ABSTRACT
Objective
This study aimed to evaluate the efficacy of azacitidine (AZA) combined with danazol (DNZ) and thalidomide (THD) maintenance therapy after intensive chemotherapy (IC) in patients with acute myeloid leukemia (AML).
Methods
we retrospectively analyzed the clinical data of 11 patients treated with AZA combined with DNZ and THD as maintenance therapy after IC at the Baiyun Hospital were between February 2017 and March 2021. The patients' clinical features, relapse-free survival (RFS), and overall survival (OS) were analyzed.
Results
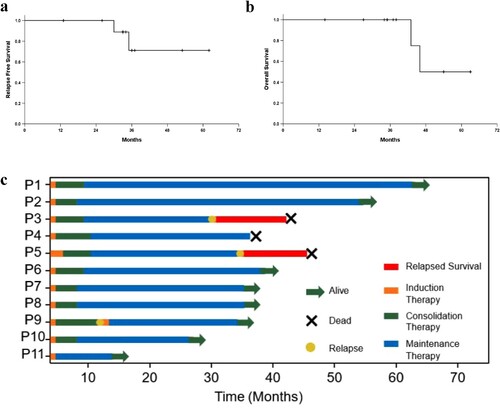
Eleven cases fulfilled the AML criteria per the 2016 World Health Organization classification. Of the 11 patients, five were females, and six were males, with a median age of 45 years (range, 23-65 years). Ten patients were in the first complete remission (CR1), and one patient was in the second complete remission (CR2). All patients received AZA combined with DNZ and THD maintenance therapy after IC. The median number of AZA cycles received was 7 (6-12). Until June 2022, the median follow-up period was 37 (14-63) months; one patient had a relapse, and three died. RFS at 1 year and 3 years was 100% and 71.1%, respectively, and OS at 3 years was 100%.
Conclusion
AZA combined with DNZ and THD maintenance therapy is effective for patients with AML who are ineligible for allogeneic hematopoietic stem cell transplantation. Further studies with large sample sizes and randomized are needed to verify these findings.
Introduction
Although more patients with acute myeloid leukemia (AML) have had better outcomes in recent decades, the relapse rates after intensive chemotherapy (IC) remain high. This is especially true for patients who are ineligible for allogeneic hematopoietic stem cell transplantation (allo-HSCT) for various reasons (financial constraints, lack of an acceptable donor, comorbidities, etc.) [Citation1–3]. The cost of allo-HSCT is one of the most important reasons in our country. Most of the patients with AML cannot affort the cost, some patients even have to borrow the money to pay for chemotherapy. Outcomes in patients with relapse remain poor. Thus, how to prevent relapse in patients with AML is still a major therapeutic challenge.
No maintenance therapy is used as a standard treatment for AML because of its insufficient efficacy [Citation4]. Previous studies have shown that the clinical benefits of maintenance therapies with interleukin-2 (IL-2), low-dose chemotherapy, and gemtuzumab ozogamicin are controversial [Citation5–7]. Recently, several drugs, including hypomethylating agents (azacitidine (AZA) and decitabine) [Citation8,Citation9], immunomodulating agents (lenalidomide) [Citation10], androgen [Citation11], kinase inhibitors (sorafenib, midostaurin, etc.) [Citation12–15], which are maintenance therapies in patients with AML, have shown better event-free survival (EFS). Particularly, oral azacytidine significantly improves the overall survival (OS) and relapse-free survival (RFS) in patients with AML and recommended by NCCN guidelines as maintenance therapy in AML indicating that maintenance therapy is an important aspect of AML treatment [Citation16].
Previous reports had shown that maintenance therapy with AZA, androgen and lenalidomide alone could improve AML patients DFS through different mechanisms [Citation8,Citation10,Citation11]. Let us considered that AZA in combination with danazol (DNZ) and thalidomide (THD) as maintenance therapy may have a synergistic anti-leukemia effect, and DNZ may reduce the adverse effects of AZA and THD, such as cytopenia, fatigue,etc. So we designed maintance therapy including those three medicines.
Here, the clinical data of 11 AML patients treated with AZA combined with DNZ and THD as maintenance therapy were retrospectively analyzed. This study aimed to observe the efficacy of maintenance therapy, along with RFS and OS.
Patients and methods
Patients
Eleven patients diagnosed with AML and treated at the Baiyun Hospital between February 2017 and March 2021 were treated with this maintenance treatment. All patients met the AML criteria of the 2016 World Health Organization classification. All patients signed an informed consent document before treatment and complied with the Declaration of Helsinki and institutional guidelines.
