ABSTRACT
Objectives:
To describe clinical characteristics, factor consumption, and events of interest in patients with haemophilia A without inhibitors receiving prophylaxis in France, and the clinical impact of switching to Elocta® in this population.
Methods:
This retrospective, observational study using the Système National des Données de Santé database, analysed data from patients with haemophilia A without inhibitors using prophylactic factor VIII (FVIII) replacement therapy during 2016–2019. Clinical characteristics, treatment patterns and switches, factor consumption, and rate of events of interest were determined. In a sub-cohort of patients treated with Elocta®, clinical characteristics, factor consumption, and rate of events of interest before and after switching to Elocta® were compared.
Results:
For 545 patients, with mean age (standard deviation [SD]) 25.4 (17.8) years, Elocta® was the most used treatment. Bleeding events and articular non-bleeding events leading to hospitalization occurred in 15.4% and 13.9% of patients, respectively, and 9.9% of patients had surgeries or procedures related to haemophilic arthropathy. The mean (SD) FVIII product consumption was 344 (93) IU/kg/month for extended half-life treatment, and 331 (98) IU/kg/month for standard half-life products. For the sub-cohort of 146 patients, bleeding events (SD) decreased from 0.32 (2.2) to 0.09 (0.42) events/patient/year (p = 0.227) after switching to Elocta®. There was no statistically significant difference in rates of factor consumption or articular non-bleeding events before and after initiation of Elocta®.
Conclusion:
This study provides real-world insights that advance the understanding of treatment patterns and events of interest in patients with haemophilia A on prophylactic regimens in France.
Introduction
Haemophilia A is a rare disease with an estimated prevalence at birth of 24.6 per 100,000 males [Citation1]. In France, haemophilia A is present at birth in an estimated 25.6 cases per 100,000 males [Citation1]; as of January 2023, FranceCoag reported 7,243 cases and, of these, 29% of patients had severe haemophilia A [Citation2].
A deficiency of clotting factor VIII (FVIII) results in bleeding episodes in patients with haemophilia A, which can occur spontaneously or when caused by trauma [Citation3]. Joint bleeds can lead to arthropathy and internal organ bleeds can be life-threatening [Citation3].
Treatment for haemophilia A includes recombinant or plasma-derived FVIII replacement therapy [Citation3] that is administered prophylactically or on-demand [Citation4]. Current guidelines for the treatment of patients with severe haemophilia A, or a severe phenotype, recommend prophylactic treatment [Citation3]. However, patients can develop anti-FVIII inhibitors, which severely impact the effectiveness of treatment [Citation3]. In France, there have been 10 approved and reimbursed FVIII replacement products with standard half-lives (SHLs; approximately 8–12 h [Citation5,Citation6]) and one with an extended half-life (EHL; approximately 20 h in patients 15 years or older [Citation7]), which is Elocta® (efmoroctocog alfa), a rFVIIIFc (recombinant FVIII Fc fusion protein) [Citation7]. No pegylated FVIII products have been authorized for reimbursement in France [Citation8–10].
The French National Claims Database, Système National des Données de Santé (SNDS), provides longitudinal patient-level data covering approximately 99% of the French population [Citation11] and has been previously utilized to study the haemophilia population of France. The MOTHIF-II study, for example, utilized the SNDS paired with the BERHLINGO database to assess the impact of switching to treatment with Elocta® in patients with severe haemophilia A in France [Citation12]. The study reported that patients who switched to Elocta® typically had a severe bleeding profile and, following switch, reductions in mean FVIII prescription and annual bleeding rate (ABR) were observed [Citation12]. Similarly, a French real-world study found switching from SHL to EHL FVIII treatment resulted in reductions in ABR, factor consumption, injection frequency, and cost [Citation13]. These real-world results support the previous evidence that EHL FVIII replacement products decrease FVIII consumption while maintaining bleeding protection [Citation14].
However, there are few real-world insights into the clinical characteristics, treatment patterns, and occurrence rate of events of interest in the French population of patients with haemophilia A receiving prophylactic treatment, or the clinical impact of EHL FVIII replacement therapy in these patients. Our study aimed to assess these outcomes using data from the SNDS database.
