ABSTRACT
Objectives
This study aimed to evaluate the prognostic significance of the revised European LeukemiaNet (ELN)−2022 risk stratification model for 123 elderly acute myeloid leukemia (AML) patients treated with decitabine chemotherapy.
Results
Based on the ELN-2022 risk stratification, 15 (12.2%), 51 (41.5%), and 57 (46.3%) patients were classified as having favorable, intermediate, and high-risk AML, respectively. In comparison with the ELN-2017 risk stratification, the ELN-2022 risk stratification re-assigned 26 (21.1%) and three (2.4%) patients to the adverse and favorable risk groups, respectively. Survival analysis revealed distinctive overall survival (OS) outcomes among the ELN-2022 risk groups (6-month OS rate: 73.3%, 52.9%, and 47.7% for favorable, intermediate, and adverse risk, respectively; P = 0.101), with a parallel trend observed in the event-free survival (EFS) (6-month EFS rate: 73.3%, 52.9%, and 45.6% for favorable, intermediate, and adverse risk, respectively; P = 0.049). Notably, both OS and EFS in the favorable risk group were significantly superior in comparison to that of the adverse risk group (OS: P = 0.040, EFS: P = 0.030). Although the ELN-2022 C-index (0.559) was greater than the ELN-2017 C-index (0.539), the result was not statistically significant (P = 0.059). Based on the event net reclassification index, we consistently observed significant improvements in the ELN-2022 risk stratification for overall survival (0.21 at 6 months).
Conclusion
In conclusion, the revised ELN-2022 risk stratification model may have improved the risk classification of elderly AML patients treated with hypomethylating agents compared to the ELN-2017 risk stratification model.
Introduction
Acute myeloid leukemia (AML), marked by morphological and genetic diversity, predominantly affects individuals aged 70 years and older [Citation1–4]. Elderly AML patients exhibit distinct genetic profiles, often influenced by antecedent hematologic conditions like myelodysplastic syndrome (MDS) or myeloproliferative disorders [Citation5,Citation6]. Advances in understanding AML's genetic landscape have led to innovative therapies, combining low-intensity chemotherapy with targeted agents (FLT3 inhibitors, IDH1/2 inhibitors), anti-apoptotic protein inhibitors (such as venetoclax), antibody–drug conjugates (like gemtuzumab ozogamicin), or immune checkpoint inhibitors, such as PD-1, PD-L1, and/or CTLA-4 inhibitors with good results [Citation7–12]. While these treatments broaden the therapeutic options in relapsed or refractory disease [Citation9,Citation10,Citation13,Citation14], elderly AML patients face challenges due to their unsuitability for high-intensity chemotherapy and allogeneic hematopoietic stem cell transplantation (alloHSCT), which are often unsuitable due to elevated treatment-related mortality in contrast to younger patients [Citation2]. Additionally, these patients frequently develop resistance to chemotherapy attributed to genomic mutations that activate alternative signaling pathways [Citation15–17]. Prognosis is further influenced not only by these disease-specific characteristics but also by patient-related factors, such as medical comorbidities and performance status [Citation18,Citation19].
To devise an optimal treatment strategy, a comprehensive understanding of prognostic stratification in AML is necessary. Genomic advances have enabled the categorization of AML based on genetic characteristics [Citation20,Citation21]. In 2017, the European LeukemiaNet (ELN) introduced risk stratification guidelines that improved AML patient management, particularly for those eligible for hematopoietic stem cell transplantation (HST) [Citation15,Citation16,Citation21,Citation22]. Recently, the ELN released the ELN-2022 recommendations for AML diagnosis and management in adults, bringing notable changes, especially in risk stratification [Citation1]. Particularly, the ELN-2022 model excludes the FLT3-ITD allelic ratio due to standardization challenges, and the FLT3-ITD without nucleophosmin 1 (NPM1) mutation is no longer classified as adverse risk due to the impact of FLT3 inhibitors [Citation11,Citation23]. In-frame mutations in the basic leucine zipper region (bZIP) of CCAAT/enhancer binding protein alpha (CEBPα) are now considered favorable risk, regardless of their allelic status [Citation24,Citation25]. Additionally, myelodysplasia-related gene mutations are now classified as adverse risk, marking a significant change from the previous ELN risk stratification model [Citation1].
