recently prescribed eltrombopag or romiplostim. In addition, 60% (n = 41/68) of respondents felt their condition had worsened since ITP diagnosis ().
Figure 2. Respondents’ perceived change in ITP condition since diagnosis. Percentages may not add up to 100 due to rounding.
Only one participant was unable to provide their last recorded platelet count. The median (interquartile range [IQR]) last recorded platelet count was 91 × 109/L (57–147 × 109/L), with 81% (n = 55/68) of respondents’ platelet counts >50 × 109/L. When asked whether their condition had been stable over the preceding 3 months, 52% (n = 34/65) reported a stable platelet count >30 × 109/L. Of the 48% (n = 31/65) who reported an unstable platelet count, 25% (n = 16/65) reported an unstable platelet count >30 × 109/L and 14% (n = 9/65) reported an unstable platelet count <30 × 199/L. Overall, 25 respondents reported experiencing some adverse events. Stability of condition was a self-assessment by participants, criteria defining stability were not provided.
3.3. Treatment patterns
Just under two-thirds (62%, n = 43/69) of respondents reported their current or most recent TPO-RA treatment was eltrombopag, with the remaining respondents (38%, n = 26/69) reporting this to be romiplostim. TPO-RA switching was common; of the 21 (30%) respondents who had switched TPO-RA, 11 (52%) users had previously taken eltrombopag and 10 (48%) users had previously taken romiplostim. The most common reasons for TPO-RA switching were no change in ITP symptoms (29%, n = 6/21), TPO-RA stopped working (29%, n = 6/21) and adverse events (24%, n = 5/21). Preference was not provided as an option for TPO-RA switching.
The majority of respondents (76%, n = 48/63) were receiving only one treatment for their ITP (range 0–5), this being their TPO-RA. Twelve patients were taking two or more medications for their ITP (nine patients taking two medications, two patients taking three medications and one patient taking five medications, including their TPO-RA). Corticosteroid co-use was reported, with 8% (n = 5/63) of respondents stating they were currently taking a corticosteroid as well as a TPO-RA. Prednisolone was the most frequently prescribed corticosteroid; one respondent on eltrombopag was taking dexamethasone and no respondents were currently taking methylprednisolone. Additionally, 26% (n = 11/43) of respondents on eltrombopag and 23% (n = 6/26) of respondents on romiplostim had received a splenectomy.
3.4. Treatment preferences
3.4.1. Patient burden survey
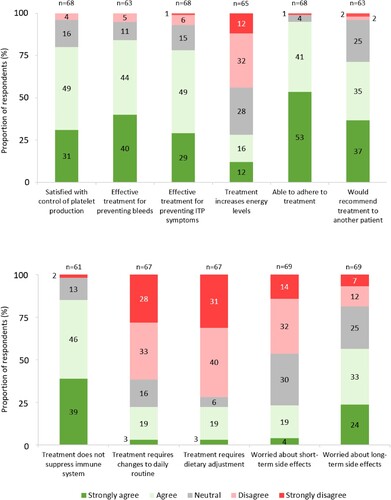
All respondents treated with eltrombopag (100%, n = 42/42) and most respondents treated with romiplostim (92%, n = 23/25) were satisfied with their TPO-RA method of administration. Further, 78% (n = 53) agreed their TPO-RA effectively treated their ITP symptoms. Commonly reported themes for treatment satisfaction were improved response and lifestyle with eltrombopag, and less frequent dosing with romiplostim. Reasons reported for therapy dissatisfaction included food restrictions with eltrombopag and subcutaneous delivery with romiplostim. Importantly, patients were able to report reasons for therapy dissatisfaction as free text answers, even if they had reported overall satisfaction with therapy.
Responses demonstrated high treatment adherence to eltrombopag (95%, n = 41/43) and romiplostim (100%, n = 26/26). However, less than one-third (28%, n = 18/65) of respondents felt their TPO-RA increased energy levels. Most patients agreed their TPO-RA controlled platelet production (80%, n = 54/68), prevented bleeds (84%, n = 53/63), prevented ITP symptoms (78%, n = 53/68) and that they would recommend their TPO-RA to a friend (72%, n = 45). Additionally, 22% (n = 15/67) of respondents reported making changes to their daily routine to accommodate their TPO-RA ().
