ABSTRACT
Background and objective:
Graft versus host disease (GVHD) is the common complication seen after allogeneic hematopoietic stem cell transplantation (HSCT) and a pleomorphic syndrome that resembles autoimmune and other immunologic disorders, leading to profound immune dysregulation and organ dysfunction. The most common targets of GVHD are skin, gastrointestinal tract and liver. GVHD is classified as acute graft versus host disease (aGvHD) if it occurs within the first 100 days after HSCT and chronic graft versus host disease(cGVHD) if it occurs after day 100. The skin is most frequently and earliest affected by aGvHD, followed by the gastrointestinal tract and liver. An ideal biomarker would predict the onset and severity of clinical acute GVHD and help to direct management, and this is an area of active research regarding the use of biomarkers for diagnosis and prognosis of acute GVHD. Recently, elafin has been identified as a potential plasma biomarker for aGVHD.
Method:
We searched the databases PubMed, Cochrane library, and medRxiv for all studies investigating the Diagnostic or prognostic role of elafin in GVHD. We set the search strategy incorporating the search terms, ‘elafin’, ‘graft versus host’, and ‘GVHD’, and operated using the Boolean operators ‘AND’, and ‘OR’. Thus, retrieved articles were then exported on an Excel® sheet, and duplicates were removed. The systematic review was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. After selecting the study based on inclusion criteria, data on study characteristics and biomarker description was extracted on a pre-determined data extraction table on the Microsoft Excel version. The quality assessment of the included studies was determined using the QUIPS tool.
Result:
The search revealed 547 studies and 6 studies that met the eligibility criteria of this review have been included. The major finding of our study is the significant elevation of elafin in skin aGVHD.
Conclusion:
Elafin is a significant biomarker for diagnosis and prognosis of skin aGVHD and should be assessed within 2 weeks of the onset of the disease.
Introduction
Graft versus host disease (GVHD) is a life-threatening complication, which occurs commonly after allogeneic hematopoietic stem cell transplantation (HSCT) [Citation1]. It is an adverse immunological phenomenon in which the donor cell recognizes the host cell as a foreign antigen resulting in damage to the healthy host tissue. GVHD may also be seen following blood products and solid organ transplantation [Citation1,Citation2].
Skin is the most commonly involved organ in aGVHD characterized by rashes which begin as a central erythematous, maculopapular eruption that spreads to the extremities, and in severe cases, it may lead to the formation of bullae [Citation3]. In addition to the skin, the gastrointestinal (GI) tract and liver may also be affected by GVHD [Citation4]. Based on clinical manifestation, GVHD is classified as acute if it occurs within the first 100 days after HSCT and chronic if it occurs after day 100 [Citation5].
Diagnosis of GVHD is based on clinical manifestations, laboratory data, and pathological findings [Citation6]. However, there is a need for active research in GVHD biomarkers for diagnosis and prognosis of acute GVHD. Some of the biomarkers that are being investigated are Suppression of Tumorigenicity 2 (ST2), Regenerating islet-derived 3-alpha (REG3α), Tumor Necrosis factor Receptor 1 (TNFR1), and elafin. Those biomarkers are classified by the phases of GVHD. There are biomarkers of early inflammation, biomarkers of GVH reaction, and biomarkers that reflect organ damage due to GVHD [Citation7].
The first and most common clinical feature is a maculopapular skin rash in acute GVHD patients. Initially, the rash involves the nape of the neck, ears, shoulders, palms, and soles. In cases of severe GVHD, the maculopapular rash becomes bullous lesions with Stevens-Johnson syndrome-like toxic epidermal necrolysis affecting the majority of the skin.
Currently, there are no biomarkers that can foresee the progression of skin involvement in GVHD. Recently, studies have been performed on the potential role of a novel marker, elafin in the diagnosis and prognostication of GVHD manifestations. Elafin has been identified as an important plasma biomarker that reflects skin damage due to GVHD [Citation8]. It is also known as Skin-derived antileukoproteinase (SKALP) which is a serine proteinase inhibitor that specifically inhibits human leukocyte elastase and proteinase. It is absent in normal human epidermis but produced by epidermal keratinocytes under hyperproliferative conditions and inflammatory disorders such as psoriasis [Citation9,Citation10]. Immunohistochemistry study of skin biopsy of aGVHD has also revealed the overexpression of elafin [Citation11]. Elafin has been recognized as a potential biomarker that differentiates skin aGVHD from other etiologies of skin rashes following allogeneic HSCT [Citation12]. The secretion of elafin at the skin protects the epidermis against damage leading to continuous reepithelization and interfering with PMN migration which leads to healing [Citation9].