Methods
Objectives
The primary objectives of the study were to: (1) describe the French haemophilia A population without inhibitors on a prophylactic regimen; (2) describe the monthly factor consumption in this population; (3) describe the switches between FVIII products in this population; and (4) assess the rate of bleeding events and articular non-bleeding events requiring hospitalization, as well as surgeries or procedures related to haemophilic arthropathy, in this population (events of interest; Supplementary methods 1). The secondary objective was to compare factor consumption and the occurrence rate of events of interest before and after initiation of Elocta® in this population.
Database
Data from the SNDS were analysed in the study. The SNDS database includes longitudinal patient-level data and links data from French national databases for health insurance, hospital discharges, and the death registry (Supplementary methods 2) [Citation15]. All databases are linked using a pseudonymised French unique national identifier.
Study design and population
This was a retrospective, observational, longitudinal, descriptive study based on data from the SNDS database. The time period 2010–2019 was used to capture all patients with haemophilia A, and 2016–2019 to capture medication use after the launch of Elocta®.
Overall population
Eligible patients had a haemophilia A diagnosis and at least one delivery claim of FVIII replacement therapy between 2010 and 2019, and were on a prophylactic regimen, having received no on-demand treatment, between 2016 and 2019. Patients were considered to be on a prophylactic regimen if they received eight or more delivery claims of FVIII replacement therapy during each 12-month period after treatment initiation and were treated for at least 12 months with an average dose of more than 150 international units (IU)/kg/month. Patients were excluded if they had acquired haemophilia or a diagnosis of von Willebrand disease, and any claims for von Willebrand factor, during 2010–2019 (Supplementary methods 3). In addition, patients were excluded if they had anti-FVIII inhibitors or any claim for bypassing agents, or a diagnosis of obesity, during 2016–2019 (Supplementary methods 3).
The study period for the analysis of the overall population covered 1 January 2016–31 December 2019, with Elocta® being launched at the end of 2015. Index date was defined as the date of first claim of delivery of FVIII replacement therapy during the study period. The follow-up period ran until 31 December 2019 or death of the patient.
Within-patient comparison population
From the overall population, a sub-cohort of patients treated with Elocta® was studied before and after initiation of Elocta® in the within-patient comparison analysis. Eligible patients were male, treated for at least 12 months post-index date and, during a 3-year pre-index period, received no immune tolerance induction (ITI) therapy (patients receiving ≥800 IU/kg/month of FVIII replacement therapy [i.e. ≥ 2,400 IU/kg/trimester] for at least one trimester were excluded) and were receiving prophylactic therapy with Elocta® only, with no on-demand treatments, using the prophylaxis criteria as per the overall population. The study period for the comparison covered 1 January 2016 to 31 December 2019, with the index period covering 3 years from 1 January 2016–1 January 2019. A 3-year pre-index period and a 1- to 3-year post-index period were considered for the analysis. The index date was defined as the first claim of Elocta® delivery during the study period.
Analyses
Patient demographics and clinical characteristics
Age and sex were described at index date. Vital status and comorbidities were identified during the follow-up period (Supplementary methods 4). For the within-patient analysis population, clinical characteristics including comorbidities were evaluated 12 months prior to index date.
Monthly factor consumption
Monthly factor consumption was calculated only for patients treated with the product for at least 12 months. An algorithm was used to convert dosage for factor replacement therapy to IU/kg/month and to determine start and end dates of products consumed (Supplementary methods 4).
Duration and switches of treatment
Duration of treatment and switches of product were calculated from the start and end dates of each treatment sequence (Supplementary methods 4).
Events of interest
Events of interest were defined using International Classification of Disease (ICD)−10 codes for diagnoses, and ‘classification commune des actes médicaux’ codes for surgeries or procedures (Supplementary methods 1). Only events that required hospitalization were available in the SNDS database.
Statistical analyses
Statistical analyses were performed as outlined in Supplementary methods 4.
Results
Overall population
Patient selection
From 66 million people in the SNDS database of France, 4,642 patients were identified with haemophilia A between 2010 and 2019 within the inclusion criteria. During 2016–2019, 545 patients were identified as without inhibitors and with no claims for obesity, treated for haemophilia A and on a prophylactic regimen only, composing the overall study population ().