The prognostic impact of the prior ELN risk stratification for elderly patients on low-intensity chemotherapy is not well-established. Only limited studies have assessed whether the updated ELN-2022 recommendations provide better precision in predicting the prognosis for elderly patients compared to the ELN-2017. Consequently, this retrospective cohort study aims to evaluate the prognostic significance of the updated ELN-2022 risk stratification for elderly patients on decitabine chemotherapy.
Methods
Patients
From 2013 to 2020, we enrolled 153 AML patients aged 65 years or older who met the 2016 World Health Organization (WHO) criteria and were receiving first-line decitabine therapy. Exclusions comprised acute promyelocytic leukemia cases and 30 individuals lacking diagnostic bone marrow samples for targeted sequencing. All included patients received standard decitabine monotherapy (20 mg/m2 intravenous infusion for 5 consecutive days) every 4 weeks. Patients on combined hypomethylating agents with novel therapies like venetoclax or FLT3 inhibitors were not included. Written informed consent was not received from the patients because of the retrospective design of the study. The study adhered to the Declaration of Helsinki and was approved by the Institutional Review Boards of the institution (CNUHH-2022-242).
Molecular analysis: next generation sequencing (NGS)
Genomic DNA was extracted using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA) from cryopreserved bone marrow samples obtained at diagnosis. Deep sequencing targeted the coding regions of 51 genes with recurrent driver mutations, selected based on large cohort studies investigating AML and other myeloid malignancies [Citation26,Citation27]. A custom Agilent (Santa Clara, CA, USA) probe set was used for panel construction, and a variant allele frequency (VAF) ≥ 2.0% defined positivity. The gene list of the NGS panel is provided in Supplementary Table 1.
Statistical analysis
Clinical characteristics were assessed using the chi-square test for categorical variables and the two-sided Student’s t-test for quantitative variables. Genetic risk stratification and definitions of complete remission (CR), CR with incomplete hematologic recovery (CRi), morphologic leukemia-free state (MLFS), partial remission (PR), and no response followed the ELN recommendations [Citation1]. The CR rate and overall response rate (ORR) in patients treated with decitabine monotherapy were calculated for the entire cohort of 123 patients, including those who died before completing the fourth cycle of chemotherapy. The ORR included patients achieving CR, CRi, PR, and MLFS. Treatment response assessments were based on the results after the 4th cycle of treatment. Overall survival (OS) was measured from AML diagnosis to death or last follow-up, while event-free survival (EFS) was defined as the time between the start of therapy and the date of determination of lack of response, loss of response, progression, or death, whichever occurred first. The Kaplan-Meier method and log-rank test were employed for OS and EFS analysis. Cox regression models were used for univariate and multivariate analysis of independent prognostic factors. Significance was set at P < 0.10. C-statistics and the net reclassification index (NRI) statistics were utilized to compare the survival predictive ability of each risk stratification model [Citation28,Citation29]. SPSS Statistics (version 26.0; IBM Corp., Armonk, NY, USA) and EZR software (version 1.54) [Citation30] were used for data analyses.
Results
Characteristics of patients and ELN-2022 re-stratification
A total of 123 elderly AML patients were included in the study, with a median age of 75 years (range: 67–89). The median number of decitabine cycles was six (range: 1–41), and the median follow-up for survivors was 15.0 months (range: 9.5–48.6 months). Among the patients, 59 died before completing the 4th decitabine cycle, primarily due to infection (52.5%) and disease progression (30.5%). Those who died before completing the 4th cycle were categorized as non-responders to the hypomethylating agent (Supplementary Table 2).
The risk distribution between the ELN-2017 and ELN-2022 risk stratification models is shown in . Based on the ELN-2017 risk stratification, 22 (17.9%), 62 (50.4%), and 39 (31.7%) patients were classified as having favorable, intermediate, and adverse risk, respectively. In the ELN-2022 risk stratification, patients were also classified as having favorable (n = 15, 12.2%), intermediate (n = 51, 41.5%), and adverse (n = 57, 46.3%) risk. Notably, the ELN-2022 adverse risk group showed a 15% increase compared to the ELN-2017 group. No significant differences were observed among the three ELN-2022 risk groups concerning age, gender, disease type, and decitabine cycles (). Additionally, the ELN-2022 adverse risk group in elderly AML patients exhibited a higher prevalence of myelodysplasia-related mutations compared to other adverse genetic abnormalities. provides details on the distribution of patients with different genetic abnormalities based on the ELN-2022 risk stratification model.
Figure 1. Risk distribution between the European LeukemiaNet (ELN)−2017 and ELN-2022 risk stratification models. Fav, Favorable; Int, Intermediate; Adv, adverse.