3.4.2. Discrete choice experiment
A total of 76 DCE responses were collected. The DCE portion of this study elicited patient preference towards TPO-RA product attributes. Method of administration (Odds Ratio [OR] 4.33; 95% CI 2.88–6.52), frequency of dosing (OR 2.33; 95% CI 1.86–2.92) and drug–food interactions (OR 1.91; 1.54–2.37) were the product attributes most associated with respondents’ choice of TPO-RA therapy ((A)).
Figure 4. (A) Association between TPO-RA attributes and respondent preference towards TPO-RA treatments (n = 76). The red line indicates no effect (odds ratio = 1). The black lines indicate the lower to upper confidence intervals. (B) Analysis of respondent preference between TPO-RA attribute levels (n = 76). The red line indicates no effect (odds ratio = 1). For each attribute category, the first attribute level plotted is the reference level. The black lines indicate lower to upper confidence intervals. *Two separate tests: one for measuring platelet count and one for liver function. **Must be taken 2 h before or 4 h after, food containing dairy products or calcium; indigestion remedies (antacids); or mineral supplements.
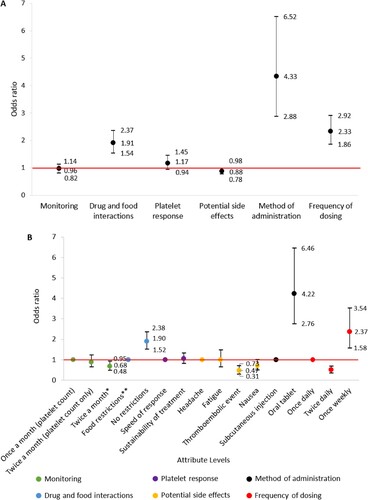
When analyzing preference between attribute levels, respondents were 4.22 (95% CI 2.76–6.46) times more likely to select an orally administered TPO-RA over subcutaneous injection. A TPO-RA delivered once weekly was 2.37 (95% CI 1.58–3.54) times more likely to be preferred over once daily delivery. Respondents were also 1.90 (95% CI 1.52–2.38) times more likely to select a TPO-RA without food restrictions over with restrictions and were less likely (OR 0.47 [95% CI 0.73–0.31]) to select thromboembolic event over headache as a potential side effect ((B)).
3.5. Views on side effects
A notable number of respondents more or less worry about short-term (23%, n = 16/69) and about long-term (57%, n = 39/69) side effects ().
3.6. Healthcare resource utilization
The mean number of specialist hospital consultant visits reported for both October 2019–March 2020 and March 2020–November 2021 was 4, despite a difference of 14 months between timeframes. In the 12 months preceding the study, 29% (n = 20/69) of respondents had seen a general practitioner and 16% (n = 11/69) of respondents had used a mental health service due to ITP. Similarly, 15% (n = 10/69) of respondents had attended hospital in the previous 12 months, due to their ITP, with the mean number of nights spent in hospital per admittance being 2 (range 0–5).
3.7. Work and productivity
The majority of female (71%, n = 32/45) and male (75%, n = 18/24) respondents were employed when completing the study. Of employed respondents, 41% (n = 13/32) of female and 72.2% (n = 13/18) of male respondents were employed full time. Female respondents reported a greater spread in weekly working hours compared with male respondents ((A)). Just under half of respondents (46%, n = 23/50) reported reducing weekly working hours due to ITP, with 12% (n = 6/50) of respondents reducing working hours by >20 h ((B)). When asked about symptoms which influenced their ability to fulfill employment responsibilities, fatigue (76%, n = 38/50) and anxiety about platelet count (24%, n = 12/50) were the most common reasons reported by respondents, followed by bruising or bleeds (16%, n = 8/50) and pain (10%, n = 5/50). Additionally, respondents reported having to make changes to their workplace behaviour due to ITP, such as avoiding certain physical activities due to bleeding risk (16%, n = 8/50), temporarily or permanently work from home (16%, n = 8/50), increase regularity of rest breaks (16%, n = 8/50) or ‘other’ (20%, n = 10). A further 44% (n = 22/50) of respondents did not report making changes to their workplace behaviour due to ITP.