Figure 1. Flow chart depicting the selection of the overall population. FVIII, factor VIII; rFVIIa, recombinant factor VIIa; SNDS, Système National des Données de Santé.
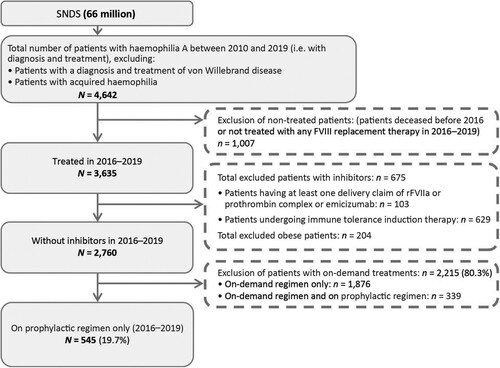
Patient characteristics and demographics are shown in Supplementary Table 1. The mean (standard deviation [SD]) age at index date was 25.4 (17.8) years and most patients (99.6%) were male. The mean (SD) follow-up period was 3.8 (0.6) years and, overall, 14 patients (2.6%) died. The most frequent comorbidities during the follow-up period were hepatitis C (14.7%), human immunodeficiency virus (HIV) (12.1%), hypertension (3.5%), and cancer (3.5%).
Treatment patterns
Of the FVIII replacement therapies prescribed in the study population, Elocta® was used in the greatest proportion of patients (47.3%), followed by Advate® (octocog alfa) (41.7%), and Kogenate® (octocog alfa) (17.6%) (Supplementary Figure 1).
The mean (SD) duration of treatment per product ranged from 13.6 (6.0) months to 33.4 (14.8) months (Supplementary Table 2). For Elocta®, the mean (SD) duration of treatment was 23.9 (11.4) months.
Monthly factor consumption
The mean monthly factor consumption of SHL FVIII products was 331 (98) IU/kg/month (), with a range of 279–385 IU/kg/month (Supplementary Table 2). For plasma-derived products, Octanate® (coagulation factor VIII) and Factane® (coagulation factor VIII), mean (SD) consumption was 279 (88) IU/kg/month and 298 (98) IU/kg/month, respectively. Mean (SD) monthly factor consumption ranged from 327 (94) to 385 (138) IU/kg/month for recombinant SHL FVIII products.
Table 1. Monthly consumption of FVIII replacement therapy per patient in IU/kg/month for the overall population.
The mean (SD) monthly consumption of the EHL FVIII product, Elocta®, was 344 (93) IU/kg/month ().
Treatment switch
Over 97% of patients treated with Helixate® (octocog alfa) (n = 81) and Kogenate® (n = 96) switched to another product, followed by 67.2% of patients treated with ReFacto® (moroctocog alfa) (n = 64), 51.5% of patients treated with Factane® (n = 68), and 49.3% of patients treated with Advate® (n = 227) (Supplementary Figure 2). Among those patients treated with Elocta® (n = 258), 2.3% switched to another product.
The majority of patients who switched from SHL FVIII products, including Advate® (86.6%), Factane® (80.0%), NovoEight® (turoctocog alfa) (81.8%), Nuwiq® (simoctocog alfa) (100.0%), Kovaltry® (octocog alfa) (70.0%), and ReFacto® (63.6%), switched to Elocta® (Supplementary Table 3).
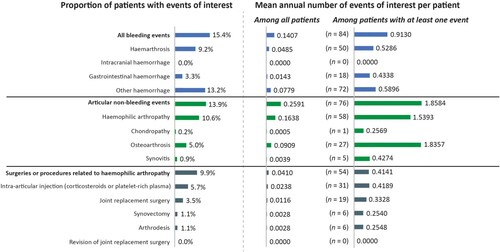
Events of interest
During the follow-up period, 15.4% of patients were hospitalized with a bleeding event, with a mean (SD) annual number of 0.14 (0.56) events/patient (). Articular non-bleeding events were experienced by 13.9% of patients, and 9.9% had surgeries or procedures related to haemophilic arthropathy.
Within-patient comparison population
Patient selection
Of the 545 patients in the overall study population, 146 met the inclusion criteria for the within-patient comparison population ().