Although several studies have been performed in this regard, the results from these studies have not been uniform. Although studies have observed supportive findings for elafin to be used as a biomarker in GVHD [Citation13–16], someother studies have generated conflicting results [Citation4,Citation17–19]. Therefore, the actual truth behind the potential biomarker utility of elafin is yet to be established so that clinicians having to deal with GVHD can be aided significantly in the diagnosis and treatment of these patients. To accomplish this, we performed a study aimed to determine the prognostic and diagnostic role of elafin in skin GVHD based on the findings of previous studies performed in this context.
Methodology
Search strategy
We searched the databases PubMed and Google Scholar for all studies investigating the diagnostic or prognostic role of elafin in GVHD, published between 1 January 2000 and 10 September 2022. We set the search strategy incorporating the search terms, ‘Elafin’, ‘graft versus host disease’, and ‘GVHD’, and operated using the Boolean operators ‘AND’, and ‘OR’. Authors GD and RN searched the databases. Thus, retrieved articles were then exported on an Excel® sheet, and duplicates were removed. Disagreement if any was resolved through discussion among the primary reviewers and third reviewer (AB).
Study selection
The systematic review was performed in accordance to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. First, the search results were exported as a CSV file, and screening by title and abstract was performed. Duplicates were removed both automatically and manually. The search results were thus narrowed down and full-text screening was performed based on the eligibility criteria of our review. References of the relevant studies were also screened to find more studies relevant to the context. Those studies that met our eligibility criteria were included in the review.
Eligibility criteria
We included all studies that met the following features:
The study involved the role of Elafin in aGVHD.
Subjects with GVHD of any age, gender, or nationality in whose elafin was evaluated using any available techniques were eligible.
The study evaluated the diagnostic and prognostic role of elafin in aGVHD.
All studies measured serum elafin levels in aGVHD patients.
Studies that involve any of the following were excluded: Studies that demonstrated elafin on skin biopsy, those with unrelevant and insufficient information on elafin for diagnosis and prognosis of GVHD, Non-English, Full text not available, review articles, correspondences, editorials, and poster/ abstract presentations.
Data extraction
After selecting the study based on inclusion criteria, data extraction was done on a pre-determined data extraction table on the Microsoft Excel version. The data extraction sheet was designed to extract the data under the following headings 1. Study Characteristics include (author, publication year, study country, study design, sample size, gender, age, and major finding of the study), 2. Assay description (includes the number of patients developing aGVHD, Prophylaxis, Sample, Method of detection of Elafin, Period for measurement of Elafin, Elafin concentration, Elafin Positivity, and Grade of aGVHD). GD and RN performed data extraction whereas any conflict confronted was resolved through discussion with AB.
Risk of bias and quality assessment
Quality assessment was carried out independently by two authors using the QUIPS tool.
Outcome of interest
The primary outcome of interest was to compare the level of elafin at different periods in aGVHD and non-aGvHD samples and compare mortality based on initial elafin levels.
Diagnostic outcomes of interest: Serum elafin level.
Prognostic outcomes of interest: Overall survival and non-relapse mortality.
Data synthesis
From the included studies, information on the diagnostic and prognostic results was retrieved and included in the descriptive summary. The data were summarized using descriptive statistics, such as means and percentages.
Results
Study search results and study selection
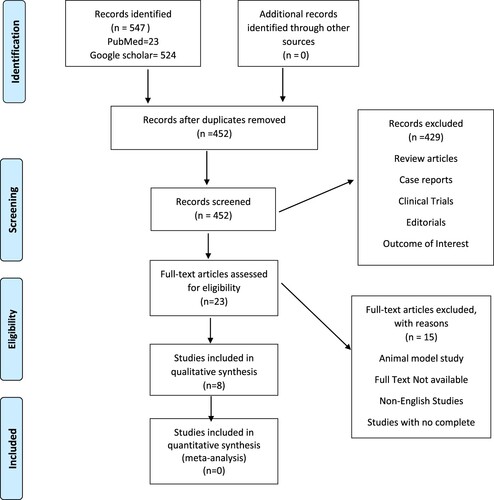
A total number of 547 studies were retrieved after a systematic literature search using keywords. After screening the studies by title and abstract, and excluding the studies that do not meet our eligibility criteria, six original studies were included in our systematic review. The details regarding the study selection process according to PRISMA guideline is given in .
Quality assessment
Two primary reviewers (GD and RN) evaluated the quality of the included studies. The quality of each study was assessed using the QUIPS tool. The detailed results of the quality assessment of the included studies are shown in .
