ABSTRACT
Aim
Hemophilia A (HA) is an inherited bleeding disorder caused by a deficiency of clotting factor VIII in the blood. In resource-limited settings like India, affordability is a significant challenge in managing patients with severe HA. This study aims to assess the cost-effectiveness of intermediate-dose prophylaxis versus on-demand factor therapy in adult and pediatric populations with moderate-to-severe congenital HA without inhibitors in India.
Method
We conducted a prospective cost-effectiveness analysis from a societal perspective, categorizing patients into a base state and a joint disease state (patients with Hemophilia suffering extensive bleeds leading to chronic joint disease). Using targeted literature search and primary market research, we developed a Markov model measuring the total cost of Hemophilia treatment and health outcomes, including life-years (LYs), quality-adjusted life-years (QALYs), incremental cost-utility ratio (ICUR), and incremental cost-effectiveness ratio (ICER). The model extended over a lifetime horizon of 70 years with a one-year cycle length. Sensitivity analyses assessed study robustness.
Results
Low-dose prophylactic therapy was cost-effective for adults (>18 years) and pediatric populations (<18 years), yielding better health outcomes (adults: 0.15 LYs and 2.43 QALYs gained; pediatric: 0.40 LYs and 3.12 QALYs gained). Intermediate-dose prophylaxis showed positive net monetary benefits in terms of Quality-Adjusted Life Years (QALYs) for both adult and pediatric populations, with dominant ICER and ICUR values in both cases.
Conclusion
Using intermediate-dose prophylactic factor VIII therapy is a cost-effective approach that improves clinical outcomes compared to on-demand therapy in the Indian adult and pediatric HA populations without inhibitors.
Introduction
Hemophilia A (HA), an X-linked inherited bleeding disorder, is marked by a deficiency of clotting factor VIII in the plasma. In individuals with severe deficiency, it results in spontaneous and/or traumatic bleeding episodes, predominantly affecting joints, soft tissues, muscles, and body cavities. [Citation1]. Patients with hemophilia are categorized as mild, moderate, and severe—depending on the percent of normal clotting factor present [Citation2]. As per the World Federation of Hemophilia annual report, 2020, a total of 347,026 patients globally had bleeding episodes, with 165,379 patients suffering from HA [Citation3]. In India, 18,928 patients have been identified with HA, with the majority of patients in the age range of 19–44 years (43%) [Citation3].
Treatment approaches for HA in India include lifestyle modification, physiotherapy, and on-demand or prophylactic treatment of bleeding events using replacement therapy with clotting factor VIII concentrates [Citation2,Citation4]. While prophylaxis has become the standard of care for severe hemophilia, the utilization of prophylactic clotting factor treatment in India remains limited to just 9% of individuals under the age of 18 with severe hemophilia [Citation1,Citation3]. In developed countries, the established approach to treating hemophilia involves intermediate-dose prophylaxis (injection of factor VIII at a dose of 15–25 units/kg, administered intravenously two to three times a week). This regimen has demonstrated remarkable joint health benefits when implemented over extended periods.
It has been observed that HA significantly hampers the quality of life (QoL) and holistic wellbeing of patients and their family members [Citation2]. Prophylactic treatment has demonstrated a substantial enhancement in both clinical and economic outcomes for patients when contrasted with on-demand therapy. [Citation1,Citation5–20].
In India, patients with HA often experience inadequate medical insurance coverage. However, this issue is currently being addressed through government support initiatives.
Presently, approximately 85% of partial or complete financial assistance is provided by the government for on-demand therapy, while prophylaxis support is offered to a limited extent in a few states. The substantial treatment costs continue to pose a significant barrier and source of concern for all stakeholders [Citation21,Citation22].
The guidelines set forth by the Indian Academy of Pediatrics (IAP) also emphasize the potential benefits of low-dose prophylaxis (based on research) commencing at an early age, aiming to enhance the quality of life for patients [Citation23,Citation24]. Furthermore, to facilitate hemophilia care, the government has established hemophilia treatment centers that offer free anti-hemophilic factor. However, accessibility to these centers remains limited [Citation25]. In an effort to address accessibility issues, a pilot hematology program under the National Rural Health Mission has demonstrated a 21% reduction in out-of-pocket (OOP) expenses [Citation22,Citation26].