Treatment: induction and consolidation treatment
The induction and consolidation therapies for newly diagnosed patients with AML were based on the Chinese guidelines for diagnosing and treating adult patients with AML (not acute promyelocytic leukemia) [Citation17]. Induction therapy involved daunorubicin 60 mg/m2/day intravenously on days 1–3 and cytarabine (Ara-c) 100 mg/m2/day on days 1–7. Acceptance reinduction therapy was administered if the patient did not achieve complete remission (CR). Three post-remission cycles of consolidation chemotherapy with high-dose Ara-c (3 g/m2/q12h d1–3) and two cycles of a reduced dose of chemotherapy (DA/HA/EA) were administered for five days.
Maintenance therapy with AZA combined with DNZ and THD
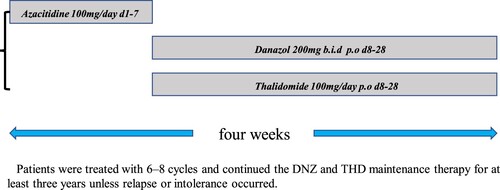
After the completion of consolidation therapy, patients remained in CR and were treated with 6–8 cycles of AZA combined with DNZ and THD maintenance therapy: AZA 100 mg/day (approximately 50–75 mg/m2/day) d1–7 by subcutaneous injection, DNZ 200 mg b.i.d., and THD 100 mg/day p.o., followed by AZA every four weeks. After completing the 6–8 cycles, patients continued the DNZ and THD maintenance therapy for at least three years unless relapse or intolerance occurred().
Disease evaluation included bone marrow examinations every three months for the first two years and then every six months until a CR duration of more than five years.
Criteria for response and clinical follow-up
A patient was considered to have achieved CR after meeting all the following conditions: absence of leukemic blasts in blood and <5% blasts in the bone marrow; absolute neutrophil count (ANC) > 1 × 109/L and platelet count (PLTC) > 100 × 109/L; lack of evidence of extramedullary disease. Minimal residual disease (MRD) negative refers to the failure to detect residual leukemia cells in bone marrow specimens by flow cytometric analysis (FCM) and real-time quantitative polymerase chain reaction (RT-qPCR). Relapse was the presence of bone marrow blast cells (>5%) or extramedullary disease. OS was calculated from the time of diagnosis to death or the last follow-up. RFS was calculated from the first remission to the first relapse (during maintenance therapy) or the last follow-up.
Statistical analysis
RFS and OS rates were extracted based on Kaplan–Meier estimates. The results were analyzed using Statistical Package for Social Sciences version 20.
Results
Patient characteristics
Eleven patients treated with AZA combined with DNZ and THD maintenance therapy between February 2017 and March 2021. shows the baseline patient and disease characteristics. Of the 11 patients, five were females, and six were males, with a median age of 45 years (range, 23–65 years) at the time of diagnosis. The median white blood cell count was 37.55 × 109/L (range: 1.04–132.96). Before starting maintenance therapy, 10 patients were in the first complete remission (CR1), and one patient was in the second complete remission (CR2). Patients were classified as favorable (4/11), intermediate (5/11), and adverse (2/11) according to the European Leukemia Net genetic risk stratification(2017).
Table 1. Characteristics of the 11 patients with AML.
All patients were treated with DA induction therapy: 10 patients achieved remission after one course of treatment, whereas one patient achieved remission after salvage therapy. Ten patients completed all consolidation therapy courses, including three courses of high-dose Ara-c and two courses of a low-dose 5-day regimen. One patient diagnosed with AML with t(8;21) refused to continue chemotherapy because of renal failure after remission and was directly switched to maintenance therapy. Ten patients completed all consolidation chemotherapy cycles, nine of whom began maintenance therapy. One patient with AML with t(8;21) relapsed after stopping chemotherapy for two months and achieved CR2 by FLAG reinduction therapy, refused continuous chemotherapy and transplantation, and directly switched to maintenance therapy. Only one patient was not evaluated for MRD, and all others were MRD negative before starting maintenance therapy.
All patients received a median of seven (6–12) courses of AZA combined with DNZ and THD continuous therapy. Treatment was well tolerated and accepted by the patients. All patients received continuous DNZ and THD maintenance therapy after finishing AZA. Common adverse events included mild neutropenia, thrombocytopenia, and elevated aminotransferase(). One patient had a significant increase in urea nitrogen and creatinine because the patient was diagnosed with renal failure after remission before maintenance therapy. No patient discontinued the maintenance therapy due to adverse events. The patient in CR2, after reinduction therapy, received 12 courses of AZA. AZA was given every two months after 10 cycles.