Table 1. Bias of included studies.
Descriptive characteristics of the included studies
A total of six studies were included in our study. Three studies were from Asia, one study was from Europe and one study was from North America. Five studies were cohort, one study was retrospective cohort, and one study was observational. A total of 1163 HSCT recipients were included in our review. All participants were adults above 18 years of age with a median age of 19–61 years and males proportion were more than females. The detailed descriptive characteristics of the included studies are elucidated in .
Table 2. Study characteristics.
Elafin assay in aGVHD
The description of the assay used in the measurement of elafin is shown in . In our review, a total of 753 patients develops aGVHD among HSCT recipients. In most of the studies, calcineurin inhibitor-based drugs were given for prophylaxis [Citation4,Citation15,Citation16,Citation18]. However, in two studies, recombinant thrombomodulin and antithymocyte globulin were given [Citation13,Citation14]. Elafin assay was done by ELISA in all studies.
Table 3. Description of the assay used in the measurement of Elafin in the included studies.
Outcomes measurement
Diagnostic outcomes
Elafin level: Serum elafin level was raised and measured in all 753 patients who developed GVHD out of 1163 HSCT patients. The most of the selected studies reported the plasma Elafin being significantly elevated in aGVHD as compared to non-GVHD cases. Li X et al. reported increased elafin levels in severe GVHD as compared to less severe GVHD [Citation14]. Furthermore, Metafuni et al. found increased elafin level in patients with aGVHD as compared to non-GVHD cases and elafin level was lower in treatment responsive aGVHD than resistant aGVHD [Citation15]. Nelson PR Jr et al. demonstrated the association between increased risk of GVHD and the serum elafin level [Citation16]. But some studies have given contrasting results. GVHD and no rash group had statistically distinct increases in elafin from baseline (p = 0.000), whereas GVHD and non-GVHD rash did not (p = 0.441) [Citation17]. When compared to individuals who did not suffer from GVHD, patients with aGvHD had higher median elafin levels (32,344 vs. 10,229 pg/ml; p = 0.001) [Citation15].
Mortality or prognostics outcomes (overall survival and non-relapse mortality (NRM))
Despite the paucity of strong evidence of the prognostic significance of serum elafin in GVHD, the prognostic value of elafin has been reported Response to treatment was high in the low elafin group as compared to the high elafin group. Statistical significance was not obtained (Gray, p = 0.07), but the cumulative incidence of response was higher in the low elafin group (68%, 95% CI 26% to 86%) than in the high elafin group (23%, 95% CI 1% to 39%) [Citation15]. Similarly, study by Nomura et al. [Citation13] found evidence of endothelial dysfunction which was related to elevated serum elafin levels [Citation13]. Moreover, elafin levels were high in 59% (23/39) of aGvHD patients compared to 42% (16/38) of HSCT patients who did not develop GVHD (χ2 p = 0.001) [Citation15]. A study by Zewde et al. found contrasting results. Although the difference between patients in the low-risk elafin group unexpectedly showed a greater incidence of 6-month nonrelapse mortality(NRM) (17% vs. 11%, p = 0.19), the difference was not statistically significant [Citation4]. Nelson Jr et al. did not find the association between serum elafin and overall survival and NRM [Citation16]. Additionally, higher elafin levels at the onset of aGvHD predicted a four times higher likelihood of developing steroid resistance [Citation15].
Discussion
This systematic review aimed to determine the diagnostic and prognostic role of elafin in skin aGVHD. Based on the above data, 65.74% of individuals developed aGVHD after HSCT. Skin is the most commonly affected organ in aGVHD in about 80% of cases of aGVHD [Citation20]. Previously, the diagnosis of skin aGVHD was based upon the clinical features and pathological examination. However, numerous studies have been done to determine the role of biomarkers in the diagnosis and prognosis of aGVHD [Citation20]. Elafin is one of the most studied biomarkers associated with the diagnosis and prognosis of skin aGVHD [Citation21].
Elafin as such is not specific for GVHD as it has been linked to various inflammatory conditions like inflammatory bowel disease, celiac disease, intestinal fibrosis, dermatitis herpetiformis, and psoriasis [Citation22–25]. In the majority of the included studies, there was a significant elevation of elafin in GVHD. There was a distinct difference in elafin levels between GVHD and non-GVHD patients. Moreover, the serum elafin level differed according to the severity of GVHD. Nelson et al. demonstrated that there was a significant elevation of elafin at day 14 which coincides with the result shown by other studies [Citation16,Citation21]. Thereafter, the elafin concentration gradually declined. Therefore, the measurement of plasma elafin at various period of onset of aGVHD symptoms can potentially help in the diagnosis and prognosis of GVHD.