The prevalence of inhibitors that neutralize the effects of recombinant clotting factors in India ranges from 8.2% to 13%, further contributing to the overall economic burden [Citation2,Citation27]. Direct costs of hemophilia encompass expenses related to medications, laboratory tests, hospitalization, surgical procedures, and consultation fees. Additionally, there are indirect associated costs such as loss of work productivity, inability to attend educational institutions, disability due to illness, and even mortality [Citation5].
Many economic evaluations have been conducted to compare on-demand versus prophylactic treatment in terms of cost-effectiveness (CE) and/or cost-utility [Citation6,Citation15]; however, there is a dearth of such analysis from an Indian perspective. The objective of the current pilot study is to conduct a CE analysis to evaluate prophylaxis versus on-demand factor treatment in people with moderate-to-severe congenital HA without inhibitors in India.
Methods
Study population
A CE analysis from the societal perspective was conducted from October 2021–May 2022 among patients with moderate-to-severe HA without inhibitors (adult: 18 years and above; pediatric: 2–18 years of age). The model was conceptualized to evaluate the treatment flow of hemophilia-A patients with the two modes of factor VIII therapy. A virtual cohort was introduced into this model concept with intermediate-dose prophylaxis and on-demand therapy.
Treatment regimens
Treatment regimens were analyzed based on the information provided by various published studies on the accepted prophylactic dosages. For intermediate prophylaxis 15–25 IU/kg twice a week were used for calculations [Citation28]. In case of low dose prophylaxis quantity of 10 IU/kg once or twice a week was used for analysis [Citation9]
For on demand therapy, treatment regime according to the WFH 2020 guidelines was followed where FVIII is given only after bleeding. Severe bleeds, such as intracranial or psoas bleeds, require a more intensive and extended period of clotting factor replacement (80–100% correction) to ensure effective control and healing. Moderate bleeds are managed with a lower level of correction (50% correction) for a shorter duration with specific amount of clotting factor administered being FVIII 25 IU/kg.
Targeted literature review
A targeted literature review was conducted for the development of the model from the societal perspective. Literature on treatment guidelines; available clinical data (Indian/global)—meta-analysis; published economic data; cost and resource use; and other model inputs (utilities, disutilities, adverse events, morbidity, and mortality) were considered. Inclusion and exclusion criteria were based on Population, Intervention, Comparison, and Outcome criteria. The population included were patients with moderate-to-severe congenital HA without inhibitors across all age groups; the intervention/comparator was intermediate-dose prophylaxis with clotting factor VIII versus on-demand factor therapy; and outcomes were clinical and economic data, QoL, bleed-free model studies, and India-specific budget impact model studies (). Limited use of secondary data was a key parameter to obtaining directional results.
Table 2. Inclusion parameters for targeted literature review.
Model structure and outcomes derived
The Markov model [Citation29] was developed with three discrete health states (base, joint disease, and death) and two transitory health events with a model cycle length of 1 year (). The movement of patients from different states was determined by transition probabilities. The model was run with secondary literature inputs to collect primary data. The post collection of primary data, final inputs were used to generate the results for the analysis. Cost-associated outcome from the study is the total direct and indirect cost of prophylactic and on-demand factor, while the health outcomes included life-years (LYs) and quality-adjusted life-years (QALYs). Aggregated outcomes, including ICUR (cost/QALY) and ICER (cost/LY), were also determined.
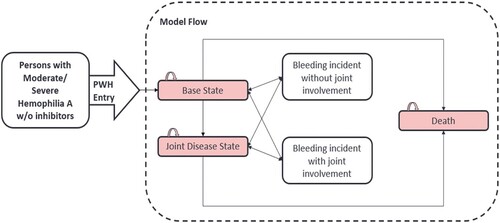
Figure 1. Model structure and different states of patients. Abbreviations: PWH: Patients with hemophilia: Base state in which persons with hemophilia A (PWH) are entering and starting therapy; Joint disease state: in which PWH have extensive bleeds leading to joint disease (requiring major surgery). Model flow: Individuals diagnosed with hemophilia initially experience a bleeding incident, which can occur with or without joint involvement. When a bleeding incident transpires without joint involvement, patients may either revert to the base state or advance to the joint disease state. Conversely, when a bleeding incident involves joints, patients may either revert to the joint disease state or show improvement and return to the base state. Both the base state and the joint disease state are associated with the possibility of death. The minimum age for entering the model was 2 years for the pediatric population and 18 years for the adult population.