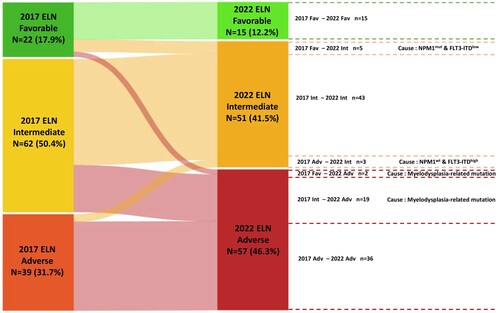
Table 1. Clinical characteristics of acute myeloid leukemia (AML) patients.
Table 2. ELN-2022 model risk distribution according to genetic abnormalities at initial diagnosis.
In comparison to the ELN-2017 model, 76.4% of patients remained in the same risk group, while the ELN-2022 model reassigned 26 (21.1%) patients to the adverse risk group and three (2.4%) to the favorable risk group; these reclassifications were driven by specific mutations in the elderly cohort. Among the initial ELN-2017 favorable risk group (n = 22), seven patients were reclassified, with five patients placed in the ELN-2022 intermediate risk group due to NPM1 mutations with a low FLT3-ITD allelic ratio. The remaining two patients were moved to the adverse risk group due to myelodysplasia-related gene mutations (). Regarding the ELN-2017 intermediate risk group (n = 62), 30.6% were reclassified as adverse risk according to the ELN-2022 model, mainly due to myelodysplasia-related gene mutations. Within the ELN-2017 adverse risk group (n = 39), three patients were shifted to the ELN-2022 intermediate risk group due to having wild-type NPM1 with a high FLT3-ITD allelic ratio.
Response outcomes according to the ELN-2022 risk stratification
After the 4th cycle of decitabine therapy, the CR and CRi rate was 11.4% (14/123, CR = 8, CRi = 6), and the overall response rate was 34.1% (42/123, CR/CRi = 14, MLFS = 6, PR = 22). Subsequent response evaluations were conducted after 8 and 12 cycles of decitabine monotherapy, ultimately resulting in 15 patients achieving CR and 5 patients achieving CRi. The median time to the first first CR/CRi was 4.2 months (range, 3.1-12.4) (Supplementary figure 1).
Although no statistical significance was observed, the CR/CRi rate after 4 cycles of treatment showed a trend with the highest occurrence in the favorable risk group (26.7%) based on the ELN-2022 risk stratification. The overall response rate was highest in the favorable risk group (46.7%), followed by the intermediate risk group (37.3%), and the adverse risk group (28.1%); however, statistical significance was not detected (P = 0.333, ). In comparison with the ELN-2022 risk stratification, the overall response rates of elderly AML patients were not significantly different according to the ELN-2017 risk stratification (favorable risk, 31.8%; intermediate risk, 38.7%; adverse risk, 28.2%, P = 0.533).
Survival outcomes according to the ELN-2022 risk stratification
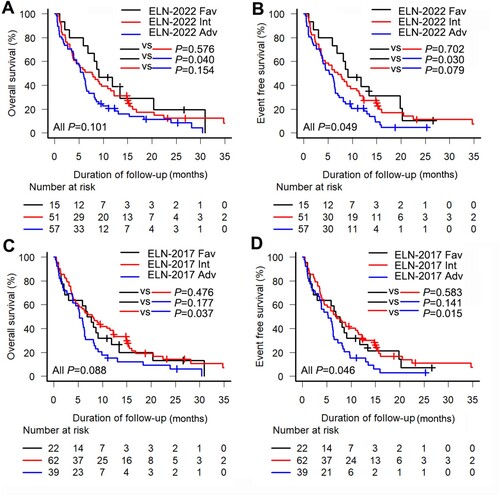
The median OS was 6.3 months (95% CI, 5.1–8.1 months), and the median EFS was 6.3 months (95% CI, 4.5–7.7 months). Survival analyses based on the ELN-2022 and ELN-2017 risk stratification models () demonstrated that the ELN-2022 model exhibited a tendency to distinguish prognostic differences between the risk groups. Specifically, the ELN-2022 favorable, intermediate, and adverse risk groups showed 6-month OS rates of 73.3%, 52.9% and 47.7%, respectively (P = 0.101; favorable OS vs adverse OS, P = 0.040, (a)). The 6-month EFS rate for the favorable, intermediate, and adverse risk groups was 73.3%, 52.9%, 45.6%, respectively (P = 0.049; favorable EFS vs adverse EFS, P = 0.030, (b)). In comparison, the ELN-2017 risk stratification resulted in a 6-month OS and 6-month EFS rates of 59.1% in the favorable risk group, 56.5% and 54.8%, respectively, in the intermediate risk group, and 43.6% for both in the adverse risk group.