Regarding the prognostic role, the four out of six studies highlight the prognostic significance of elafin in aGVHD and one study has shown significant elevation of elafin in patients with aGVHD. Response to treatment was higher in the low elafin group than high elafin group indicating prognostic significance of elafin. Additionally, higher elafin levels at the start of aGvHD predicted a four times higher likelihood of developing steroid resistance [Citation15]. There are studies in the literature that support the prognostic role of elafin [Citation8,Citation17,Citation18,Citation21]. However, in this review, elafin did not significantly predict overall survival or NRM in the study by Nelson et al. [Citation16], and Zewde et al. [Citation4]. In the study by Nelson PR et al., the discrepancy was presumably because the study's sample size was insufficient to detect these endpoints of NRM and overall mortality. This discrepancy in the study by Zewde et al. [Citation4] can be explained by the multicenter nature of the data, the bigger patient population, the scarcity of individuals with severe stage 4 skin GVHD, and, most crucially, the exclusion of patients with skin GVHD who did not need systemic treatment.
Elafin can also be demonstrated in the skin biopsy by the IHC method. It is supported by a pilot study done by Mahabel et al. where there was a direct correlation between elafin expression in tissue and GVHD diagnosis [Citation8]. Mahabel et al. recruited 23 patients with skin rash within 100 days of HSCT. Among 27 episodes of rash, 16 episodes (59.26%) were diagnosed as GVHD by histopathological examination. Elafin was positive in all patients with GVHD and negative in 8 patients without GVHD concluding the sensitivity and specificity of Elafin immunohistochemistry to be 100% and 75%, respectively. Thus, the tissue elafin is also a useful immunohistochemical marker for the diagnosis and prognosis of acute skin GVHD.
Various biomarkers have been studied for the diagnosis of aGVHD such as IL-2R alpha, TNFR-1, IL-8, hepatocyte growth factor (HGF), and so on. However, the elafin has been recognized as the foremost biomarker which has greater specificity for differentiating skin aGVHD from others [Citation26,Citation27]. In a study by Pacszeny et al. 2010 performing a Receiver Operating Characteristics (ROC) curve of different biomarkers, the area under curve (AUC) of the elafin was highest among ROC curves for elafin, TNFR1, IL2Ra, HGF, and IL-8 but less than composite curve of all five biomarkers (AUC = 0.77, 0.73, 0.65, 0.62, 0.54, and 0.84, respectively) showing elafin as best single discriminator for diagnosis of skin GVHD from another nonGVHD skin rash [Citation12].
To our knowledge this is the first systematic review, emphasizing the diagnostic and prognostic role of elafin in skin aGVHD. Our study had certain limitations. The study population was limited. We excluded the studies that demonstrated elafin in skin biopsy rather than serum. The meta-analysis couldn’t be performed because all the studies were single arm and data on prognosis were not adequately retrieved.
Conclusion
Elafin can potentially be the specific biomarker associated with the diagnosis and prognosis of skin aGVHD and it should be investigated within 14 days of onset of aGVHD.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All the required data is in the manuscript itself.
References
- Ramachandran V, Kolli SS, Strowd LC. Review of graft-versus-host disease. Dermatol Clin. 2019;37(4):569–582. doi:10.1016/j.det.2019.05.014
- Shantanam S. MUELLER. 乳鼠心肌提取 HHS public access. Physiol Behav. 2018;176(1):139–148.
- Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi:10.1016/S0140-6736(09)60237-3
- Zewde MG, Morales G, Gandhi I, et al. Evaluation of elafin as a prognostic biomarker in acute graft-versus-host disease: M. G. Zewde et al. Transplant Cell Ther. 2021;27(12):988.e1–988.e7.
- Lee SJ, Schubert MM. Graft-vs.-host disease. Crit Rev Oral Biol Med. 1997;8(2):201–216.