Model parameters
Model clinical parameters were bifurcated into prophylactic and on-demand therapy, further subdivided into the adult and pediatric populations. The time horizon selected for the study was 70 years (computed based on the life expectancy in India). The minimum age for entering the model was 2 years for the pediatric population and 18 years for the adult population, with male population being 96.7% (based on the available literature). The adult population comprised of pediatric population diagnosed in early stages and were transitioned into adult after attaining 18 years or patients in rare scenario who have been diagnosed at the age of 18 years or above. Based on Key Opinion Leader (KOL) inputs, average doses and frequency values for prophylaxis and on demand treatment were used in the model. Intermediate-dose prophylaxis of 14.6 IU/kg/dose, 2.2 times a week for pediatric population and 14.2 IU/kg/dose, 1.6 times a week for adults was considered for the model. The values for on-demand were 27.5 IU/kg/dose, 2.1 times per bleed event for adults; and 24.6 IU/kg/dose, 1.9 times per bleed event for pediatric population. The cycle length for the model was 1 year, with half-cycle corrections being made. Bleed events within this 1 year were mapped in this morbidity-driven model. Based on KOL inputs, for prophylaxis therapy and on-demand therapy, annualized overall bleed rates of 1.9 and 11.6 bleed/year respectively were used for the model in base case for adult population. The annualized bleed rates of 2.5 and 12.9 bleed/year were used for the pediatric population for prophylaxis therapy and on-demand therapy respectively. The discounting factor considered in the study for costs and effects was 3.5%, as per the National Institute for Health and Clinical Excellence (NICE) standard [Citation30].The cost-effective threshold taken as per gross domestic product (GDP) per capita for India was INR (Indian Rupee) 140,462 [Citation31] and CE was determined at less than or equal to one time the GDP for India. For the baseline set taken in the model, the cost considered was a mix of literature review and public sector cost [Citation32–35]. The direct costs accrued in the model were medication, hospitalization, surgery, physician’s visit, and laboratory investigation while the indirect costs were absenteeism from school and work, wage, and productivity loss.
Study gaps were identified based on the dry run and proposed model. These gaps were the baseline to conduct market research to collate the required information on the gaps from physician KOLs treating hemophilia in India. The primary objective of market research conducted was to determine the healthcare resource use and cost in HA. After incorporating the inputs generated in the model, a finalized model was developed to run the CE analysis.
Sensitivity analysis outputs
Sensitivity analysis of the outcomes was performed for any variation in the input parameters and uncertainty in results. It was evaluated using one-way sensitivity analysis illustrated as a Tornado diagram and a probabilistic sensitivity analysis illustrated with an ICER scatter plot compared to a CE plane and a cost-effectiveness acceptability curve (CEAC). Economic model eliminates the use of p-values and significance is found through sensitivity analysis.
Results
Cost-associated outcomes
The total ‘per patient costs’ seen at a lifetime horizon for the intermediate-dose prophylactic therapy in comparison to on-demand therapy were established in the adult (INR 13,771,037.24 [USD 166,837.74] vs. 16,528,040.40 [USD 200239.16]) and pediatric (INR 15,846,745.00 [USD 191,985.18] vs. 18,779,924.5 1[USD 227,521.00]) populations. The cost difference was observed to be INR 2,757,002.79 [USD 33,401.41] for the adult population and INR 2,933,179.52 [USD 35,535.82] for the pediatric population (). The highest cost was attributable to the drug cost at ‘per patient level’ (adult: prophylaxis, INR 12,453,265.45 [USD 150,872.78]; on demand, INR 10,374,296.03 [USD 125,685.82]; pediatrics: prophylaxis, INR 13,602,155.15 [USD 164,791.71]; on demand, INR 11,059,180.60 [USD133,983.28]) followed by physician’s visit (adult: prophylaxis, INR 935,670.89 [USD 11,335.76]; on demand, INR 318,071.04 [USD 3,853.47]; pediatrics: prophylaxis, INR 1,402,177.58 [USD 1,6987.55]; on demand, INR 501,490.95 [USD 6,075.62]). The surgery costs at ‘per patient level’ were higher in on-demand therapy (Adult: INR 5,168,580.95 [USD 6,2617.97]; Pediatric: INR 6,243,111.17[USD 75,636.03]) (). The annual cost of factor VIII was found to be higher in adults than pediatric patients at ‘per patient level’ (Adult: prophylaxis, INR 685,235.20 (USD 8,309.15); on demand, INR 388,542[USD 4,711.46]; Pediatrics: prophylaxis, INR 286,654.94 [USD 3,475.97]; on demand, INR 103,480.61[USD 1,254.80]).