Figure 2. Kaplan-Meier curves for elderly acute myeloid leukemia patients according to the European LeukemiaNet (ELN) risk stratification. (a) Overall survival (OS) and (b) event-free survival (EFS) according to the ELN-2022 risk stratification; (c) OS and (d) EFS according to the ELN-2017 risk stratification. Fav, Favorable; Int, Intermediate; Adv, adverse.
In the univariate analysis, the variables considered included the ELN-2022 risk stratification, age, sex, and blast percentage of the bone marrow at diagnosis. Age (OS, HR [95% CI] 1.78 [1.16–2.74], P = 0.009; EFS, HR 1.66 [1.09–2.55], P = 0.020) and the ELN-2022 risk stratification (OS, HR 1.42 [1.06–1.86], P = 0.033; EFS, HR 1.42 [1.06–1.86], P = 0.019) were identified as poor prognostic factors for OS and EFS in the univariate analysis. In the multivariate analysis, the ELN-2022 risk stratification (OS, HR 1.40 [1.05–1.86], P = 0.022; EFS, HR 1.44 [1.08–1.92], P = 0.013) and age (OS, HR 1.82 [1.19–2.78], P = 0.006; EFS, HR 1.70 [1.20–2.58], P = 0.013) were confirmed as independent inferior prognostic factors for OS and EFS ().
Table 3. Univariate and multivariate Cox-proportional hazard regression analyses for elderly acute myeloid leukemia (AML) patients.
Using C-statistics, Harrell’s C-indices for the ELN-2017 and ELN-2022 risk stratification models were 0.54 and 0.56, respectively. Although the ELN-2022 model C-index was numerically higher, the difference was not statistically significant (P = 0.059). For the 29 patients reclassified by the ELN-2022 guidelines, the C-indices were 0.55 (95% CI, 0.46–0.59) and 0.57 (95% CI, 0.53–0.67) for the ELN-2017 and ELN-2022 models, respectively (P = 0.098). The NRI for OS and EFS at 6, 9, and 12 months did not show statistical significance overall (Supplementary Table 3). However, considering the high event prevalence (death, n = 109/123, 88.6% and event, n = 110/123, 89.4%), the event NRI was more meaningful. Based on the event NRI, the ELN-2022 model consistently demonstrated significant improvements at 6, 9, and 12 months for both OS and EFS (event NRIs for OS: 0.21 [95% CI, 0.09–0.33] at 6 months, 0.22 [95% CI, 0.11–0.34] at 9 months, and 0.20 [95% CI, 0.10–0.30] at 12 months; event NRIs for EFS: 0.22 [95% CI, 0.09–0.35] at 6 months, 0.21 [95% CI, 0.11–0.31] at 9 months, and 0.19 [95% CI, 0.09–0.29] at 12 months). In summary, the ELN-2022 risk stratification model significantly improved the risk classification of elderly AML patients by reassigning a notable number (n = 26/123, 21.1%) to higher risk groups.
Survival outcomes of the ELN-2022 reclassified risk groups
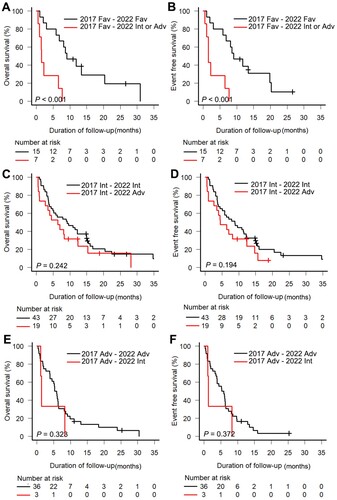
Patients consistently in the favorable risk group (n = 15) exhibited significantly longer survival than those reclassified into other risk groups (n = 7; 6-month OS rate, 73.3% vs. 28.6%; 6-month EFS rate, 73.3% vs. 28.6%, all P < 0.001, (a,b)). Although not statistically significant, patients reassigned to the ELN-2022 adverse risk group displayed poorer survival compared to those consistently in the intermediate risk group (6-month OS rate, 58.1% vs. 52.6%, P = 0.242; 6-month EFS rate, 58.1% vs. 47.4%, P = 0.194, (c,d)). Patients consistently in the adverse risk group showed poor survival outcomes, with no difference between those reassigned based on the ELN-2022 recommendations and the original group (OS, P = 0.323; EFS, P = 0.372, (e,f)).