Figure 3. Flow chart depicting the selection of the within-patient comparison population. SHL, standard half-life
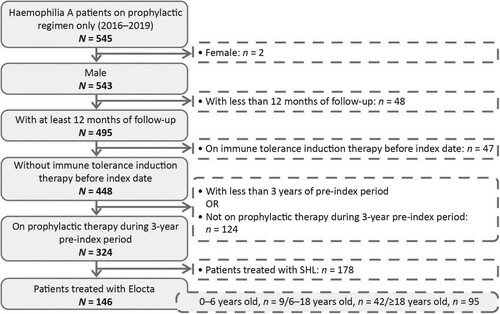
Patient clinical characteristics and demographics are shown in Supplementary Table 4. The mean (SD) age at index was 27.3 (15.9) years, and the mean follow-up period was 2.2 (0.6) years. The most common comorbidity during the 12 months prior to index date was HIV (17.1%) followed by hepatitis C (11.0%). No patients died during the study follow-up period.
Monthly factor consumption
Mean (SD) monthly factor consumption was 338 (93) IU/kg/month before initiation of Elocta® and 345 (93) IU/kg/month after switch to Elocta® treatment (Supplementary Table 5). The difference was not found to be statistically significant (p = 0.400).
Events of interest
The mean (SD) annual number of bleeding events requiring hospitalization was lower after the initiation of Elocta® compared with before initiation (0.09 [0.42] versus 0.32 [2.2] events/patient/year). However, no statistically significant difference was detected (p = 0.227).
No statistically significant differences were found in the mean (SD) annual number of articular non-bleeding events before and after the initiation of Elocta®; 0.16 (0.73) versus 0.47 (1.96) events/patient/year, p = 0.078.
Similarly, no statistically significant differences were found in the mean (SD) annual number of surgeries or procedures related to haemophilic arthropathy events, before and after the initiation of Elocta®; 0.08 (0.23) versus 0.11 (0.42) events/patient/year, p = 0.413.
Discussion
This observational database study sought to describe a French population of patients with haemophilia A receiving prophylactic treatment, including treatments received, switches made between treatments, monthly factor consumption, and occurrence of events of interest. In addition, the effect of treatment switch from SHL FVIII products to Elocta® on factor consumption and events of interest was analysed in a sub-cohort of patients.
The population of patients with haemophilia A without inhibitors on prophylactic regimens identified in the French SNDS database (19.7%) is similar to that reported by the retrospective Hemraude study (approximately 21%), which utilized the SNDS database to describe patients with haemophilia A in France, along with the economic burden of the disease and disease management [Citation16].
The mean age at index for the overall study population was 25.4 years old, which is lower than, but similar to, that published by FranceCoag in 2021 for patients with haemophilia A (36.1 years old) [Citation17], and that of the French cohort of patients with moderate/severe haemophilia A (29.9 years) included in a European retrospective study [Citation18]. The lower age in our current study might reflect that all patients were on prophylaxis, which is considered the gold standard for treatment of young patients.
Our analysis found that the most common comorbidities in the overall population were hepatitis C (14.7%) and HIV (12.1%) and that these were only seen in patients aged 18 years or more. This may be attributable to the utilization of contaminated plasma-derived factor products over 40 years ago [Citation19]. These figures differ slightly to those reported in the FranceCoag in 2021, where overall 22.7% and 5.9% of patients with haemophilia A had hepatitis C and HIV, respectively [Citation17]. This may be related to under-reporting of these comorbidities in the SNDS database, particularly for patients now cured of hepatitis C.
During 2016–2019, Elocta® was the most prescribed FVIII product (47.3%) in our study population, followed by Advate® (41.7%) and Kogenate® (17.6%); notably patients may have been treated with more than one FVIII replacement product during the follow-up period. This frequency of Elocta® use is higher than the 33.1% reported in a study describing a Swedish population of patients with moderate or severe haemophilia A [Citation20]. In addition, a European study reported that Elocta® was routinely prescribed in 76% of 33 haemophilia treatment centres assessed in 2018; the remaining centres stated the product was either not used or not yet available [Citation21]. These differences may reflect that in France, unlike in other European countries, Elocta® is the only EHL FVIII product available.