Figure 2. Study outcomes in terms of quality adjusted life years gained and total cost. Abbreviation: QALY: Quality adjusted life year.
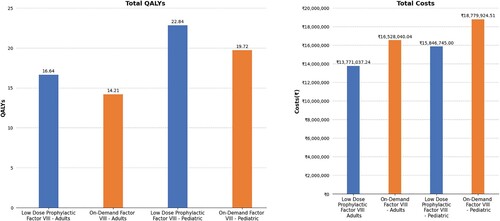
Table 1. Breakdown of cost distribution for adult and pediatric patients with hemophilia at ‘per patient level’.
Health-associated outcomes
QALY is a standardized measure of disease burden combining both the survival and QoL attributes in a single index [Citation36], while LYs gained represents only the quantity or number of years of life gained with the healthcare intervention [Citation37]. Both QALY and LY provide overall wellbeing of the patients, thereby giving a true representation of improvement in patient’s life.
Total LYs gained at ‘per patient level’ in a lifetime horizon after intermediate-dose prophylactic factor VIII was 19.683 years compared to on-demand therapy (19.529 years), with difference gained in LYs being 0.153 in adults. Total QALYs gained after intermediate-dose prophylactic factor VIII in adults was 16.644, while for on-demand factor VIII it was 14.213; the difference gained was 2.432. Total LYs gained after intermediate-dose prophylactic factor VIII in pediatric patients was 28.375 years, while for on-demand factor VIII, it was 27.972 years, with the difference being 0.403. The total QALYs gained at ‘per patient level’ after intermediate-dose prophylactic factor VIII in pediatric patients was 22.845, while for on-demand factor VIII, it was 19.721, with the difference of 3.124. Thus intermediate-dose prophylactic therapy was demonstrated to be superior in terms of QALYs, in both the adult and pediatric populations ().
Aggregated outcomes
The intermediate-dose prophylactic factor VIII therapy was found to be cost-effective as compared to on-demand therapy for both the adult and pediatric populations at ‘per patient level’. The net monetary benefits (per QALY) were positive for adult and pediatric population (INR 3,098,591 and INR 3,372,013 respectively) favoring intermediate-dose prophylactic therapy over the lifetime horizon. The ICUR and ICER gained throughout the life span at ‘per patient level’ were found to be dominant in low-dose prophylactic therapy (ICUR: on demand -INR 1,133,691[USD 13747.13] and -INR 938,856 [USD 11384.56] for adult and pediatric respectively; ICER: on demand -INR17,997,533 [USD 218237.91]and -INR7,270,668 [USD 88164.05] for adult and pediatric respectively).
Sensitivity analysis
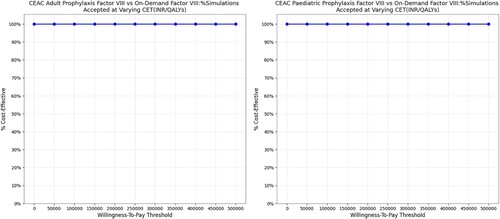
A tornado diagram for one-way sensitivity analysis established that the annualized joint bleed rate (adults) and overall annualized overall bleed rate (adults) in on-demand therapy along with the cost of surgery (at ‘per patient level’) caused the maximum change in the results (). In probabilistic sensitivity analysis, the scatter plot indicated that low-dose prophylactic factor was dominant over on-demand factor VIII therapy, both for the adult and pediatric populations (). Similarly, CEAC plots showed that prophylactic therapy was cost-effective at any willingness-to-pay level, in both the adult and pediatric populations ().
Figure 3. One-way sensitivity analysis representation. Abbreviations: ICUR: Incremental cost-utility ratio; QALY: Quality adjusted life year.