Figure 3. Kaplan-Meier curves for elderly acute myeloid leukemia patients according to the European LeukemiaNet (ELN) risk stratification. (a) Overall survival (OS) and (b) event-free survival (EFS) in the ELN-2017 favorable risk group; (c) OS and (d) EFS in the ELN-2017 intermediate risk group; (e) OS and (f) EFS in the ELN-2017 adverse risk group. Fav, Favorable; Int, Intermediate; Adv, adverse.
To obtain more information, a subgroup analysis of the genes leading to the ELN-2022 model reclassification was conducted. For patients with NPM1-mutated AML with FLT3-ITDlow, survival was inferior compared to those with other ELN-2022 favorable genotypes (6-month OS rate, 73.3% vs 20.0%, P < 0.001; 6-month EFS rate, 73.3% vs 20.0%, P < 0.001). No survival difference was observed between patients with mutated NPM1 with FLT3-ITDlow and those with FLT3-ITDhigh, although this analysis was limited by small sample numbers (6-month OS rate, 20.0% vs 25.0%, P = 0.796; 6-month EFS rate, 20.0% vs 25.0%, P = 0.796). Moreover, the subgroup analysis of patients with myelodysplasia-related genes showed no difference in survival compared to those with other ELN-2022 adverse genotypes (6-month OS rate, 50.0% vs 45.7%, P = 0.891; 6-month EFS rate, 45.5% vs 45.7%, P = 0.735).
Discussion
This study aimed to assess the prognostic significance of the ELN-2022 risk stratification model for elderly AML patients after decitabine treatment by comparing it with the previous ELN-2017 risk stratification model. Of the 123 patients analyzed, 29 elderly AML patients (23.5%) were reclassified into other risk groups, especially higher risk groups (n = 26, 20.2%), according to the ELN-2022 risk stratification. The distribution of elderly AML patients in Molica et al.'s study (favorable 10.2%, intermediate 56.3%, and adverse 33.3%) [Citation31] resembled that of our study (favorable 17.9%, intermediate 50.4%, and adverse 31.7) for the ELN-2017 model. However, the ELN-2017 model, validated in intensively treated AML cohorts, may not effectively predict outcomes for elderly patients undergoing less intensive chemotherapy. This disparity explains why the ELN-2017 intermediate risk patients under decitabine monotherapy show better survival than the ELN-2017 favorable risk patients and significantly differ from those with an adverse risk. Krzysztof M. et al. reported the ELN-2022 model outcomes for younger and elderly AML patients and found no significant survival differences between the intermediate and adverse risk elderly groups, with only the favorable group showing better results [Citation32]. In our study, applying the revised ELN-2022 model revealed pronounced survival differences across all risk groups, not just the favorable group. Compared to the ELN-2017, there was a distinct difference in OS and EFS between the favorable and adverse risk groups. Notably, 31% of the ELN-2017 favorable risk patients were shifted to the ELN-2022 intermediate or adverse risk groups, which was a significant change from the ELN-2017 model.
In contrast to unclear and generally inferior survival outcomes in elderly patients receiving less intensive treatment in other studies based on the ELN-2022 stratification, our results aligned better with the characteristics of elderly patients in the ELN-2022 model than in the ELN-2017 model [Citation32,Citation33]. This is partly due to the ELN-2022 model no longer classifying mutated NPM1 with a low FLT3-ITD allelic ratio as a favorable risk indicator. Studies evaluating the prognostic effect of a low FLT3-ITD allelic ratio and mutant NPM1 [Citation33–36] have reported intermediate outcomes for AML with these characteristics [Citation35,Citation36]. Schnittger et al. showed minimal survival differences between high and low FLT3-ITD allele ratios in age-dependent NPM1-mutant AML with an FLT3-ITD mutation, especially in patients over 60 years old [Citation33]. Our study, though limited by small sample numbers, found no significant survival difference between elderly patients with this mutation and a low FLT3-ITD allele ratio and those with a high ratio. Moreover, patients with mutated NPM1 and a low FLT3-ITD allelic ratio had shorter survival outcomes compared to those with other favorable genetic abnormalities. Changes in FLT3-ITD mutations with an NPM1 co-mutation status could better stratify elderly AML patients on hypomethylating agent (HMA) monotherapy into favorable and intermediate risk groups under the revised risk stratification. In addition, only three patients in the ELN-2017 adverse risk group were reclassified into the ELN-2022 intermediate risk group. Although no significant survival differences were observed for these patients, the small sample size limits the validation of prognostic significance. Overcoming the poor prognosis of FLT3-ITD-mutated AML with decitabine monotherapy for elderly patients is challenging. FLT3-ITD mutations are frequently detectable in up to 25% of elderly AML patients [Citation37]. Consequently, considering venetoclax combination therapy or FLT3-targeted agents over HMA monotherapy alone is advisable for these patients [Citation14,Citation38].