3.8. Partner and family experience
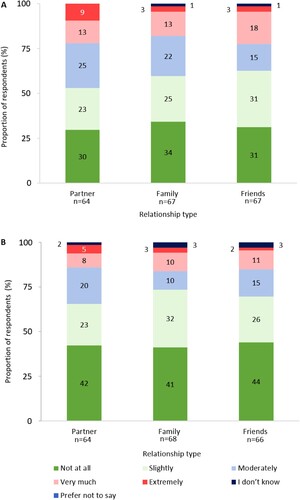
Respondents stated that ITP had an impact on their relationships with their partners, family and friends ((A)), and that this impact had worsened as their disease had progressed since diagnosis ((B)). The degree of impact of ITP was reported to be relatively consistent between each relationship type. Of the 43 respondents who provided text responses on the impact ITP had on their relationships, 19% (n = 8/43) mentioned adjustments to parenting responsibilities and 60% (n = 26/43) mentioned fatigue negatively impacting their relationships.
Figure 6. (A) Degree of impact ITP has had on relationships (excluding responses: ‘not applicable’). (B) Degree that the impact of ITP on relationships has changed as disease has progressed (negatively) since diagnosis (excluding responses: ‘not applicable’). Percentages may not sum to 100 due to rounding.
4. Discussion
While investigations into the patient perspective in ITP have been conducted previously [Citation26,Citation33], the European TRAPeze study is the first to analyze patient preferences towards TPO-RAs. Participant demographics from the Dutch TRAPeze cohort were reflective of the Dutch ITP patient population in clinical practice [Citation34]. The majority of participants were female, the mean age was over 50 years and two-thirds of patients had most recently taken eltrombopag [Citation32].
Self-reported health states indicated respondents felt they were in reasonable health, and scores were notably the same between eltrombopag and romiplostim. Despite this, the majority of ITP respondents also felt their condition had worsened, as compared with their situation before diagnosis. Fatigue was the most commonly reported symptom and also the symptom ranked as being the most negatively impactful on QoL by the majority of respondents. The notable negative impact of fatigue is consistent with the UK and Italian TRAPeze cohorts [Citation27,Citation30]. Additionally, previous studies such as Rovó et al. confirm our findings. Here, patients with ITP reported, via a questionnaire, no treatment induced reduction in fatigue despite overall satisfaction with their TPO-RA therapy [Citation33]. Free text responses in the TRAPeze survey further contextualized the impact of fatigue on respondent QoL: the negative impact of fatigue on relationships was a frequently mentioned theme. This highlights that a clear unmet need remains for patients treated with TPO-RAs.
Respondent satisfaction with mode of administration of their TPO-RA therapy was observed. Consistent with respondent satisfaction, 94% reported being able to adhere to their TPO-RA. Despite this, issues relating to food restrictions with eltrombopag and subcutaneous administration with romiplostim were highlighted by a number of respondents in free text responses. A minority of respondents reported making changes to their daily routine (21%) and dietary adjustments (22%) to accommodate their TPO-RA regimen. While PBS responses demonstrate TPO-RAs were adhered to and well tolerated overall, this image differs from previous research outlining TPO-RA adherence issues with romiplostim and eltrombopag [Citation20,Citation35].
Less than a third of respondents felt their TPO-RA increased their energy levels and over half were concerned with long-term side effects of their TPO-RA. Given the reported negative impact of fatigue in this population, this result appears somewhat at odds with high treatment satisfaction levels reported. This may be due to respondents taking a holistic view towards treatment satisfaction, and may also be influenced by previously unsuccessful lines of therapy making their TPO-RA seem more satisfactory in comparison. In addition, the striking concern of a large majority of respondents surrounding long-term side effects may be alleviated by improved patient education, as type and frequency of TPO-RA adverse events do not appear to change over time [Citation36]. As more treatment options come to market, patient preference may become more pronounced in therapy selection. The Dutch cohort DCE identified method of administration, frequency of dosing and drug–food interactions as primary drivers of therapy choice. These results are aligned with other TRAPeze cohorts and provide further context to experiences related in the PBS arm of this study [Citation27,Citation30].