Table 2. Adverse events of patients during maintenance treatment.
Outcomes
The median follow-up period was 37 (14–63) months until June 2022. The expected RFS at 1 and 3 years was 100% and 71.1%, respectively, and OS at 3 years was 100% ((a and b)). Three of the 11 patients died, and eight were alive with EFS. Of the three patients who died, one relapsed at 36 months and died of disease progression at 46 months. The blood test of the second patient was abnormal at 30 months, and a bone marrow puncture was performed, confirming the diagnosis of mixed phenotypic acute leukemia (T/My, NOS), and the patient died at 43 months. The third patient died of a traffic accident at 37 months while in CR1. Notably, two cases of AML with t(8; 21) (one patient relapsed after consolidation chemotherapy, and another patient developed renal failure after DA induction therapy) remained in CR by the end of the follow-up period. The CR duration was 24 and 13 months, respectively. The latest bone marrow MRD test showed AML/ETO 0%. (c) shows the Swimmer plot of the 11 patients.
Discussion
Relapse is still a major problem for patients with AML, especially for those who cannot undergo allo-HSCT. Thus, an effective and safe post-remission maintenance therapy is needed to improve RFS and OS for patients with AML remission after induction and consolidation.
Although clinicians have been using various maintenance therapies after IC or HSCT to improve RFS and OS for many years, maintenance therapies are not a part of the definite treatment schedule for AML. Multiple recent studies evaluating maintenance therapies after IC or HSCT using different agents, such as lenalidomide, androgens, HMAs (AZA, oral azacitidine, and decitabine), kinase inhibitors (sorafenib, midostaurin, and gilteritinib), and BCL-2 inhibitors, have shown encouraging results with improved RFS and OS.
In this study, we evaluated the efficacy and safety of AZA combined with DNZ and THD as maintenance therapy after IC for patients with AML who cannot undergo allo-HSCT. This combination has never been reported. We combined the three drugs for the following reasons: (i) AZA was used because previous studies have shown its anti-leukemia effect, and a recent study has shown that AZA maintenance therapy after IC improves disease-free survival in elderly patients with AML [Citation8]. (ii) DNZ was used due to Pigneux reported that maintenance therapy with norethandrolone improved survival in elderly patients with AML [Citation11], on the other hand DNZ may reduce adverse events such as hemocytopenia, fatigue caused by AZA and THD so that patients would not interrupt treatment. (iii) THD’s precise mechanism of action in hematological tumors remains unclear. Previous studies have shown that THD has the following effects: anti-cell proliferation, induction of apoptosis, blocking tumor-stroma interactions, and changes in immune responses. We are now conducting pre-clinical work to investigate the synergistic effect or potential synergistic mechanism of these three drugs.
This study demonstrated that AZA combined with DNZ and THD could be safely administered to patients after IC. All our patients received a median of seven courses of AZA (6–12) combined with DNZ and THD continuous therapy and continued DNZ and THD oral maintenance therapy. The major side effects included mild neutropenia, thrombocytopenia, and elevated aminotransferase. None of the patients interrupted treatment because of adverse events.
By the end of the follow-up period, three patients had died. However, only one case died of disease relapse and progression. Of the remaining two patients, one was diagnosed with mixed phenotypic acute leukemia (T/My, NOS) and died, while the other died in a road accident. RFS at 1 and 3 years was 100% and 71.1%, respectively; OS at 3 years was 100%. The reasons for this result are as follows: (1) our maintenance treatment; (2) most of the patients entering maintenance therapy belonging to the favorable (4/11) or intermediate (5/11) risk group and being MRD negative (10/11). Due to most of our patients did not proceed the NGS test, the ELN risk of some patients may not precise. Two patients were diagnosed with AML with t(8;21). One relapsed, and the other received only one induction therapy because of renal failure but remained in CR until the end of the follow-up period. The CR duration was 24 and 13 months, respectively, which implies that AZA combined with DNZ and THD is an effective treatment. Although the number of patients was small, AZA combined with DNZ and THD maintenance therapy showed efficacy and safety. It provides an excellent treatment for patients who cannot undergo allo-HSCT.
In conclusion, this is the first study to report the efficacy and safety of AZA combined with DNZ and THD as maintenance therapy in patients with AML. Based on the results of this study, we are conducting a randomized trial in order to verify these findings.
Author's contributions
Fang Zheng and Qianqian Li collected the data and wrote the manuscript. Sisi Yang, Zhen Zhou, Qingfan Zeng and Fang Zheng were involved in patient management and clinical data collection. Kaiqi Liu and Fangzheng designed therapeutic regimen. Kaiqi Liu revised the manuscript and provided valuable advice.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Consent for publication
All authors have read and approved the manuscript for publication.