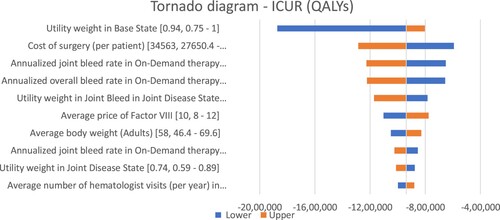
Figure 4. Probabilistic sensitivity analysis representing the cost-effectiveness cloud. Abbreviations: CE: Cost-effectiveness; QALY: Quality adjusted life year. The study analyzed certain probable factors that may affect the results of the study model. 1000 simulations were run to check the impact of these factors on the comparison between low-dose prophylactic factor and on-demand factor VIII therapy and the incremental scatter plot graph was generated. In majority of the simulations (99.8%), the low-dose prophylactic factor was better than on-demand factor VIII therapy which means that the low-dose prophylactic factor is not only less expensive but also more effective than on-demand factor VIII therapy. Hence, the factors tested had very little effect on the results of our study.
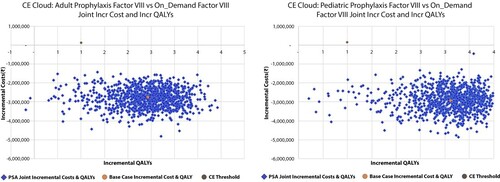
Figure 5. Probabilistic sensitivity analysis representing cost-effectiveness acceptability curve. Abbreviations: CEAC: Cost-effectiveness acceptability curve; INR: Indian Rupee; QALY: Quality adjusted life year. The PSA results were analyzed using the cost-effectiveness acceptability curve. The curve showed that Low-dose prophylactic factor therapy was below the cost-effectiveness threshold in all the simulations (100%) which indicates a cost-effective medication strategy. The prophylactic therapy was cost-effective for both adults and children, no matter what willingness-to-pay level was considered.
Discussion
Continuous prophylactic therapy is associated with decreased recurrent joint bleed incidence and reduced risk of development of arthropathy in children without preexisting joint damage [Citation18,Citation20], thereby demonstrating to improve the health-related QoL of the patients with HA over time. In this analysis, we have explored the costs and health outcomes of intermediate-dose prophylaxis versus on-demand therapy of factor VIII in the context of Indian adult and pediatric patients with HA.
The key outcomes of the study highlighted the CE of intermediate-dose prophylactic therapy in adult and pediatric populations in comparison to on-demand therapy with factor VIII, which leads to improved clinical and health outcomes, including ICER and ICUR. There was a gain in LYs and QALYs with intermediate-dose prophylaxis as compared to on-demand therapy along with net monetary benefits for both adult and pediatric populations. This was in line with the WFH 2020 and IAP guidelines [Citation38,Citation24].
Early prophylaxis reduces physical impairment from hemophilic arthropathy, maintains joint health throughout life, reduces hospitalization, surgeries, and bleeding episodes [Citation38–41].
Previously published literature demonstrated that low-dose prophylaxis therapy significantly reduces bleeding episodes (64.86%–68.99%), improves joint function (18.18% moderate improvement, with 55.76% mild improvement), improves QoL of patients, reduces hospitalization (60.34%), decreases absenteeism from work, and is cost-effective when administered to adult patients with moderate-to-severe hemophilia [Citation1,Citation6,Citation16]. For males aged 12–65 years with severe hemophilia A, administering prophylaxis two or three times a week resulted in a 97% reduction in the annualized occurrence of all bleeds compared to on-demand therapy[Citation18]. Adult patients-maintained adherence to late prophylaxis therapy, with benefits gained in terms of improved joint health (improved CAJAS[Colorado Adult Joint Assessment Scale] scores – 0.31 [n = 42] vs. + 0.63 [n = 42]) and health-related QoL (HAEMO-QoL-A scores + 3.98 [n = 41] versus – 6.00 [n = 42]), decreased bleeding episodes (reduced by 94%), less chronic pain (reduced by 50%), less health care resource utilization (twofold reduction), and improved satisfaction scores (64.3% patients were extremely satisfied with the treatment) [Citation19].