Just under one-third of respondents had previously taken a different TPO-RA, in line with the prevalence of TPO-RA switching seen in clinical practice [Citation19]. Switching to eltrombopag from romiplostim was more common than the reverse, also representative of clinical practice [Citation19]. There is growing evidence that response, or lack of, to the first TPO-RA received does not predict response when switching to a second TPO-RA agent. In their pooled analysis, Gonzalez-Porras et al. found overall response to subsequent TPO-RAs was 78% and that responses were similar whether switching from either romiplostim or eltrombopag [Citation19]. Overall response to a secondary TPO-RA agent was higher in patients who had switched due to preference than due to lack of effectiveness. This demonstrates the clinical and QoL benefits of shared decision-making, between clinician and patient, in ITP management.
Uncertainty remains regarding the specific impact of patient preference on TPO-RA adherence and, therefore, response to treatment. A recent study by Al-Samkari et al. suggests that TPO-RA adherence can influence effectiveness [Citation23]. Patients in this retrospective study switched to avatrombopag, from eltrombopag or romiplostim for one of three reasons: lack of response, adverse event(s) or preference; that is to avoid injections or food restrictions. Of the three reasons provided for switching, response (platelet count ≥50 × 103) to avatrombopag was highest in those who switched due to preference (23/23), followed by lack of efficacy (12/14) and adverse event(s) (6/7). Likewise, in TRAPeze Netherlands, lack of effectiveness and adverse events were the most common reasons for discontinuation, although preference as a reason for discontinuation was not included here. Similar to results published by Gonzalez-Porras et al., these data underscore that switching TPO-RA can be beneficial in cases where the first agent provides insufficient response, causes adverse events or where the patient prefers. In the future, reasons for lack of adherence should be explored in tandem with response rates, to determine any impact TPO-RA attributes may have on adherence and, thus, effectiveness.
ITP-related healthcare resource utilization was modest in the Dutch cohort. Only a minority of respondents had seen a GP or used mental health services due to their ITP in the 12 months preceding the study. This is of potential concern given anxiety and depression are suspected to be more prevalent in people with ITP compared with healthy individuals [Citation37,Citation38]. Reported frequency of specialist hospital appointments was also lower from March 2020–February 2022, compared with the period October 2019–March 2020. Low healthcare resource use may be due to disruption caused by the COVID-19 pandemic. However, as the recall time for this was >2 years, this data should be interpreted with caution.
The Dutch TRAPeze cohort reported a significant social impact of ITP. A majority of respondents felt their condition had impacted relationships with their families, partners and friends, and that this impact had increased since diagnosis. This was reflected in free text response where 19% of respondents who provided text responses mentioned adjusting parental responsibilities as a result of their ITP.
Respondents also indicated that the impact of ITP extended to their working life. Indeed, the employment rate in this cohort (72.5%) was lower than the national average in the Netherlands (96%) [Citation39]. It is important to note that four respondents who reported being employed, were over the Dutch retirement age. As a result no age correction was applied because to do so would have excluded some employed respondents. Although most respondents were in full time employment, ITP did cause some to reduce their working hours, with reductions of >20 h per week for some respondents. Furthermore, nearly two-thirds of respondents felt fatigue had influenced their ability to fulfill employment responsibilities. Together these findings underscore the indirect cost of productivity loss seen in ITP and highlight the unmet need for therapies that better resolve fatigue in ITP.
The Dutch cohort enriches the European TRAPeze study and further supports the importance of patient choice of TPO-RA therapy to improve QoL.
5. Limitations
An uneven number of respondents received eltrombopag and romiplostim in this study. This is consistent with clinical practice in the Netherlands [Citation32]. As a consequence, responses in this survey may be skewed towards the patient experience on eltrombopag. Although this may limit the representativeness of these results for the patient experience on romiplostim, this confirms that the survey is capturing real world ITP patient experience and preference.