Ethics approval and consent to participate
All studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki Declaration 2013. Written informed consent was obtained from the patients or patients’ parents/legal guardians.
Acknowledgements
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bailey C, Richardson LC, Allemani C, et al. Adult leukemia survival trends in the United States by subtype: a population-based registry study of 370,994 patients diagnosed during 1995–2009. Cancer 2018 Oct 1;124(19):3856–3867. doi:10.1002/cncr.31674
- Laura FN, Rachel JC. Advances in acute myeloid leukemia. Br Med J. 2021;375:n2026.
- Hagop MK, Nicholas JS, Amir TF, et al. Acute myeloid leukemia: historical perspective and progress in research and therapy over 5 decades. Clin Lymphoma Myeloma Leuk. 2021 Sep;21(9):580–597. doi:10.1016/j.clml.2021.05.016
- dL M, Gail JR, Uwe P, et al. AML and the art of remission maintenance. Blood Rev. 2021 Sep;49:100829. doi:10.1016/j.blre.2021.100829
- Marc B, Pierre S, Beverly JL, et al. Individual patient data meta-analysis of randomized trials evaluating IL-2 monotherapy as remission maintenance therapy in acute myeloid leukemia. Blood. 2011 Jun 30;117(26):7007–7013. doi:10.1182/blood-2011-02-337725
- Robles C, Kim KM, Oken MM, et al. Low-dose cytarabine maintenance therapy vs observation after remission induction in advanced acute myeloid leukemia: an eastern cooperative oncology group trial (E5483). Leukemia. 2000;14:1349–1353. doi:10.1038/sj.leu.2401850
- Lowenberg B, Beck J, Graux C, et al. Gemtuzumab ozogamicin as postremission treatment in AML at 60 years of age or more: results of a multicenter phase 3 study. Blood. 2010;115:2586–2591. doi:10.1182/blood-2009-10-246470
- Huls G, Chitu DA, Havelange V, et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133:1457–1464. doi:10.1182/blood-2018-10-879866
- Blum W, Sanford BL, Klisovic R, et al. Maintenance therapy with decitabine in younger adults with acute myeloid leukemia in first remission: a phase 2 cancer and leukemia group B study (CALGB 10503). Leukemia. 2017;31:34–39. doi:10.1038/leu.2016.252
- Xie CH, Wei M, Yang FY, et al. Efficacy and safety of lenalidomide for the treatment of acute myeloid leukemia: a systematic review and meta-analysis. Cancer Manag Res. 2018;10:3637–3648. doi:10.2147/CMAR.S168610
- Pigneux A, Béné MC, Guardiola P, et al. Addition of androgens improves survival in elderly patients with acute myeloid leukemia: a GOELAMS study. J Clin Oncol. 2017;35:387–393. doi:10.1200/JCO.2016.67.6213
- Burchert A, Bug G, Fritz LV, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3–internal tandem duplication mutation (SORMAIN). J Clin Oncol. 2020;38:2993–3002. doi:10.1200/JCO.19.03345
- Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377:454–464. doi:10.1056/NEJMoa1614359
- Larrosa-Garcia M, Baer MR. FLT3 inhibitors in acute myeloid leukemia: current status and future directions. Mol Cancer Ther. 2017;16:991–1001. doi:10.1158/1535-7163.MCT-16-0876
- Sandmaier BM, Khaled S, Oran B, et al. Results of a phase 1 study of quizartinib as maintenance therapy in subjects with acute myeloid leukemia in remission following allogeneic hematopoietic stem cell transplant. Am J Hematol. 2018;93:222–231. doi:10.1002/ajh.24959
- Roboz GJ, Dohner H, Pocock C, et al. Health-related quality of life (HRQoL) in the phase III QUAZAR-AML-001 trial of CC-486 as maintenance therapy for patients with acute myeloid leukemia (AML) in first remission following induction chemotherapy (IC). J Clin Oncol. 2020;38:7533. doi:10.1200/JCO.2020.38.15_suppl.7533
- Leukemia & Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association. Chinese guidelines for diagnosis and treatment of adult acute myeloid leukemia (not APL) (2017). Chin J Hematol. 2017;38(3):177–182.
- Schreiber AD, Chien P, Tomaski A, et al. Effect of danazol in immune thrombocytopenic purpura. N Engl J Med. 1987;316:503–508. doi:10.1056/NEJM198702263160903