- Levine JE, Logan BR, Wu J, et al. Acute graft-versus-host disease biomarkers measured during therapy can predict treatment outcomes: a blood and marrow transplant clinical trials network study. Blood. 2012;119(16):3854–3860. doi:10.1182/blood-2012-01-403063
- Nagasawa M. Biomarkers of graft-vs-host disease: understanding and applications for the future. World J Transplant. 2021;11(8):335–343. doi:10.5500/wjt.v11.i8.335
- Mahabal GD, George L, Peter D, et al. Utility of tissue elafin as an immunohistochemical marker for diagnosis of acute skin graft-versus-host disease: a pilot study. Clin Exp Dermatol. 2019;44(2):161–168. doi:10.1111/ced.13678
- Van Bergen BH, Andriessen MPM, Spruijt KIJ, et al. Expression of SKALP/elafin during wound healing in human skin. Arch Dermatol Res. 1996;288(8):458–462. doi:10.1007/BF02505235
- Alkemade HAC, van Vlijmen-Willems IMJJ, van Haelst UJGM, et al. Demonstration of skin-derived antileukoproteinase (skalp) and its target enzyme human leukocyte elastase in squamous cell carcinoma. J Pathol. 1994;174(2):121–129. doi:10.1002/path.1711740208
- Chacon AH, Farooq U, Shiman MI, et al. Elafin: a possible new biomarker and immunohistochemical stain for pre-engraftment syndrome. J Am Acad Dermatol. 2013;69(2):e102–e103. doi:10.1016/j.jaad.2012.11.024
- Paczesny S, Braun TM, Levine JE, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Sci Transl Med. 2010;2(13):1–19.
- Nomura S, Ishii K, Fujita S, et al. Associations between acute GVHD-related biomarkers and endothelial cell activation after allogeneic hematopoietic stem cell transplantation. Transpl Immunol. 2017;43–44(6):27–32.
- Li X, Chen T, Gao Q, et al. A panel of 4 biomarkers for the early diagnosis and therapeutic efficacy of aGVHD. JCI Insight. 2019;4(16):e130413.
- Metafuni E, Giammarco S, De Ritis DG, et al. Changes in protein serum levels during stem cell transplantation. Eur J Clin Invest. 2017;47(10):711–718. doi:10.1111/eci.12796
- Nelson RP, Khawaja MR, Perkins SM, et al. Prognostic biomarkers for acute graft-versus-host disease risk after cyclophosphamide-fludarabine nonmyeloablative allotransplantation. Biol Blood Marrow Transplant. 2014;20(11):1861–1864. doi:10.1016/j.bbmt.2014.06.039
- George L, Mahabal G, Mohanan E, et al. Limited utility of plasma elafin as a biomarker for skin graft-versus-host disease following allogeneic stem cell transplantation. Clin Exp Dermatol. 2021;46(8):1482–1487. doi:10.1111/ced.14785
- Brüggen MC, Petzelbauer P, Greinix H, et al. Epidermal elafin expression is an indicator of poor prognosis in cutaneous graft-versus-host disease. J Invest Dermatol. 2015;135(4):999–1006. doi:10.1038/jid.2014.489
- Wegner J, Weidenthaler-Barth B, Engelbert J, et al. Immunohistochemical markers for histopathological diagnosis and differentiation of acute cutaneous graft-versus-host disease. Exp Dermatol. 2021;30(12):1814–1819. doi:10.1111/exd.14416
- Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: secondary treatment. Blood. 1991;77(8):1821–1828. doi:10.1182/blood.V77.8.1821.1821
- Solán L, Carbonell D, Muñiz P, et al. Elafin as a predictive biomarker of acute skin graft-versus-host disease after haploidentical stem cell transplantation using post-transplant high-dose cyclophosphamide. Front Immunol. 2021;12(2):1–7.
- Elgharib I, Khashaba SA, Elsaid HH, et al. Serum elafin as a potential inflammatory marker in psoriasis. Int J Dermatol. 2019;58(2):205–209. doi:10.1111/ijd.14217
- Wang J, Ortiz C, Fontenot L, et al. High circulating elafin levels are associated with Crohn’s disease-associated intestinal strictures. PLoS One. 2020;15(4). doi:10.1371/journal.pone.0231083
- Ollague JE, Nousari CH. Expression of elafin in dermatitis herpetiformis. Am J Dermatopathol. 2018;40(1):1–6. doi:10.1097/DAD.0000000000000915
- Krawiec P, Pac-Kożuchowska E. Clinical significance of serum elafin in children with inflammatory bowel disease. Biomedicines. 2022;10(12):3267.
- Solán L, Carbonell D, Muñiz P, et al. Elafin as a predictive biomarker of acute skin graft-versus-host disease after haploidentical stem cell transplantation using post-transplant high-dose cyclophosphamide. Front Immunol. 2021;12:516078.
- Ali AM, DiPersio JF, Schroeder MA. The role of biomarkers in the diagnosis and risk stratification of acute graft-versus-host disease: a systematic review. Biol Blood Marrow Transplant. 2016;22(9):1552–1564. doi:10.1016/j.bbmt.2016.04.022