Due to survey recruitment methods, the TRAPeze Netherlands study is a convenience sample: respondents were engaged with ITP Patiëntenvereniging Nederland and were motivated to complete the survey. This is likely to select for respondents who have more disposable time, access to technology and those that are highly motivated, given the length of the survey. Furthermore, as TRAPeze was disseminated via ITP Patiëntenvereniging Nederland, a potential selection bias is present for patients engaged enough to have membership with a PAG. To overcome this potential bias, similar surveys in the future could be circulated during medical appointments, in combination with PAGs as described here.
Specific survey questions required respondents to provide information from >2 years prior to completing the survey. Such instances may have introduced inaccuracy in responses and should, therefore, be interpreted carefully. In addition, while the survey was professionally translated from English to Dutch by a native Dutch speaker, and reviewed by the Dutch pharmaceutical affiliate, a second reverse translation was not conducted. In future, patient preference surveys fielded in multiple geographies may benefit from including a reverse translation step, to further optimize consistency of messaging.
As avatrombopag had only recently been approved in the Netherlands at time of survey fielding, no respondents had been taking avatrombopag for >3 months. As a result, no respondents taking avatrombopag were included. This will have limited the results to the patient experience on eltrombopag and romiplostim.
Finally, though highly useful, the TRAPeze PBS format does not constitute a validated instrument to measure the patient experience, such as EQ-5D or SF-12 [Citation40,Citation41]. This reduces how comparable TRAPeze survey results are with those of other studies.
6. Conclusion
While TPO-RAs are an effective second-line therapy for ITP, many patients’ QoL and daily functioning remain adversely impacted by persistent symptoms of disease. This study highlights fatigue in particular as a persistent symptom that impacts social relationships and hinders patients’ ability to fulfill employment responsibilities. An indirect societal cost can be attributed to the loss of productivity seen in some ITP patients, demonstrating the unmet patient need for tackling fatigue as a symptom of ITP. Consistent with other TRAPeze cohorts, TPO-RA preference in the Dutch cohort was primarily driven by method of administration, drug and food interactions, and frequency of dosing. TPO-RAs with attribute profiles which would encourage treatment adherence may, therefore, yield improved response rates. Insight from the Dutch TRAPeze cohort elucidates unmet patient needs in ITP and may facilitate improved management by promoting the role of patient choice in treatment selection and improving QoL.
Supplemental Material
Download MS Word (66 KB)Acknowledgements
The authorships are grateful for the contributions of Ineke Steetskamp from the Dutch ITP patient association (ITP Patiëntenvereniging Nederland) and thank the members of the association for their participation in this study. Thanks also to Sjoerd Visser-Peereboom, Sobi and Evemie Schutyser, Sobi for their support throughout survey fielding and publication.
Disclosure statement
AJGJ reports compensation for acting as a speaker on behalf of 3sBio, Amgen, Novartis, fees for international advisory board attendance from Novartis, and research funding from CSL, Behring, Argenx, Principia Biopharma. VM has received consultancy fees from Amgen, Bayer, Novartis and Sobi, and research funding from Grifols. AN has received honoraria from Amgen, Angle, Argenx, Dova, Novartis, Ono and Shionogi, and consultancy fees from Amgen, Angle, Argenx and Dova. MM has received consultancy fees from Novartis, Sobi and UCB. MB, KW and DE are employees of Sobi. SP, EG and ODP are employees of Wickenstones, which received funding from Sobi for this research. Sobi reviewed and provided feedback on the manuscript.
Data availability statement
Data supporting the findings presented here are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Kashiwagi H, Tomiyama Y. Pathophysiology and management of primary immune thrombocytopenia. Int J Hematol 2013;98:24–33. doi:10.1007/s12185-013-1384-y
- Cines DB, Bussel JB, Liebman HA, et al. The ITP syndrome: pathogenic and clinical diversity. Blood J Am Soc Hematol. 2009;113(26):6511–6521.