Recent studies emphasize myelodysplasia-related gene mutations, more frequently detected in secondary AML or AML with myelodysplasia-related changes [Citation39,Citation40]. Rausch et al. demonstrated a significant correlation between these mutations and older age [Citation41]. As elderly AML patients often have a history of MDS or myelodysplastic/myeloproliferative neoplasm, prognosis with the revised ELN-2022 risk stratification model likely reflects adverse genetic abnormalities [Citation42]. In our study, 15.4% of ELN-2017 intermediate risk patients and 1.6% of favorable risk patients were re-assigned to the ELN-2022 adverse risk group due to myelodysplasia-related gene mutations. Those reclassified showed relatively poor survival outcomes with decitabine treatment, though not statistically significant. Additionally, the myelodysplasia-related gene mutations did not significantly differ in response rate and survival from other adverse risk genotypes. However, another study reported better survival for patients with myelodysplasia-related gene mutations, which was possibly influenced by a cohort with mostly younger patients [Citation41]. For transplant-eligible patients, myelodysplasia-related mutations may be overcome through alloHSCT [Citation43], as suggested by Rausch et al. [Citation41]. However, suitable therapeutic options for an adverse prognosis associated with myelodysplasia-related genetic mutations in elderly AML patients (who are often ineligible for transplantation) remain limited.
This retrospective cohort study has inherent limitations in data collection and analysis. To mitigate selection bias, we consecutively collected data from 2013 to 2020. Our focus on elderly AML patients undergoing decitabine monotherapy reflects the standard of care during the data collection period. As the preferred therapeutic approach for elderly AML patients now involves HMAs plus venetoclax or other novel agents, additional follow-up is required to assess the impact of the ELN-2022 stratification on patients treated with various regimens. Despite challenges in accessing novel agents for elderly patients, HMAs continue to be a valuable therapeutic option. Therefore, we believe it is meaningful to validate the ELN-2022 risk stratification in patients receiving decitabine monotherapy, especially elderly AML patients. Lastly, none of the observed comparisons showed statistical significance except for the favorable versus adverse groups. Nevertheless, the ELN-2022 risk stratification showed a better ability to stratify risk groups compared to the ELN-2017 model, suggesting that statistical significance may become evident as more patients become eligible for analysis.
Despite advancements in new drugs, managing elderly AML patients poses ongoing challenges, compounded by various clinical hurdles in drug administration to this vulnerable population. This study, grounded in real-world data with a prolonged follow-up, uniquely demonstrates the prognostic impact of ELN-2022 risk stratification in elderly AML patients receiving less intensive treatment. While ELN risk stratification traditionally relies on data from intensively treated patients, our study offers valuable clinical insights and proportions of elderly AML patients based on the new ELN risk stratification model. These findings enhance our understanding of the prognosis in elderly AML patients and contribute positively to clinical trial design.
Our study demonstrated that the revised ELN-2022 risk stratification model may be better than the ELN-2017 risk stratification model in predicting the prognosis of elderly AML patients treated with HMAs. As interest grows in novel AML therapies, further validation with larger cohorts receiving these agents is necessary to better validate the significance of the revised ELN-2022 risk stratification for elderly AML patients.
Availability of data and materials
The data may be obtained from the corresponding authors on reasonable request.
Ethical approval and consent to participate
This study was approved by the institutional ethics committees of all participating institutions and was conducted in accordance with the Declaration of Helsinki. The committees waived the need for informed consent given the retrospective nature of the study.
Author contributions
MK, JHA and DDHK designed the study; MK and SYA prepared the manuscript; TK, SHJ, GYS, DHY, JJL, MYK, JHP, MGH, JSA, HJK and DDHK critically reviewed the manuscript. All authors have read and approved the final manuscript.