Prophylaxis that is initiated in adulthood (>18 years), primarily when arthropathy is already prevalent is often termed as tertiary prophylaxis. Tertiary prophylaxis is considered an effective treatment strategy in preventing progression of joint damage even though it is initiated after the onset of joint disease. The goal of tertiary prophylaxis is to minimize life threatening bleeds and bleeding frequency, improve QoL of the patients and improve activity/autonomy levels along with reducing worsening of arthropathy [Citation1,Citation42,Citation43]. Tertiary prophylaxis reduces the overall bleed episodes (8 vs. 25) and joint bleed episodes (3 vs. 9) and demonstrates improvement in the Hemophilia Joint Health Score (2 vs. 6 in 12 months) in comparison to on-demand therapy in pediatric population [Citation21]. Studies conducted in China (patients aged 2–18 years), exhibited decreased bleeding (63.3–68.9%), improved daily activity (60%), superior joint health outcomes (Joint-specific Hemophilia Joint Health Score: prophylaxis, 9.0 ± 10.3; on-demand, 11.9 ± 12.5), and improvement in Canadian Hemophilia Outcomes-Kids’ Life Assessment Tool score (57.6–61.9) [Citation7,Citation8,Citation13,Citation14]. Similarly, in Iran, prophylactic therapy was found to be cost-effective along with having higher effectiveness and utility in patients under 12 years of age (for prophylaxis: costs being $478,963.1 purchasing power parity [PPP], joint health score of 96.67, QALY of 11.98; and for on-demand regime: cost being $521,797.2 PPP, joint health score of 93.46, QALY of 10.99). [Citation15].
Secondary prophylaxis or tertiary prophylaxis is favored over on-demand therapy for the pediatric and young adult populations, owing to improvements in school activity participation score, daily activity, reduction in annual bleeds, reduction in hospitalizations, and minimal school absenteeism [Citation11,Citation12,Citation17,Citation20,Citation44]. The CE of secondary or tertiary prophylaxis using factor VIII is still an underexplored area in terms of the pediatric population in the Indian context.
The current study includes both adult and pediatric population to give a holistic view regarding clinical decision making. Despite promising CE and clinical outcomes, the study was associated with certain limitations. The utility weights were not specific to India but belonged to countries with similar socioeconomic backgrounds, e.g. the Southeast Asian region was used in the study to be consistent with the study requirements. For age stratified bleed rates and age stratified disease specific mortality, a pooled value has been used for all age groups. Further, inhibitor cost was not considered in the study since the inclusion criteria were only patients without inhibitors.
Thus, based on the outcomes obtained from the study, early low-dose prophylaxis with factor VIII is recommended. The analysis was suggestive of a robust reimbursement system required in an India setting. This system should provide a continued support to the patients in getting rapid reimbursements. Additionally, improved health infrastructure is required, and initiatives might be undertaken to reduce OOP expenditure. Creating awareness regarding the benefits of prophylactic therapy is of paramount importance in the current scenario.
Conclusion
The research found that intermediate-dose prophylactic factor VIII therapy is cost effective and offers better clinical outcomes than on-demand therapy for both adult and pediatric hemophilia-A patients without inhibitors. The study suggests that early prophylaxis could lead to improved pediatric outcomes. In resource-limited contexts like India, personalized prophylaxis tailored to bleeding phenotype and activity level could enhance cost-effectiveness and joint health preservation.
Author contributions
All authors participated in the conception and design, acquisition, analysis and interpretation of data, critical manuscript revision for significant intellectual content, final approval, and consensus.
Acknowledgments
The authors would like to thank Dr. Vandana Govindan, Dr. Anisha Raju, Dr. Abheet Sharma and Neha Dwivedi from IQVIA, India, for their medical writing, editing and technical support which was funded by Takeda Biopharmaceuticals India Private Limited.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be made available within three months from initial request to researchers. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection and requirements for consent and anonymization
Additional information
Funding
References
- Mandal P, Phukan A, Bhowmik A, et al. Effect of tertiary prophylaxis with low-dose factor VIII in quality of life in adult patients with severe hemophilia A. J Appl Hematol. 2019;10:88. doi:10.4103/joah.joah_37_19
- Kar A, Phadnis S, Dharmarajan S, et al. Epidemiology & social costs of haemophilia in India. Indian J Med Res. 2014;140(1):19–31.
- Report on the Annual Global Survey 2020 is published by the World Federation of Hemophilia. (2020). https://www1.wfh.org/publications/files/pdf-2045.pdf.
- Sachdeva A, Gunasekaran V, Ramya HN, et al. Consensus statement of the Indian academy of pediatrics in diagnosis and management of hemophilia. Indian Pediatr. 2018;55(7):582–590. doi:10.1007/s13312-018-1302-8
- Goudarzi R, Seraji S, Amiresmaili M, et al. Safety and efficacy of secondary prophylactic in patients with severe hemophilia type A and B: a systematic review and meta-analysis study. Health Technol Assess Action. 2020;3(1):1–7.