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–3866. doi:10.1182/bloodadvances.2019000966
- Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780–3817. doi:10.1182/bloodadvances.2019000812
- Mithoowani S, Arnold DM. First-line therapy for immune thrombocytopenia. Hämostaseologie. 2019;39(03):259–265. doi:10.1055/s-0039-1684031
- Park YH, Kim D-Y, Kim S, et al. Management of immune thrombocytopenia: 2022 update of Korean experts recommendations. Blood Res. 2022;57(1):20–28. doi:10.5045/br.2022.2022043
- Cuker A, Prak ETL, Cines DB. Can immune thrombocytopenia be cured with medical therapy? Semin Thromb Hemost 2015;41(4):395–404.
- Mithoowani S, Gregory-Miller K, Goy J, et al. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 2016;3(10):e489–ee96. doi:10.1016/S2352-3026(16)30109-0
- Alharbi MG, Alanazi N, Yousef A, et al. COVID-19 associated with immune thrombocytopenia: a systematic review and meta-analysis. Expert Rev Hematol. 2022;15(2):157–166. doi:10.1080/17474086.2022.2029699
- Lozano ML, Segú-Vergés C, Coma M, et al. Elucidating the mechanism of action of the attributed immunomodulatory role of eltrombopag in primary immune thrombocytopenia: an in silico approach. Int J Mol Sci. 2021;22(13):6907. doi:10.3390/ijms22136907
- Bussel JB, Soff G, Balduzzi A, et al. A review of romiplostim mechanism of action and clinical applicability. Drug Des Devel Ther. 2021;15:2243–2268.
- Markham A. Avatrombopag: a review in thrombocytopenia. Drugs. 2021;81:1905–1913.
- FDA. Eltrombopag full prescribing information; 2015. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207027s000lbl.pdf.
- EMA. Eltrombopag summary of product characteristics; 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/revolade-epar-product-information_en.pdf.
- FDA. Romiplostim full prescribing information; 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125268s163lbl.pdf.
- EMA. Romiplostim summary of product characteristics; 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/nplate-epar-product-information_en.pdf.
- FDA. Avatrombopag full prescribing information; 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210238s001lbl.pdf.
- EMA. Avatrombopag summary of product characteristics; 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/doptelet-epar-product-information_en.pdf.
- González-Porras JR, Godeau B, Carpenedo M. Switching thrombopoietin receptor agonist treatments in patients with primary immune thrombocytopenia. Ther Adv Hematol. 2019;10:2040620719837906.
- Al-Samkari H, Kuter DJ. Optimal use of thrombopoietin receptor agonists in immune thrombocytopenia. Ther Adv Hematol. 2019;10:2040620719841735.
- NICE. Avatrombopag for treating primary chronic immune thrombocytopenia. Technology appraisal guidance [TA853]; 2022. Available from: https://www.nice.org.uk/guidance/ta853.
- Gilbert MM, Grimes AB, Kim TO, et al. Romiplostim for the treatment of immune thrombocytopenia: spotlight on patient acceptability and ease of use. Patient Prefer Adherence. 2020;14:1237–1250.