Supplementary material.docx
Download MS Word (336.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345–1377. doi: 10.1182/blood.2022016867
- Prassek VV, Rothenberg-Thurley M, Sauerland MC, et al. Genetics of acute myeloid leukemia in the elderly: mutation spectrum and clinical impact in intensively treated patients aged 75 years or older. Haematologica. 2018;103(11):1853–1861. doi: 10.3324/haematol.2018.191536
- Park E-H, Lee H, Won Y-J, et al. Nationwide statistical analysis of myeloid malignancies in Korea: incidence and survival rate from 1999 to 2012. Br. 2015;50(4):204–217.
- Shin D-Y. Human acute myeloid leukemia stem cells: evolution of concept. Blood Res. 2022;57:S67–S74.
- Erba HP. Prognostic factors in elderly patients with AML and the implications for treatment. ASH Education Program Book. 2007;2007(1):420–428.
- Kim DH, Byun JM, Shin DY, et al. Concomitant ruxolitinib with cytarabine-based induction chemotherapy in secondary acute myeloid leukemia evolving from myeloproliferative neoplasm. Blood Res. 2023;58(3):155–157. doi: 10.5045/br.2023.2023136
- Short NJ, Konopleva M, Kadia TM, et al. Advances in the treatment of acute myeloid leukemia: new drugs and new challenges. Cancer Discov. 2020;10(4):506–525. doi: 10.1158/2159-8290.CD-19-1011
- Wu RH, Zhu CY, Yu PH, et al. The landscape of novel strategies for acute myeloid leukemia treatment: therapeutic trends, challenges, and future directions. Toxicol Appl Pharmacol. 2023;473:116585. doi: 10.1016/j.taap.2023.116585
- Yilmaz M, Kantarjian H, Short NJ, et al. Hypomethylating agent and venetoclax with FLT3 inhibitor “triplet” therapy in older/unfit patients with FLT3 mutated AML. Blood Cancer J. 2022;12(5):77. doi: 10.1038/s41408-022-00670-0
- Wang QQ, Wang HF, Zhao JZ, et al. Venetoclax for arsenic-resistant acute promyelocytic leukaemia. Br J Haematol. 2022;197(5):e58–e60.
- Ahn J-S, Kim H-J. FLT3 mutations in acute myeloid leukemia: a review focusing on clinically applicable drugs. Blood Res. 2022;57:S32–S36.
- Byun JM, Yoo S-J, Kim H-J, et al. IDH1/2 mutations in acute myeloid leukemia. Blood Res. 2022;57(1):13–19. doi: 10.5045/br.2021.2021152
- Konopleva M, Thirman MJ, Pratz KW, et al. Impact of FLT3 mutation on outcomes after venetoclax and azacitidine for patients with treatment-naïve acute myeloid leukemia. Clin Cancer Res. 2022;28(13):2744–2752. doi: 10.1158/1078-0432.CCR-21-3405
- Ohanian M, Garcia-Manero G, Levis M, et al. Sorafenib combined with 5-azacytidine in older patients with untreatedFLT3-ITD mutated acute myeloid leukemia. Am J Hematol. 2018;93(9):1136–1141. doi: 10.1002/ajh.25198
- Ossenkoppele G, Löwenberg B. How I treat the older patient with acute myeloid leukemia. Blood. 2015;125(5):767–774. doi: 10.1182/blood-2014-08-551499
- Klepin HD. Elderly acute myeloid leukemia: assessing risk. Curr Hematol Malig Rep. 2015;10(2):118–125. doi: 10.1007/s11899-015-0257-2
- Rao AV, Valk PJ, Metzeler KH, et al. Age-Specific differences in oncogenic pathway dysregulation in patients With acute myeloid leukemia. J Clin Oncol. 2009;27(33):5580–5586. doi: 10.1200/JCO.2009.22.2547
- Wang ES. Treating acute myeloid leukemia in older adults. Hematology. 2014;2014(1):14–20.
- Hourigan CS, Karp JE. Development of therapeutic agents for older patients with acute myelogenous leukemia. Curr Opin Investig Drugs. 2010;11(6):669–677.
- Hwang SM. Bibliometric analysis of studies about acute myeloid leukemia conducted globally from 1999 to 2018. Blood Res. 2020;55(S1):1–9. doi: 10.5045/br.2020.55.1.1
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196
- Wang B, Liu Y, Hou G, et al. Mutational spectrum and risk stratification of intermediate-risk acute myeloid leukemia patients based on next-generation sequencing. Oncotarget. 2016;7(22):32065–32078.