The majority of patients previously treated with Helixate® or Kogenate® in our study switched, which was inevitable since these two products were replaced during this period by a third-generation product (Kovaltry®) [Citation22] and Helixate® was withdrawn from the market [Citation23]. Notably, 38.1% of patients on Kogenate® and 49.4% of those on Helixate® switched directly to Elocta®. In contrast, only 2.3% of patients switched from Elocta® to an SHL FVIII product, suggesting the treatment was well tolerated and effective. Most patients who switched their FVIII replacement treatment switched from an SHL FVIII product to Elocta®. Other studies have reported reasons patients switch from SHL FVIII product to Elocta®, including fewer injections, and improved bleeding protection, quality of life, and treatment adherence [Citation24,Citation25]. The MOTHIF-II study, which also utilized data from the SNDS database, reported that by the end of the study period, 54.5% of patients with severe haemophilia A had switched to Elocta®, benefiting from reductions in injections frequency and ABR, and factor consumption [Citation12]. Analysis of data from haemophilia treatment centres in Europe in early 2018 showed that in 72% of centres (18/25), < 10% of patients were switched from standard products to Elocta® [Citation21]. However, the study was around the time EHL FVIII was first introduced. Moreover, in France, the list price of Elocta® is the same as SHL FVIII [Citation13]. A switch to Elocta® was, therefore, of no economic consequence and, because of the interest in Elocta®, physicians may have been more inclined to choose this product.
Mean monthly consumption per patient receiving EHL FVIII product was 344 IU/kg/month compared with SHL FVIII, 331 IU/kg/month, in the overall population. Analysis of our within-patient comparison population showed no statistically significant difference in monthly factor consumption before and after Elocta® initiation (p = 0.400). These results are similar to those reported in Austrian and French studies that showed no significant change in factor consumption for prophylaxis following switch to Elocta® [Citation13,Citation26]. However, the results of the MOTHIF-II study reported a reduction in mean prescribed factor treatment for patients, typically with high initial ABRs, who switched from SHL FVIII to Elocta® only during the study [Citation12]. Similarly, real-world studies in Germany and Sweden reported a reduction in factor consumption following switch to Elocta® from SHL FVIII [Citation20,Citation27]. In addition, the European multicentre prospective A-SURE study reported significantly lower factor consumption in patients receiving Elocta® compared with the matched cohort of patients who received SHL FVIII [Citation28]. A real-world study of a combined US and European population also found weekly consumption of FVIII replacement products was lower for those using EHL than SHL products [Citation29]. The differences between our study and the current literature are likely related to the SNDS data limitations, which were collected only from medication dispensed from reimbursed prescriptions (including initial stock in case of trauma or bleeds not requiring hospitalization), and the need to estimate monthly factor consumption considering patient weights are not available in the SNDS database.
Around 15% of the overall population in our study experienced a major bleeding event requiring hospitalization, with a mean annual number of 0.14 events/patient. Whilst not statistically significant, a trend towards fewer bleeding events (p = 0.227), and articular non-bleeding events (p = 0.078) requiring hospitalization, was observed in the within-patient comparison population after Elocta® initiation, compared with before. This suggests that, as observed in the MOTHIF-II study [Citation12], those patients with the most severe bleeding phenotypes might have been switched for improved protection. A Swedish real-world study reported that ABR was similar for patients before (1.3 bleeds per year) and after (1.4 bleeds per year) switching from SHL FVIII to Elocta® treatment [Citation20]. However, other real-world studies analysing the impact of such a switch have reported a significant reduction in ABR or bleeding episodes [Citation12,Citation13,Citation27]. Similarly, the A-SURE study noted that the ABR was significantly lower in patients who received Elocta® compared with a matched cohort of patients receiving SHL FVIII [Citation28]. Conversely, a real-world study of a combined US and European population found that the mean ABR was 1.8 events/patient/year for patients receiving EHL FVIII compared with 1.7 events/patient/year for SHL FVIII [Citation29]. The difference seen in the results of our study may be due to the SNDS database only capturing bleeding events requiring hospital admission, indeed, most bleeding events, particularly haemarthrosis, do not require hospitalization and patients can treat them effectively at home.
A strength of our study is that the use of the SNDS database provided a large sample size, approximately 99% of the French population, with continuous long-term patient data [Citation11,Citation15]. In addition, the longitudinal study design without loss to follow-up allows enough time to observe any changes in the outcomes of interest. Access to data from such databases allows for more comprehensive studies than analysis of registries based on self-reporting by physicians.