- Singh A, Mehta S, Goyal LK, et al. Low dose prophylaxis vis-a-vis on-demand treatment strategies for hemophilia: a cost effective and disability attenuating approach. J Assoc Physicians India. 2019;67(11):52–55.
- Tang L, Xu W, Li CG, et al. Describing the quality of life of boys with haemophilia in China: results of a multicentre study using the CHO-KLAT. Haemophilia. 2018;24(1):113–119. doi:10.1111/hae.13349
- Wu R, Sun J, Xiao J, et al. A prospective study of health-related quality of life of boys with severe haemophilia A in China: comparing on-demand to prophylaxis treatment. Haemophilia. 2017;23(3):430–436. doi:10.1111/hae.13198
- Poon MC, Lee A. Individualized prophylaxis for optimizing hemophilia care: can we apply this to both developed and developing nations? Thromb J. 2016;14(Suppl. 1):32. doi:10.1186/s12959-016-0096-y
- Sidharthan N, Sudevan R. Low dose prophylaxis in hemophilia care. Indian J Hematol Blood Transfus. 2020;36(1):16–25. doi:10.1007/s12288-019-01147-0
- Verma S, Tripathi A, Sharma G, et al. Low dose long-acting factor VIII prophylaxis in pediatric and young adult patients with hemophilia A: short-term single-center experience from a developing country. J Hematol Allied Sci. 2021;1:75–80. doi:10.25259/JHAS_13_2021
- Verma SP, Dutta TK, Mahadevan S, et al. A randomized study of very low-dose factor VIII prophylaxis in severe haemophilia - A success story from a resource limited country. Haemophilia. 2016;22(3):342–348. doi:10.1111/hae.12838
- Zhao Y, Xiao J, Yang R, et al. Efficacy of standard prophylaxis versus on-demand treatment with Bayer's sucrose-formulated recombinant FVIII (rFVIII-FS) in Chinese children with severe hemophilia A. Pediatr Hematol Oncol. 2017;34(3):146–148. doi:10.1080/08880018.2017.1313921
- Tang L, Wu R, Sun J, et al. Short-term low-dose secondary prophylaxis for severe/moderate haemophilia A children is beneficial to reduce bleed and improve daily activity, but there are obstacle in its execution: a multi-centre pilot study in China. Haemophilia. 2013;19(1):27–34. doi:10.1111/j.1365-2516.2012.02926.x
- Zahedi Z, Karimi M, Keshavarz K, et al. A cost-effectiveness analysis of the prophylaxis versus on-demand regimens in severe hemophilia A patients under 12 years old in southern Iran. Hematology. 2021;26(1):240–248. doi:10.1080/16078454.2021.1885123
- Sudevan R, Beenakumari AA, Ganapathy R, et al. Intermediate dose prophylaxis in adults with haemophilia: a clinical audit from a resource limited setting. Indian J Hematol Blood Transfus. 2020;36(2):374–376. doi:10.1007/s12288-019-01189-4
- Gulshan S, Mandal PK, Phukan A, et al. Is Low dose a New dose to initiate hemophilia A prophylaxis? - A systematic study in eastern India. Indian J Pediatr. 2020;87(5):345–352. doi:10.1007/s12098-019-03179-w
- Kavakli K, Yang R, Rusen L, et al. Prophylaxis vs. on-demand treatment with BAY 81-8973, a full-length plasma protein-free recombinant factor VIII product: results from a randomized trial (LEOPOLD II). J Thromb Haemost. 2015;13(3):360–369. doi:10.1111/jth.12828
- Manco-Johnson MJ, Lundin B, Funk S, et al. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. J Thromb Haemost. 2017;15(11):2115–2124. doi:10.1111/jth.13811
- Debanjana BPK M, Manik M, Tapan Kumar S. Study on secondary versus tertiary prophylaxis in hemophilia children under 12 years of Age in a tertiary care hospital in eastern India. J Indian Med Assoc. 2020;118(12):49–53.
- Chozie NA, Primacakti F, Gatot D, et al. Comparison of the efficacy and safety of 12-month low-dose factor VIII tertiary prophylaxis vs on-demand treatment in severe haemophilia A children. Haemophilia. 2019;25(4):633–639. doi:10.1111/hae.13770
- Ghosh K, Ghosh K. Management of haemophilia in developing countries: challenges and options. Indian J Hematol Blood Transfus. 2016;32(3):347–355. doi:10.1007/s12288-015-0562-x
- Representation of State-wise AHF Support, Chapters and HTCs as on 1st March 2020. Hemophilia Federation (India); [Available from: https://hemophilia.in/index.php/ahf-status.