- Döhner K, Thiede C, Jahn N, et al. Impact of NPM1/FLT3-ITD genotypes defined by the 2017 European LeukemiaNet in patients with acute myeloid leukemia. Blood. 2020;135(5):371–380. doi: 10.1182/blood.2019002697
- Sierra J, Nomdedeu JF. CEBPA bZip mutations: just a single shot. Blood. 2021;138(13):1091–1092. doi: 10.1182/blood.2021011263
- Taube F, Georgi JA, Kramer M, et al. CEBPA mutations in 4708 patients with acute myeloid leukemia: differential impact of bZIP and TAD mutations on outcome. Blood. 2022;139(1):87–103. doi: 10.1182/blood.2020009680
- Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–2221. doi: 10.1056/NEJMoa1516192
- Ley TJ, Miller C, Ding L, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–2074. doi: 10.1056/NEJMoa1301689
- Kang L, Chen W, Petrick NA, et al. Assessing correlation of clustered mixed outcomes from a multivariate generalized linear mixed model. Stat Med. 2015;34(4):704–720. doi: 10.1002/sim.6374
- Pencina MJ, D’Agostino RB, Steyerberg EW. Editorial. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4183
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244
- Molica M, Mazzone C, Niscola P, et al. Identification of predictive factors for overall survival and response during hypomethylating treatment in very elderly (≥75 years) acute myeloid leukemia patients: a multicenter real-life experience. Cancers (Basel). 2022;14(19):4897.
- Mrózek K, Kohlschmidt J, Blachly JS, et al. Outcome prediction by the 2022 European LeukemiaNet genetic-risk classification for adults with acute myeloid leukemia: an Alliance study. Leukemia. 2023;37(4):788–798. doi: 10.1038/s41375-023-01846-8
- Schnittger S, Bacher U, Kern W, et al. Prognosis in patients with MDS or AML and bone marrow blasts between 10% and 30% is not associated with blast counts but depends on cytogenetic and molecular genetic characteristics. Leukemia. 2011;25(8):1361–1364. doi: 10.1038/leu.2011.80
- Pratcorona M, Brunet S, Nomdedéu J, et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood. 2013;121(14):2734–2738. doi: 10.1182/blood-2012-06-431122
- Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111(5):2776–2784. doi: 10.1182/blood-2007-08-109090
- Sakaguchi M, Yamaguchi H, Najima Y, et al. Prognostic impact of low allelic ratio FLT3-ITD and NPM1 mutation in acute myeloid leukemia. Blood Adv. 2018;2(20):2744–2754. doi: 10.1182/bloodadvances.2018020305
- Palmieri R, Paterno G, De Bellis E, et al. Therapeutic choice in older patients with acute myeloid leukemia: a matter of fitness. Cancers (Basel). 2020;12(1):120.
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629. doi: 10.1056/NEJMoa2012971
- Montalban-Bravo G, Kanagal-Shamanna R, Class CA, et al. Outcomes of acute myeloid leukemia with myelodysplasia related changes depend on diagnostic criteria and therapy. Am J Hematol. 2020;95(6):612–622. doi: 10.1002/ajh.25769
- Yoshizato T, Nannya Y, Atsuta Y, et al. Genetic abnormalities in myelodysplasia and secondary acute myeloid leukemia: impact on outcome of stem cell transplantation. Blood. 2017;129(17):2347–2358. doi: 10.1182/blood-2016-12-754796
- Rausch C, Rothenberg-Thurley M, Dufour A, et al. Validation and refinement of the 2022 European LeukemiaNet genetic risk stratification of acute myeloid leukemia. Leukemia. 2023;37(6):1234–1244. doi: 10.1038/s41375-023-01884-2
- Koenig KL, Sahasrabudhe KD, Sigmund AM, et al. Aml with myelodysplasia-related changes: development, challenges, and treatment advances. Genes (Basel). 2020;11(8):845. doi: 10.3390/genes11080845
- Song GY, Kim T, Ahn SY, et al. Allogeneic hematopoietic cell transplantation can overcome the adverse prognosis indicated by secondary-type mutations in de novo acute myeloid leukemia. Bone Marrow Transplant. 2022;57(12):1810–1819. doi: 10.1038/s41409-022-01817-0