- Al-Samkari H, Jiang D, Gernsheimer T, et al. Adults with immune thrombocytopenia who switched to avatrombopag following prior treatment with eltrombopag or romiplostim: a multicentre US study. Br J Haematol 2022;197(3):359–366. doi:10.1111/bjh.18081
- Cooper N, Hill QA, Grainger J, et al. Tapering and discontinuation of thrombopoietin receptor agonist therapy in patients with immune thrombocytopenia: results from a modified Delphi panel. Acta Haematol 2021;144(4):418–426. doi:10.1159/000510676
- Lucchini E, Palandri F, Volpetti S, et al. Eltrombopag second-line therapy in adult patients with primary immune thrombocytopenia in an attempt to achieve sustained remission off-treatment: results of a phase II, multicentre, prospective study. Br J Haematol 2021;193(2):386–396. doi:10.1111/bjh.17334
- Cooper N, Kruse A, Kruse C, et al. Immune thrombocytopenia (ITP) World Impact Survey (iWISh): patient and physician perceptions of diagnosis, signs and symptoms, and treatment. Am J Hematol 2021;96(2):188–198. doi:10.1002/ajh.26045
- McDonald V, Newland A, Morgan M, et al. Patient preferences and experiences regarding thrombopoietin-receptor agonists for immune thrombocytopenia in the United Kingdom and Ireland (TRAPeze UK & IE study). Hematology. 2021;26(1):799–808. doi:10.1080/16078454.2021.1978689
- Efficace F, Mandelli F, Fazi P, et al. Health-related quality of life and burden of fatigue in patients with primary immune thrombocytopenia by phase of disease. Am J Hematol 2016;91(10):995–1001. doi:10.1002/ajh.24463
- Tarantino MD, Mathias SD, Snyder CF, et al. Impact of ITP on physician visits and workplace productivity. Curr Med Res Opin. 2010;26(2):319–328. doi:10.1185/03007990903451298
- Lucchesi A, Lovrencic B, McDonald V, et al. Pcr246 patient preferences regarding thrombopoietin-receptor agonists for immune thrombocytopenia in Italy (TRAPeze Italy study). Value Health. 2022;25(12):S437–S4S8.
- Nederland P. Patiëntenvereniging Nederland Homepage; 2023. Available from: https://www.itp-pv.nl/over-de-patientenvereniging.
- Database DG. Number of users 2017-2021 for ATC subgroup B02BX: Other systemic haemostatics; 2022. Available from: https://www.gipdatabank.nl/databank?infotype=g&label=00-totaal&tabel=B_01-basis&geg=gebr&item=B02BX.
- Rovó A, Cantoni N, Samii K, et al. Real-world impact of primary immune thrombocytopenia and treatment with thrombopoietin receptor agonists on quality of life based on patient-reported experience: results from a questionnaire conducted in Switzerland, Austria, and Belgium. Plos one. 2022;17(4):e0267342.
- Zwaginga JJ, van der Holt B, Te Boekhorst PA, et al. Multi-center randomized open label phase II trial on three rituximab dosing schemes in immune thrombocytopenia patients. Haematologica. 2015;100(3):e90. doi:10.3324/haematol.2014.110213
- Ghanima W, Cooper N, Rodeghiero F, et al. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019;104(6):1112–1123. doi:10.3324/haematol.2018.212845
- Kuter DJ, Bussel JB, Newland A, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol 2013;161(3):411–423. doi:10.1111/bjh.12260
- Kruse A, Kruse C, Potthast N, et al. Mental health and treatment in patients with immune thrombocytopenia (ITP); data from the Platelet Disorder Support Association (PDSA) Patient Registry. Blood. 2019;134:2362. doi:10.1182/blood-2019-122278
- Terrell DR, Reese J, Branesky D, et al. Depression in adult patients with primary immune thrombocytopenia. Am J Hematol 2016;91(10):E462–E4E3.
- Commission E. Labour market information: Netherlands; 2022. Available from: https://eures.ec.europa.eu/living-and-working/labour-market-information/labour-market-information-netherlands_en.
- Ruotolo I, Berardi A, Sellitto G, et al. Criterion validity and reliability of SF-12 health survey version 2 (SF-12v2) in a student population during COVID-19 pandemic: a cross-sectional study. Depress Res Treat. 2021;2021:1–10.
- Sanz MA, Aledort L, Mathias SD, et al. Analysis of EQ-5D scores from two phase 3 clinical trials of romiplostim in the treatment of immune thrombocytopenia (ITP). Value Health. 2011;14(1):90–96. doi:10.1016/j.jval.2010.10.017

![Figure 5. (A) Respondents’ typical weekly working hours, by gender (n = 50). (B) Respondents’ reduction in weekly working hours due to ITP (n = 47) (Excluding responses ‘I don’t know’ [n = 3]). Percentages may not add up to 100 due to rounding.](/cms/asset/6e5033b9-6e9a-4cf1-a03c-f2a5a7ba146f/yhem_a_2267942_f0005_oc.jpg)