Limitations of our study include the retrospective and observational nature of the study design, which is associated with potential selection bias. The population selected based on the inclusion criteria may not be representative of all patients with haemophilia A on a prophylactic regimen in France. As clinical data on the treatment regimen (prophylaxis or on-demand) are not available in the SNDS, the inclusion and exclusion criteria of patients with inhibitor and the identification of those on prophylactic regimen were based on drug dispensation rather than drug administration. The classification algorithms used to identify doses for ITI therapy and prophylactic treatments were not based on physician-reported information but had to be estimated on criteria defined within a scientific committee. Inclusion criteria required patients to receive prophylaxis only, defined as staying on a prophylactic regimen during the whole follow-up period and receiving no on-demand treatment. This criterion may have biased the population towards those with very severe haemophilia A.
A limitation of the SNDS database is that it does not include clinical information such as patient weight, injection frequency, daily/weekly dosage, and severity of disease. Other real-world studies of Elocta® usage in haemophilia A are ongoing or completed in Europe which do include clinical measures [Citation28,Citation30,Citation31], and pairing our results with those could give a more complete picture. Of note, the SNDS database does not enable identification of the patient profiles of those that were switched or the reasons for switch. In addition, bleeding event rates are calculated only from those events requiring hospitalization, which could also be connected to adverse events such as accidents, and any bleeds treated outside the hospital, or self-treated by the patient, are not captured. Analysis of haemophilia patients’ logbooks can be useful in the assessment of ABR, but this was not possible in our study. Therefore, bleeding event rates in our study may have been underestimated.
The use of national average weight data in our study may have led to incorrect exclusions of patients receiving prophylaxis from the study population. In the MOTHIF-II study, the use of the SNDS database was complemented by data from BERHLINGO, which provided data relating to disease severity, and frequency and duration of injections, as well as data from patient logbooks relating to self-treated bleeding episodes [Citation12].
Finally, anecdotal evidence suggests that miscoding errors occurring in French hospitals, where events related to haemophilia A are often coded as arthropathy and osteoarthritis, are often captured in claims databases and this may have contributed to underestimation of events of interest. However, SNDS diagnostic codes are used for patient identification by the French government for evaluations.
Conclusions
This study provides real-world insights into the French haemophilia A population without inhibitors on a prophylactic regimen, and the factor consumption rates, switches between FVIII products, and occurrence rate of events of interest (the rate of bleeding events and articular non-bleeding events requiring hospitalization as well as surgeries or procedures related to haemophilic arthropathy) in this population. Use of the SNDS database provided a large sample size and valuable real-world data, providing a nationwide perspective. While this study is limited by the lack of clinical data available in the SNDS database, it might be complemented by completed or ongoing European studies that could add clinical insights.
Author contribution
MT and RV contributed to the study concept and study design and interpreted the data. AF interpreted the data. LG performed quality control of data and algorithms, analysed and interpreted the data and performed statistical analysis. NK analysed and interpreted the data and performed statistical analysis. OvH contributed to the study concept and study design, interpreted the data, and performed statistical analysis. All authors critically reviewed and revised the draft manuscript, approved the final version to be published and agreed to be accountable for all aspects of the work.
SNDS suppl updated_15Dec23 clean.docx
Download MS Word (167.7 KB)Acknowledgements
We acknowledge the contribution of Valérie Bastard, of Sobi, Paris, France, in the preparation of this article. Medical writing support, funded by Sobi, was provided by Helen Bristow, BSc, and Hayley Owen, PhD, Bioscript Group, Macclesfield, UK. Sobi and Sanofi reviewed and provided comments on the manuscript. The authors had full editorial control of the manuscript and provided their final approval of all content.
Disclosure statement
MT declares support for the present manuscript from Sobi and grants and contracts from Takeda, Novo Nordisk and LFB. AF is a Sobi employee and shareholder of Sobi. LG was an employee of Cerner Enviza who received funding from Sobi to conduct the study and is currently an employee of Janssen France. NK is an employee of Sobi and holds Sobi stock. OvH is an employee of Sobi. RV declares support for the present manuscript from Sobi, grants and contracts from LFB, Takeda, Biomarin, Novo Nordisk and Roche, and support for attending meetings and/or travel from Takeda and LFB.