- Bansal D, Totadri S. Comprehensive hemophilia management in India – miles to Go before We sleep. Indian Pediatr. 2018;55:559–560. doi:10.1007/s13312-018-1433-y
- Hemophilia Federation (India). [Available from: https://www.hemophilia.in/index.php/hemophilia-treatment-centers.
- Singh P, Mukherjee K. Cost-Benefit analysis and assessment of quality of care in patients with hemophilia undergoing treatment at national rural health mission in Maharashtra, India. Value Health Reg Issues. 2017;12:101–106. doi:10.1016/j.vhri.2016.11.003
- Ghosh K. Evolution of hemophilia care in India. Indian J Hematol Blood Transfus. 2019;35(4):716–721. doi:10.1007/s12288-018-1059-1
- Blanchette VS. Prophylaxis in the haemophilia population. Haemophilia. 2010;16:181–188. doi:10.1111/j.1365-2516.2010.02318.x
- Zhou ZY, Raimundo K, Patel AM, et al. Model of short- and long-term outcomes of Emicizumab prophylaxis treatment for persons with hemophilia A. J Manag Care Spec Pharm. 2020;26(9):1109–1120.
- National Institute for Health and Care Excellence. (2004). Guide to the methods of technology appraisal. London: National Institute for Health and Care Excellence.
- Bank TW. India [Available from: https://data.worldbank.org/country/india.].
- Jaramillo HE C, Viscaya M M, Mejia AE. Cost-utility analysis of primary prophylaxis, compared with on-demand treatment, for patients with severe hemophilia type a in Colombia. Int J Technol Assess Health Care. 2016;32(5):337–347. doi:10.1017/S0266462316000544
- A Guide to Minimum Wage in India in 2021. (2021). Available from: https://www.india-briefing.com/news/guide-minimum-wage-india-2021-19406.html/.
- National Cost database. National Health System Cost Database for India; [Available from: https://www.healtheconomics.pgisph.in/costing_web/.
- PMJAY. [Available from: https://pmjay.gov.in/sites/default/files/2020-01/HBP_2.0-For_Website_V2.pdf.
- Howren MB. Quality-Adjusted life years (QALYs). In: Gellman MD, Turner JR, editor. Encyclopedia of behavioral medicine. New York, NY: Springer New York; 2013. p. 1605–1606.
- Wichmann AB, Adang EM, Stalmeier PF, et al. The use of quality-adjusted life years in cost-effectiveness analyses in palliative care: mapping the debate through an integrative review. Palliat Med. 2017;31(4):306–322. doi:10.1177/0269216316689652
- Mancuso ME, Graca L, Auerswald G, et al. Haemophilia care in children-benefits of early prophylaxis for inhibitor prevention. Haemophilia. 2009;15(Suppl. 1):8–14. doi:10.1111/j.1365-2516.2008.01947.x
- Gringeri A, Lundin B, von Mackensen S, et al. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT study). J Thromb Haemost. 2011;9(4):700–710. doi:10.1111/j.1538-7836.2011.04214.x
- Mancuso ME, Male C, Kenet G, et al. Prophylaxis in children with haemophilia in an evolving treatment landscape. Haemophilia. 2021;27(6):889–896. doi:10.1111/hae.14412
- Saxena K. Barriers and perceived limitations to early treatment of hemophilia. J Blood Med. 2013;4:49–56. doi:10.2147/JBM.S43734
- Li P, Chen Z, Cheng X, et al. PK-tailored tertiary prophylaxis in patients with severe hemophilia A at Beijing children's hospital. Pediatr Investig. 2019;3(1):45–49. doi:10.1002/ped4.12122
- Gringeri A, Lambert T, Street A, et al. Tertiary prophylaxis in adults: is there a rationale? Haemophilia. 2012;18(5):722–728. doi:10.1111/j.1365-2516.2012.02843.x
- Sidharthan N, Sudevan R, Narayana Pillai V, et al. Low-dose prophylaxis for children with haemophilia in a resource-limited setting in south India-A clinical audit report. Haemophilia. 2017;23(4):e382–e3e4. doi:10.1111/hae.13272