Data availability
Sobi is committed to responsible and ethical sharing of data on participant level and summary data for medicines and indications approved by EMA and/or FDA, while protecting individual participant integrity and compliance with applicable legislation. Data access will be granted in response to qualified research requests. All requests are evaluated by a cross-functional panel of experts within Sobi and a decision on sharing will be based on the scientific merit and feasibility of the research proposal, maintenance of personal integrity and commitment to publication of the results. To request access to study data, a data sharing request form (available on www.sobi.com) should be sent to [email protected]. Further information on Sobi’s data sharing policy and process for requesting access can be found at: https://www.sobi.com/en/policies. The raw data were part of the SNDS and were available from the Health Data Hub under special authorization.
Additional information
Funding
References
- Iorio A, Stonebraker JS, Chambost H, et al. Establishing the prevalence and prevalence at birth of hemophilia in males: a meta-analytic approach using national registries. Ann Intern Med. 2019;171(8):540–546. doi:10.7326/M19-1208
- FranceCoag. National statistics - demographic: haemophilia A 2023 [cited 2023 Jan 20]. Available from: https://francecoag.org/SiteWebPublic/public/stats/stats_page.jsp?stat2 = on.
- Srivastava A, Santagostino E, Dougall A, et al. Wfh guidelines for the management of hemophilia. 3rd ed. Haemophilia. 2020;26(Suppl. 6):1–158. doi:10.1111/hae.14046
- Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–1939. doi:10.1111/jth.12672
- Ministère de la santé et de la prévention - base de données publique des médicaments. Factane - Summary of Product Characteristics [cited 2023 Jan 24]. Available from: https://base-donnees-publique.medicaments.gouv.fr/affichageDoc.php?specid = 66716833&typedoc = R
- European Medicines Agency. Advate - summary of product characteristics [cited 2023 Jan 24]. Available from: https://www.ema.europa.eu/en/documents/product-information/advate-epar-product-information_en.pdf.
- European Medicines Agency. Elocta - summary of product characteristics. 2019 [cited 2022 March]. Available from: https://www.ema.europa.eu/en/documents/product-information/elocta-epar-product-information_en.pdf.
- Haute Authorité de Santé. Adynovi (rurioctocog alfa pegol). Commission de le Transparence Avis, 10 avril 2019 [cited 2023 June 20]. Available from: https://www.has-sante.fr/jcms/c_2965047/en/adynovi-rurioctocog-alfa-pegol.
- Haute Autorité de Santé. Jivi (damoctacog alfa pegol). Commission de la Transparence Avis, 5 juin 2019 [cited 2023 June 20]. Available from: https://www.has-sante.fr/upload/docs/evamed/CT-17559_JIVI_PIC_INS_Avis3_CT17559.pdf.
- Haute Autorité de Santé. Esperoct (turoctocog alfa pegol). Commission de la Transparence Avis, 10 mars 2021 [cited 2023 June 20]. Available from: https://www.has-sante.fr/jcms/p_3242180/en/esperoct-turoctocog-alfa-pegol.
- Bezin J, Duong M, Lassalle R, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26(8):954–962. doi:10.1002/pds.4233
- Horvais V, Wargny M, Repesse Y, et al. rFVIII-Fc in severe haemophilia A: the incentive switch in case of high risk of joint bleedings. Eur J Clin Invest. 2022;52(10):e13824. doi:10.1111/eci.13824
- Giraud R, Delmotte N, Gensollen S, et al. Recombinant factor VIII Fc fusion protein (rFVIIIFc) in real life: one-year clinical and economic outcomes. Drugs Real World Outcomes. 2021;8(4):527–535. doi:10.1007/s40801-021-00259-2
- Nummi V, Lehtinen AE, Iorio A, et al. Switching from standard to extended half-life FVIII prophylaxis in haemophilia A: comparison of factor product use, bleed rates and pharmacokinetics. Haemophilia. 2022;28(6):e237–e244. doi:10.1111/hae.14649
- Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the systeme national d'information interregimes de l'Assurance Maladie (SNIIRAM) to the systeme national des donnees de sante (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(Suppl 4):S149–S167. doi:10.1016/j.respe.2017.05.004
- Laurendeau C, Goudemand J, Trossaert M, et al. Costs and management of patients with hemophilia A in France: the Hemraude study. Eur J Health Econ. 2022;23(1):23–32. doi:10.1007/s10198-021-01339-4
- Dispositif FranceCoag. Cohorte française des patients vivant avec une maladie hemorragique constitutionnelle. Données descriptives au 31 décembre 2021 2022. Available from: https://francecoag.org/SiteWebPublic/pdfs/rapports_annuels_publics/2021_Rapport_annuel_FC.pdf.
- Berntorp E, Dolan G, Hay C, et al. European retrospective study of real-life haemophilia treatment. Haemophilia. 2017;23(1):105–114. doi:10.1111/hae.13111
- Réseau FranceCoag. Cohorte française des patients atteints de maladies hémorragiques par déficits héréditaires en protéines de la coagulation - Données descriptives 2005 2005 [cited 2023 Jan 24]. Available from: https://www.vie-publique.fr/sites/default/files/rapport/pdf/064000522.pdf.
- Holmström M, Olsson E, Astermark J, et al. Real-world prophylactic usage of recombinant factor VIII Fc in Sweden: a report from the Swedish national registry for bleeding disorders. Haemophilia. 2021;27(4):e554–e558. doi:10.1111/hae.14316
- Peyvandi F, Garagiola I, Boscarino M, et al. Real-life experience in switching to new extended half-life products at European haemophilia centres. Haemophilia. 2019;25(6):946–952. doi:10.1111/hae.13834
- European Medicines Agency. Kovaltry - summary of product characteristics [cited 2023 Jan 24]. Available from: https://www.ema.europa.eu/en/documents/product-information/kovaltry-epar-product-information_en.pdf.
- European Medicines Agency. Helixate NextGen - summary of product characteristics [cited 2023 Jan 24]. Available from: https://www.ema.europa.eu/en/documents/product-information/helixate-nexgen-epar-product-information_en.pdf.
- van der Sluijs M, Huyghe N, Wood C, et al. A survey of physicians’ treatment switching practice in people on long-term prophylaxis for hemophilia in five European countries. Curr Med Res Opin. 2022;38(1):65–73. doi:10.1080/03007995.2021.1991901
- Tagliaferri A, Matichecchia A, Rivolta GF, et al. Optimising prophylaxis outcomes and costs in haemophilia patients switching to recombinant FVIII-Fc: a single-centre real-world experience. Blood Transfus. 2020;18(5):374–385.
- Ay C, Feistritzer C, Rettl J, et al. Bleeding outcomes and factor utilization after switching to an extended half-life product for prophylaxis in haemophilia A in Austria. Sci Rep. 2021;11(1):12967. doi:10.1038/s41598-021-92245-5
- Goldmann G, Marquardt N, Horneff S, et al. Real-world clinical experience of extended half-life recombinant factor VIII Fc fusion protein (rFVIIIFc) in comparison to conventional factor products in patients with severe hemophilia A. J Curr Med Res Opin. 2021;4(05):950–960. doi:10.15520/jcmro.v4i05.422
- Oldenburg J, Hay C, Peyvandi F, et al. Recombinant factor VIII Fc showed better prophylactic effectiveness compared to standard half-life factor VIII in haemophilia A –results from A-SURE, a 24-month prospective, non-interventional study. Res Pract Thromb Haemost. 2022;6(S1):79–80.
- Chhabra A, Spurden D, Fogarty PF, et al. Real-world outcomes associated with standard half-life and extended half-life factor replacement products for treatment of haemophilia A and B. Blood Coagul Fibrinolysis. 2020;31(3):186–192. doi:10.1097/MBC.0000000000000885
- ClinicalTrials.gov. Study identifier NCT04293523. A 48-month study to evaluate long-term effectiveness of elocta on joint health (A-MORE) [cited 2023 May 30]. Available from: https://clinicaltrials.gov/ct2/show/NCT04293523.
- ClinicalTrials.gov. Study identifier NCT03055611. A study to evaluate real-world usage and effectiveness of Elocta and Alprolix in patients with Haemophilia A or B (PREVENT) [cited 2023 May 30]. Available from: https://clinicaltrials.gov/ct2/show/NCT03055611.